Abstract
Tropomyosin plays a key role in controlling calcium regulated sarcomeric contraction through its interactions with actin and the troponin complex. The focus of this review is on striated muscle tropomyosin isoforms and the in vivo approach we have taken to define the functional differences among these isoforms in regulating cardiac physiology. In addition, we address specific regions within tropomyosin that differ among the isoforms to impart differences in the physiological performance of muscle and the sarcomere itself. There is a high degree of amino acid identity among the three striated muscle α-, β-, and γ-tropomyosin isoforms; this identity ranges from 86% – 91%. We employ transgenic mouse model systems that express the different tropomyosin isoforms or chimeric tropomyosin molecules specifically in the myocardium. Results show the three isoforms differentially regulate the rates of cardiac contraction and relaxation, along with conferring differences in myofilament calcium sensitivity and sarcomere tension development. We also found the putative troponin T binding regions of tropomyosin (amino acids 175-190 and 258-284) appear to a play significant role in imparting these physiological differences that are observed during cardiac and sarcomeric contraction/relaxation. In addition, we have successfully used chimeric tropomyosin molecules to rescue cardiomyopathic diseased mice by normalizing sarcomeric performance. These studies illustrate not only the importance of tropomyosin structure and function for understanding muscle physiology, but also demonstrate how this information can potentially be used for gene therapy.
Introduction
Tropomyosin (Tm) is an integral sarcomeric protein that functions in the calcium regulatory process of striated muscle contraction. At low calcium (Ca2+), Tm localizes to the outer domain of actin and sterically blocks the myosin binding sites on actin to prevent myosin-actin interaction and sarcomeric contraction (1, 2). Recent studies by Lehman (2) and Lehrer (3) demonstrate that at low Ca2+, the C-terminal end of troponin I drives Tm to the blocked state on actin. Ca2+ release from the sarcoplasmic reticulum and its binding by troponin C (TnC) helps to alleviate this blocked state of Tm on the myosin binding sites. Ca2+ binding by troponin also promotes Tm movement away from the blocking position and stabilizes Tm in the closed position on the inner domain of actin (2) This movement of Tm facilitates myosin binding to actin and further movement of Tm onto the inner actin domain (open state). Thus, Tm’s position on actin appears to oscillate between the inner and outer domains of actin. In addition, the binding of Ca2+ to TnC also removes the inhibitory status of troponin I (TnI) which also facilitates sarcomeric contraction. Although much is known regarding the mechanism of how calcium regulates the physical interactions of the sarcomeric thin filament proteins, there are still many unanswered questions. For example, since troponin T (TnT) is known to bind to Tm in two distinct places, are there differential functions in these binding regions? Since Tm has at least 3 principle striated muscle isoforms (α–Tm, β–Tm, and γ–Tm), are there functional differences that distinguish them and what are those differences? If and where does TnI bind to Tm? To answer some of these questions, we have employed a strategy that incorporates homogenous Tm isoforms and chimeric Tm proteins into functional sarcomeres in an in vivo setting where Tm can function in the natural environment and manifest its full effect. The in vivo settings that we employ are transgenic and knockout mice that have the innate property of regulating the total amount of Tm that is produced in the myocyte and incorporated into the sarcomere. We believe this approach provides for a comprehensive understanding of the role of Tm isoforms in the sarcomere.
Striated Muscle Tm Isoform Expression
There are 4 Tm genes: TPM1 (α-Tm), TPM2 (β-Tm), TPM3 (γ-Tm), and TPM4 (δ-Tm). Each of these genes encodes numerous tissue and developmental specific isoforms through mechanisms of differential promoters, alternative splicing, and differential 3′ end processing/polyadenylation. For example, the TPM1 gene expresses at least 10 distinct α-Tm mRNAs that are striated and smooth muscle specific, brain specific, and cytoskeletal specific isoforms. The focus of this review is on the Tm striated muscle isoforms. There are 3 principle striated muscle isoforms; for nomenclature purposes, they will be designated as α-Tm, β-Tm, and γ-Tm. There is another newly-discovered striated muscle TPM1 isoform called TPM1-κ which is solely expressed in cardiac tissue in humans, rats, and chickens, but not in the mouse (4, 5). The 3 principle Tm isoforms share a high degree of amino acid homology: α-Tm and β-Tm are 87% identical, α-Tm and γ-Tm are 91% identical, and β-Tm and γ-Tm are 86% identical (Figure 1). The differences in these 284 amino acids are scattered throughout the molecule, with 25 of the 34 differences between α- and β-Tm residing in the carboxyl half of the proteins. Striated muscle Tm isoforms are expressed early during murine development and remain transcribed throughout the lifetime of the mouse (6). That Tm is an essential protein has been demonstrated by murine knockout models of Tm. Results show that when α-Tm is removed from the murine genome by homologous recombination, the developing mouse dies between 10–14 embryonic days (7, 8), which corresponds to development of the myocardium. Furthermore, knockout of the low molecular weight non-muscle γ-Tm isoforms result in a failure of both embryonic development and cell survival (9); when β-Tm is knocked out, there is a failure in early developmental processes (Rajan and Wieczorek, unpublished data). These results illustrate that Tm expression occurs early in development, remains active throughout the lifespan of the mouse, and is essential for development and viability of the mouse.
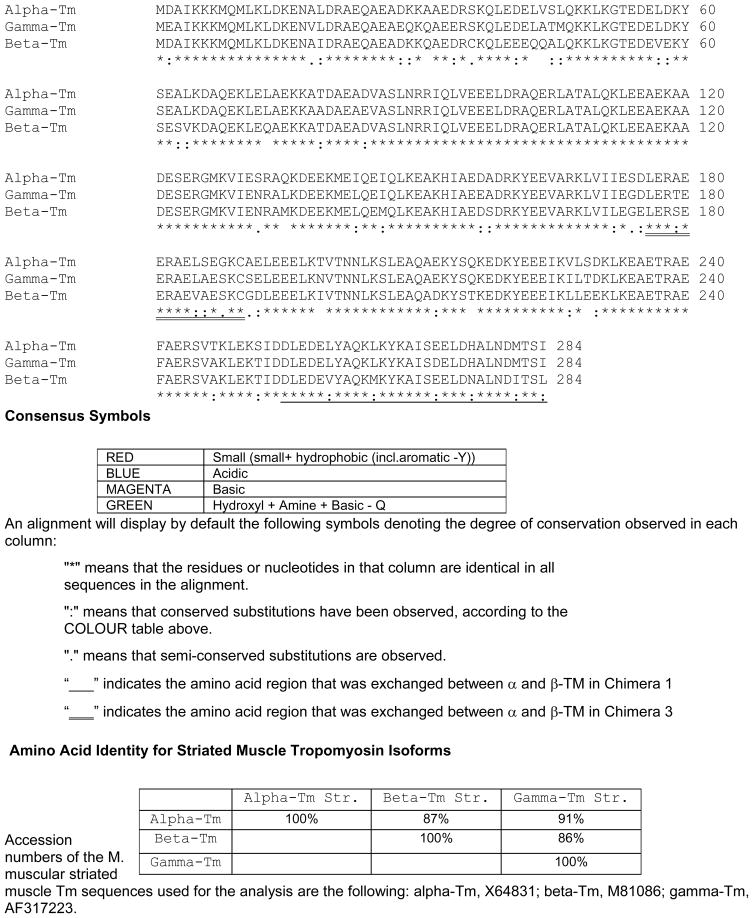
Figure 1.
Multiple sequence alignments of the mouse striated muscle Tm isoforms. The complete protein sequence (amino acids 1-284) for the striated muscle α-Tm, β-Tm, and γ-Tm isoforms were aligned. Comparisons of the amino acid biophysical properties are color coded and indicated by dots below the amino acid sequence, as designated in the legend. Chimera 1 sequences that were exchanged from α-Tm to β-Tm are underlined (amino acids 258-284). Chimera 3 sequences that were exchanged from α-Tm to β-Tm are double underlined (amino acids 175-190). Chimera 2 sequences exchanged both regions from α-Tm to β-Tm (amino acids 175-190 and 258-284). The mouse skeletal muscle α-Tm, β-Tm and γ-Tm nucleotide sequences are accession numbers X64831, M81086, and AF317223, respectively. The amino acid sequence analysis was conducted according to methods described in Larkin et al. (44).
β-Tm and γ-Tm Expression in the Heart
To address whether there are functional differences among these highly homologous Tm striated muscle isoforms, we utilized a transgenic mouse approach to investigate their in vivo function. In addition to obtaining in vivo information on these proteins, they can be evaluated in myofilament preparations where until recently, it was not possible to conduct exchange reactions with Tm proteins. Biophysical studies show that different Tm isoforms exhibit differences in their preferred position on actin (10). With Tm’s position on actin appearing to oscillate, results show skeletal muscle Tm (αβ-Tm) is usually positioned closer to the inner domain of actin (i.e. closed state), whereas cardiac (αα-Tm) and smooth muscle Tm are preferentially positioned near the outer domain of actin (blocked state). To determine whether there are functional differences between α- and β-Tm, in addition to the aforementioned structural differences, we generated transgenic (TG) mice that express the entire β-Tm protein specifically in the heart. Previous studies demonstrate the heart expresses α- and β-Tm during embryogenesis and fetal development, but β-Tm expression decreases soon after birth (6). Thus, the adult myocardium essentially has homogenous expression of only striated muscle α-Tm. By directing expression of different Tm isoforms to the heart, any morphological or physiological changes can be attributed to the exogenous Tm expression. Results demonstrate that with transgenic β-Tm expression, the total amount of Tm in the heart remains unchanged, and there are no alterations in the expression of other contractile proteins (11, 12). In addition, TG mice that express wildtype α-Tm in the heart do not exhibit any morphological nor physiological changes when compared with non-transgenic (NTG) littermate controls (13, 14). When the entire β-Tm protein is expressed at a level of 55% total striated muscle Tm, there are no morphological or pathological alterations in cardiac or sarcomeric structures (11). However, there are physiological changes that occur; this level of expression causes a decrease in the maximum rate of relaxation and an increase in the time to one-half relaxation of the heart (Table 1). In addition, there is an increase in myofilament Ca2+ sensitivity and a decrease in the rightward shift of the Ca2+-force relation induced by cAMP-dependent phosphorylation. Also, results with in vitro motility and force assays show that cardiac troponin + Tm (αα-Tm), but not skeletal troponin + Tm (αβ-Tm), limits the allosteric enhancement of force that is generated (15). In total, these results demonstrate there are physiological differences between α- and β-Tm that affect sarcomeric performance at the thin filament level and the overall functional performance of the heart.
Table 1.
Comparison of Cardiac and Sarcomeric Physiological Parameters in Various Transgenic Mouse Models with Respect to α-Tm (NTG) Hearts
| Mouse Model | Physiological parameter | |||
|---|---|---|---|---|
| Maximum rate of contraction | Maximum rate of relaxation | Myofiber Ca2+ sensitivity | Sarcomere tension development | |
| α-Tm | 100 % | 100 % | 100 % | 100 % |
| β-Tm | 100 % | ↓ | ↑ | ↓ |
| γ-Tm | ↑ | ↑ | ↓ | ↑ |
| Chimera 1: α→β switch of c-terminal TnT binding region | ↓ | ↓ | ↓ | ↓ |
| Chimera 3: α→β switch of internal TnT binding region | ↓ | ↓ | ↑ | 100 % |
| Chimera 2: α→β switch of both TnT binding regions | ↓ | ↓ | 100 % | ↓ |
| FHC α-Tm180 | 100 % | ↓ | ↑ | ↑ |
| FHC α-Tm180/Chimera 1 | 100 % | ↓ | 100 % | 100 % |
Having identified functional differences between α- and β-Tm, we next addressed whether there are physiological differences between α- and γ-Tm. As mentioned, there is a 91% amino acid identity between these isoforms. The γ-Tm isoform is not expressed in murine myocardium, but is expressed in embryonic, newborn, and adult skeletal musculature (16). In adults, γ-Tm is primarily expressed in slow twitch skeletal muscle (i.e. soleus). To identify whether there are physiological differences between α- and γ-Tm, we generated TG mice that express γ-Tm specifically in the heart (17). These mice express 50% γ-Tm and 50% α-Tm protein in their hearts, with no change in the total amount of Tm protein that is synthesized. Results show there are no morphological abnormalities in the γ-Tm TG sarcomeres or hearts. However, there are significant functional alterations in cardiac performance (Table 1). Physiological assessment of these TG mouse hearts reveals a hyperdynamic effect on systolic and diastolic performance; the maximum rates of contraction and relaxation are increased, as is the heart rate, with a concomitant decrease in the time to peak pressure and half time to relaxation. Analysis of detergent-extracted fiber bundles demonstrates a decreased sensitivity to Ca2+ in force generation and a decrease in the length-dependent Ca2+ activation with no changes in interfilament spacing (17).
In summary, the results from the TG mice expressing β- or γ-Tm in their hearts clearly illustrate that there are functional differences among the 3 striated muscle isoforms. Expressing either β-Tm or γ-Tm at ~ 50% level in the heart can influence several physiological properties to opposite effect. Transgenic expression of β-Tm leads to decreased rates of relaxation and increased myofilament Ca2+ sensitivity, whereas γ-Tm expression leads to increased rates of contraction and relaxation, with decreased myofilament Ca2+ sensitivity. The differential expression of these Tm isoforms may serve as a potential mechanism for the regulation of thin filament function in the divergent myofiber types found in skeletal musculature. Interestingly, preliminary results show that when all 3 isoforms are expressed in equimolar amounts in the heart, there is a dominant effect of the γ-Tm isoform with respect to cardiac performance; the hearts exhibit an increase in the rates of contraction and relaxation, along with a decrease in their myofilament Ca2+ sensitivity (Jagatheesan and Wieczorek, in prep.).
Identifying Regions of Functional Difference within Tm Isoforms
Recent work shows that within the Tm molecule, there is differential binding of Tm’s seven periods to actin based on destabilizing alanine amino acid clusters (18). Periods 1 and 5 are the most critical for actin binding, with the remaining periods being considered “secondary sites”. Also, the alanine clusters can positively influence cooperative actin binding (18). Further, the highly conserved aspartic acid at position 137 destabilizes the middle region of Tm which results in increased flexibility of the region (19); this region is important for the cooperative activation of the thin filament by myosin.
With the finding that there are functional differences in cardiac performance when the entire β-Tm molecule is expressed in the heart, we addressed which amino acids might confer these functional differences. We decided to initially examine the troponin T (TnT) binding regions of Tm. Previous studies determined that TnT binds to Tm in 2 distinct regions: at the carboxyl end of the molecule (amino acids 258-284), and internally in the region of amino acids 175-190 (20, 21). To determine whether there are isoform specific differences related to contractile performance at the carboxyl end of Tm, we exchanged the terminal 27 amino acids of α-Tm (amino acids 258-284) for the corresponding β-Tm sequence. NMR studies of this region indicate the amino acid strands at the carboxyl end are splayed apart to form a cleft allowing insertion of the amino terminus of the extending Tm molecule (22, 23). There are 5 amino acid differences between the terminal 27 amino acids of α-Tm and β-Tm in which 2 are highly conservative (L260V; I284L). The remaining 3 amino acids (L265M, H276N, and M281I) have substantial differences in size, and the H276N has a change in charge (one more negative charge in β-Tm). This chimeric protein (Chimera 1: α-TM: amino acids 1-257, and β-Tm: 258–284) was expressed in TG mouse hearts and results show there are no morphological or pathological changes in the hearts or myofibers of these mice (24). However, these hearts do exhibit significant functional alterations in cardiac performance; there are significant decreases in their rates of contraction and relaxation (Table 1), with concomitant increases in the time to peak pressure and end diastolic pressure (24). In myofilaments, this chimeric protein induces depression of maximum tension and ATPase rate, with a significant decrease in Ca2+ sensitivity. This data demonstrates that the Tm isoform specific carboxyl terminus is a critical determinant of sarcomere performance, tension development, and myofilament Ca2+ sensitivity.
To investigate whether the putative internal TnT binding site in Tm (amino acids 175-190) can impart Tm isoform specific differences in cardiac performance, we generated a TG mouse model with another chimeric Tm protein (25). This chimeric Tm protein (Chimera 3: replacing amino acids 175-190 of α-Tm with the corresponding sequence in β-Tm) encodes 5 amino acid changes (D175Q, A179S, L185V, S186A, Q188S). There are no significant differences in charge or size of the replacement sequences; however, there is a dramatic decrease in the hydrophobicity of this region with the amino acid substitutions. Although there were no gross morphological or pathological differences in these TG hearts, there were changes in cardiac performance (Table 1). Using the work-performing heart method, we determined there were decreases in the maximum rates of contraction and relaxation. To determine whether this decreased performance was due to blunting of the β-adrenergic response system, we checked the isoproterenol response of these hearts. Results show the TG hearts respond to increased concentrations of isoproterenol by attaining normal control rates of contraction and relaxation, indicating there is no major alteration in cAMP/PKA-dependent phosphorylation by increasing the rate of Ca2+ cycling (25). To examine the correlation between physiological results from the whole heart and the sarcomere, we conducted pCa-tension experiments using skinned fiber bundles. Results show there is a significant increase in myofilament Ca2+ sensitivity. These results demonstrate the putative internal TnT binding domain of Tm can increase Ca2+ sensitivity of the thin filament and affect sarcomeric performance at the myofilament level which culminates in altered function at the whole heart level (25).
Our studies show substitution of the α-Tm internal or carboxyl terminal TnT binding regions with β-Tm domains lead to decreases in the rates of cardiac contraction or relaxation, most likely through a mechanism involving the troponin complex. Interestingly, substitution of these regions between α- and β-Tm leads to opposite effects on myofilament Ca2+ sensitivity; substitution of the internal α-Tm region with β-Tm leads to an increase in Ca2+ sensitivity, whereas substitution of the carboxyl α-Tm region leads to a decrease in Ca2+ sensitivity. These results demonstrate that both regions play an integral role in the determination of myofilament Ca2+ sensitivity, and suggests a mechanism such that the specific Tm isoform sequence interaction with TnT is an important determinant of myofilament Ca2+ sensitivity. In addition to affecting myofilament Ca2+ sensitivity, the carboxyl region also plays a role in the development of myofilament tension in response to Ca2+, whereas the internal region does not appear to influence tension development. The precise reason for this effect on sarcomeric force may be due to the importance of the carboxyl region in cooperativity and interactions between overlapping adjacent Tm molecules. Studies by Lehman et al. show that the position of Tm on actin differs with respect to Tm isoform composition (2, 10). Whether these biophysical differences in Tm isoform interactions with actin are influenced by the troponin binding regions of Tm is currently unknown.
To test the hypothesis that both TnT binding regions of Tm act synergistically by having the same Tm isoform amino acid sequence to attain normal myofilament Ca2+ sensitivity, we generated an additional construct that simultaneously exchanged both TnT binding regions of α-Tm with the reciprocal sequence from β-Tm. Our previous chimeric Tm TG mice had either the carboxyl or internal TnT binding sequence being β-Tm while the reciprocal region was α-Tm. In the current mouse model, the exogenous chimeric Tm molecule (Chimera 2) has both TnT binding regions being β-Tm amino acid sequences (26). Results show that similar to other TG mouse hearts, these chimeric hearts also exhibit abnormalities in cardiac performance, specifically with decreases in their rates of contraction and relaxation (Table 1). However, with regard to myofilament Ca2+ sensitivity, there were no differences when compared with littermate control hearts. This shows that in the α-Tm backbone, the TnT binding sequences of the β-Tm isoform can normalize myofilament Ca2+ sensitivity. A potential reason for this ability to normalize myofilament Ca2+ sensitivity is that the slight TnT binding differences (which lead to either increased or decreased myofilament Ca2+ sensitivity) that are found with α-Tm versus β-Tm compensate when both regions are α-Tm or β-Tm. However, it should be noted that additional Tm sequences influence Ca2+ sensitivity as demonstrated by the fact that myofilaments incorporating the complete β-Tm molecule exhibit increased Ca2+ sensitivity (12). Also, mutations in α-Tm at amino acids 40 and 54 (associated with dilated cardiomyopathy) and amino acids 63 and 70 (associated with hypertrophic cardiomyopathy) lead to changes in myofilament Ca2+ sensitivity (27–29). When both TnT binding regions are substituted in the α-Tm backbone, there is also a decrease in maximum myofilament developed tension, similar to when only the carboxyl region is substituted; as such, the C-terminus of Tm plays a dominant role in determining force development in myofilaments.
What Tm Mutations Tell Us About Tm Function?
Mutations have been found to occur in human striated muscle Tm isoforms that are associated with skeletal and cardiac muscle diseases. A recent review of Tm mutations associated with skeletal myopathies, with a particular emphasis on nemaline myopathy, can be found in Kee and Hardeman (30). This current work will focus on Tm mutations and cardiomyopathies, particularly familial hypertrophic cardiomyopathy (FHC) and dilated cardiomyopathy (DCM). Eleven mutations have been found in α-Tm that lead to FHC, and three known DCM mutations are associated with α-Tm (31). For FHC α-Tm mutations, six of these reside in the TnT binding region (Ile172Thr; Asp175Asn; Glu180Gly; Glu180Val; Leu185Arg; Glu192Lys). At least five of the FHC Tm mutations confer an increased Ca2+ sensitivity of the myofilament, coupled with decreased systolic and diastolic cardiac function (31–33). To examine what physiological effects FHC mutations in the internal TnT binding domain of α-Tm might confer, we generated two transgenic mouse models that incorporate human FHC mutations (13, 34, 35). The FHC α-TmAsp175Asn TG mouse hearts exhibit a mild hypertrophic phenotype. In contrast, the FHC α-TmGlu180Gly hearts develop severe concentric hypertrophy with significant ventricular fibrosis and atrial enlargement that progressively increases from 2.5 months and results in death between 4.5 and 6 months. In vivo physiological analyses show severe impairment of both contractility and relaxation in hearts of both FHC mouse models. Both the maximum rates of contraction and relaxation are significantly depressed in these mice. In addition, myofilaments that contain the mutant FHC proteins demonstrate an increased activation of the thin filament through enhanced Ca2+ sensitivity of steady-state force (13, 35); this increase in myofiber Ca2+ sensitivity is a common feature of many of the FHC associated Tm mutations (31). There is also a correlation between increased myofiber Ca2+ sensitivity, decreased relaxation rates, and a blunted response to β-adrenergic stimulation (36). Further, isolated cardiomyocytes from TG FHC α-TmGlu180Gly hearts exhibit an increase in Ca2+ sensitivity of force production (37). These alterations of increased myofilament Ca2+ sensitivity, with additional effects on systolic and diastolic function that are found with FHC α-Tm mutations in the internal TnT binding region are in agreement with the structure-function studies of the chimeric Tm proteins described above. Thus, it appears that mutations or substitutions of amino acids in the 175–190 region of α-Tm often lead to decreased rates of contraction and relaxation, along with increases in myofilament Ca2+ sensitivity. What is interesting is that the FHC and DCM point mutations exhibit alterations in cardiac pathology, Ca2+ sensitivity, and cardiac contractile function with mutant Tm protein expression being from 30–50% of the total Tm levels. However, exchanging Tm’s internal or carboxyl TnT binding regions at a 40–60% protein level (as with Chimeras 1, 2 and 3) results in similar physiological changes without pathological alterations in either the sarcomere or the heart. This suggests that the TnT binding domains among the different Tm isoforms are largely conserved, and the FHC point mutations disrupt this conservation.
Using Targeted Tm Regions to Rescue Hypertrophic Cardiomyopathy Mice
As described above, the FHC α-TmGlu180Gly mice develop a severe cardiomyopathic condition that mimics many of the symptoms found in FHC patients, including increased Ca2+ sensitivity of myofilaments, diastolic dysfunction, cardiac hypertrophy, myocardial fibrosis, and early cardiac demise. One of the goals of generating animal systems that mimic human conditions is to develop and test potential therapeutic approaches for these diseases. To this end, we created a double-transgenic mouse model by crossing the FHC α-Tm180 mice with TG mice expressing a chimeric Tm containing α-Tm with the carboxyl β-Tm terminus (Chimera 1: α-Tm: amino acids 1-257; β-Tm amino acids 258-284) described previously. By mating mice from the two models together, we tested the hypothesis that an attenuation of myofilament calcium sensitivity would modulate the severe physiological and pathological consequences of the FHC mutation. The double-transgenic mice not only display normalized myofilament Ca2+ sensitivity, but also exhibit a normal cardiac phenotype with no pathological abnormalities and apparent normalization of life span (Table 1) (38). Physiological analyses of these rescued mice show improved cardiac function by 4 months of age and continuing up to 1 year, whereas the FHC α-Tm180 mice die between 4.5 and 6 months of age due to sudden cardiac death. These results demonstrate that alterations in myofilament response to Ca2+ by modification of contractile proteins can prevent the pathological and physiological effects of this disease.
In addition to replacement of contractile proteins as a potential therapeutic approach to the treatment of cardiac disease, other strategies are also being employed. Many of these approaches are focused on the regulation of calcium and calcium handling (39). Also, previous work shows there are Ca2+ cycling defects in the FHC α-Tm180 mice due to decreased expression of phospholamban and SERCA2a (35). Recent studies by Wolska (40) demonstrate that the FHC α-Tm180 neonates can exhibit improved cardiac morphology and in situ hemodynamic performance when administered SERCA2a by gene transfer. In addition, crossing the FHC α-Tm180 mice with phospholamban knockout mice prevents the development of hypertrophy and cardiac dysfunction (41). Also, cardiomyocytes that exhibit relaxation defects can be rectified in α-Tm180 mouse model by genetically crossing them with mice expressing parvalbumin, a calcium buffer (42). Interestingly, inhibition of calcineurin, a Ca2+-regulated phosphatase, by cyclosporine and FK506 prevents cardiac hypertrophy in transgenic mice expressing β-Tm, tropomodulin, and myosin light chain in the heart (43). However, the FHC α-Tm180 mice do not have increased levels of activated calciuneurin, suggesting a non-calcineurin hypertrophic signaling pathway in these mice (35). Regardless, increased Ca2+ sensitivity and its effect on myofilament function appear to play a critical role in the development of pathological cardiac hypertrophy, and modulation of Ca2+ sensitivity through various pathways may prove to be a viable approach for treatment of cardiovascular disease.
Conclusions
As cited above, Tm plays a major role in the regulation of sarcomeric function. Defining the physiological significance of specific Tm regions is essential for understanding its normal function, both as an integral component of the thin filament and as a potential component of a signaling complex to the cell when its function is abnormal and triggers a cascade of events culminating in myocyte hypertrophy or dilation. Striated muscle Tm isoforms have amino acid differences in key functional domains that can cause significant physiological differences in the performance of both the sarcomere and the heart. These Tm isoforms increase the plasticity of the myofiber and its ability to respond to mechanical, hormonal, and nutritional challenges. Without changes in other contractile protein expression, our studies show that differences solely in Tm isoform expression, or the TnT binding domains of Tm, can result in differences in the following physiological parameters: (1) increased or decreased rates of cardiac muscle contraction and relaxation; (2) increased or decreased myofilament sensitivity to Ca2+; and (3) changes in length-dependent Ca2+ activation. As such, Tm plays a major role in the regulation of thin filament function. Future investigations will focus on how the different Tm isoforms interact with troponin I and actin, and whether they differentially regulate steric hindrance in the myofiber.
Acknowledgments
We thank Jon Neumann for production of the transgenic mice and Maureen Bender for care of the animals. This work was supported in part by grants from the National Heart, Lung, and Blood Institute of the NIH to DFW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lehman W, Craig R. Tropomyosin and the steric mechanism of muscle regulation. Tropomyosin. In: Gunning P, editor. Adv Exp Med Biol. Vol. 644. 2008. pp. 95–109. [DOI] [PubMed] [Google Scholar]
- 2.Lehman W, Galinska-Rakocsy A, Hatch V, Tobacman L, Craig R. Structural basis for the activation of muscle contraction by troponin and tropomyosin. J Mol Biol. 2009;388:673–681. doi: 10.1016/j.jmb.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mudalige A, Tao T, Lehrer S. Ca2+-dependent photocrosslinking of tropomyosin residue 146 to residues 157-163 in the c-terminal domain of troponin I in reconstituted skeletal muscle thin filaments. J Mol Biol. 2009;389:575–583. doi: 10.1016/j.jmb.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denz CR, Narshi A, Zajdel RW, Dube DK. Expression of a novel cardiac-specific tropomyosin isoform in humans. Biochem Biophy Res Commun. 2004;320:1291–1297. doi: 10.1016/j.bbrc.2004.06.084. [DOI] [PubMed] [Google Scholar]
- 5.Rajan S, Jagatheesan G, Karam CN, Alves ML, Bodi I, Schwartz A, et al. Molecular and functional characterization of a novel cardiac specific human tropomyosin isoform. Circulation. doi: 10.1161/CIRCULATIONAHA.109.889725. In revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muthuchamy M, Pajak L, Howles P, Doetschman T, Wieczorek DF. Developmental analysis of tropomyosin gene expression in embryonic stem cells and mouse embryos. Mol Cell Biol. 1993;13:3311–3323. doi: 10.1128/mcb.13.6.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rethinasamy P, Muthuchamy M, Hewett T, Boivin G, Wolska BM, Evans CR, et al. Molecular and physiological effects of α-tropomyosin ablation in the mouse. Circ Res. 1998;82:116–123. doi: 10.1161/01.res.82.1.116. [DOI] [PubMed] [Google Scholar]
- 8.Blanchard EM, Iizuka K, Christe M, Conner DA, Geisterfer-Lowrance A, Schoen FJ, et al. Targeted ablation of the murine alpha-tropomyosin gene. Circ Res. 1997;81:1005–1010. doi: 10.1161/01.res.81.6.1005. [DOI] [PubMed] [Google Scholar]
- 9.Hook J, Lemckert F, Qin H, Schevzov G, Gunning P. Gamma tropomyosin gene products are required for embryonic development. Mol Cell Biol. 2004;24:2318–2323. doi: 10.1128/MCB.24.6.2318-2323.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehman W, Hatch V, Korman V, Rosol M, Thomas L, Maytum R, et al. Tropomyosin and actin isoforms modulate the localization of tropomyosin strands on actin filaments. J Mol Biol. 2000;302:593–606. doi: 10.1006/jmbi.2000.4080. [DOI] [PubMed] [Google Scholar]
- 11.Muthuchamy M, Grupp IL, Grupp G, O’Toole BA, Kier AB, Boivin GP, et al. Molecular and physiological effects of overexpressing striated muscle b-tropomyosin in the adult murine heart. J Biol Chem. 1995;270:30593–30603. doi: 10.1074/jbc.270.51.30593. [DOI] [PubMed] [Google Scholar]
- 12.Palmiter KA, Kitada Y, Muthuchamy M, Wieczorek DF, Solaro RJ. Exchange of β- for α-tropomyosin in hearts of transgenic mice induces changes in thin filament response to Ca2+, strong cross-bridge binding, and protein phosphorylation. J. Biol. Chem. 1996;271:11611–11614. doi: 10.1074/jbc.271.20.11611. [DOI] [PubMed] [Google Scholar]
- 13.Prabhakar R, Boivin GP, Grupp IL, Hoit B, Arteaga G, Solaro RJ, Wieczorek DF. A familial hypertrophic cardiomyopathy α-tropomyosin mutation causes severe cardiac hypertrophy and death in mice. J Mol Cell Cardiol. 2001;33:1815–1828. doi: 10.1006/jmcc.2001.1445. [DOI] [PubMed] [Google Scholar]
- 14.Wolska BM, Keller RS, Evans CC, Palmiter KA, Phillips RM, Muthuchamy M, et al. Correlation between myofilament response to Ca2+ and altered dynamics of contraction and relaxation in transgenic cardiac cells that express beta-tropomyosin. Circ Res. 1999;84:745–751. doi: 10.1161/01.res.84.7.745. [DOI] [PubMed] [Google Scholar]
- 15.Clemmens E, Entezari M, Martyn D, Regnier M. Different effects of cardiac versus skeletal muscle regulatory proteins on in vitro measures of actin filament speed and force. J Physiol. 2005;566:737–746. doi: 10.1113/jphysiol.2005.084194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pieples K, Wieczorek DF. Tropomyosin 3 increases striated muscle isoform diversity. Biochem. 2000;39:8291–8297. doi: 10.1021/bi000047x. [DOI] [PubMed] [Google Scholar]
- 17.Pieples K, Arteaga G, Solaro RJ, Grupp I, Lorenz JN, Boivin GP, et al. Tropomyosin 3 expression leads to hypercontractility and attenuates myofilament length-dependent Ca2+ activation. Am J Physiol Heart Circ Physiol. 2002;283:H1344–H1353. doi: 10.1152/ajpheart.00351.2002. [DOI] [PubMed] [Google Scholar]
- 18.Singh A, Hitchcock-DeGregori S. A peek into tropomyosin binding and unfolding on the actin filament. PLoS ONE. 2009;4:e6336. doi: 10.1371/journal.pone.0006336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumida J, Wu E, Lehrer S. Conserved Asp-137 imparts flexibility to tropomyosin and affects function. J Biol Chem. 2008;283:6728–6734. doi: 10.1074/jbc.M707485200. [DOI] [PubMed] [Google Scholar]
- 20.Pearlstone JR, Smillie LB. Effects of troponin-I plus-C on the binding of troponin-T and its fragments to alpha-tropomyosin Ca2+ sensitivity and cooperativity. J Biol Chem. 1983;258:2534–2542. [PubMed] [Google Scholar]
- 21.Zot AS, Potter JD. Structural aspects of troponin-tropomyosin regulation of skeletal muscle contraction. Annu Rev Biophy Biophy Chem. 1987;16:535–559. doi: 10.1146/annurev.bb.16.060187.002535. [DOI] [PubMed] [Google Scholar]
- 22.Greenfield NJ, Palm T, Hitchcock-DeGregori SE. Structure and interactions of the carboxyl terminus of striated muscle alpha-tropomyosin: it is important to be flexible. Biophys J. 2002;83:2754–2766. doi: 10.1016/S0006-3495(02)75285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenfield NJ, Huang YJ, Swapna GV, Bhattacharya A, Rapp B, Singh A, et al. Solution NMR structure of the junction between tropomyosin molecules: implications for actin binding and regulation. J Mol Biiol. 2006;364:80–96. doi: 10.1016/j.jmb.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 24.Jagatheesan G, Rajan S, Petrashevskaya N, Schwartz, Boivin G, Vahebi S, et al. Functional importance of the carboxyl-terminal region of striated muscle tropomyosin. J Biol Chem. 2003;278:23204–23211. doi: 10.1074/jbc.M303073200. [DOI] [PubMed] [Google Scholar]
- 25.Jagatheesan G, Rajan S, Schulz EM, Ahmed RPH, Petrashevskaya N, Schwartz, et al. An internal domain of β-tropomyosin increases myofilament Ca2+ sensitivity. Am J Physiol Heart Circ Physiol. 2009;297:H181–H190. doi: 10.1152/ajpheart.00329.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jagatheesan G, Rajan S, Petrashevskaya N, Schwartz, Boivin GP, Arteaga GM, et al. Physiological significance of troponin T binding domains in striated muscle tropomyosin. Am J Physiol Heart Circ Physiol. 2004;287:H1484–H1494. doi: 10.1152/ajpheart.01112.2003. [DOI] [PubMed] [Google Scholar]
- 27.Mirza M, Marston S, Willott R, Ashley C, Mogensen J, McKenna W, et al. Dilated cardiomyopathy mutations in three thin filament regulatory proteins results in a common functional phenotype. J Biol Chem. 2005;280:28498–28506. doi: 10.1074/jbc.M412281200. [DOI] [PubMed] [Google Scholar]
- 28.Rajan S, Ahmed R, Jagatheesan G, Petrashevskaya N, Boivin G, Urboniene D, et al. Dilated cardiomyopathy mutant tropomyosin mice develop cardiac dysfunction with significantly decreased fractional shortening and myofilament calcium sensitivity. Circ Res. 2007;101:205–214. doi: 10.1161/CIRCRESAHA.107.148379. [DOI] [PubMed] [Google Scholar]
- 29.Heller M, Nili M, Homsher E, Tobacman L. Cardiomyopathic tropomyosin mutations that increase thin filament Ca2+ sensitivity and tropomyosin N-domain flexibility. J Biol Chem. 2003;278:41742–8. doi: 10.1074/jbc.M303408200. [DOI] [PubMed] [Google Scholar]
- 30.Kee AJ, Hardeman EC. Tropomyosins in skeletal muscle diseases. Tropomyosin. In: Gunning P, editor. Adv Exp Med Biol. Vol. 644. 2008. pp. 143–157. [DOI] [PubMed] [Google Scholar]
- 31.Wieczorek DF, Jagatheesan G, Rajan S. The role of tropomyosin in heart disease. Tropomyosin. In: Gunning P, editor. Adv Exp Med Biol. Vol. 644. 2008. pp. 132–142. [DOI] [PubMed] [Google Scholar]
- 32.Tardiff J. Sarcomeric proteins and familial hypertrophic cardiomyopathy: linking mutations in structural proteins to complex cardiovascular phenotypes. Heart Fail Rev. 2005;10:237–248. doi: 10.1007/s10741-005-5253-5. [DOI] [PubMed] [Google Scholar]
- 33.Westfall MV, Borton AR, Albayya FP, Metzger JM. Myofilament calcium sensitivity and cardiac disease. Circ Res. 2002;91:525–531. doi: 10.1161/01.res.0000034710.46739.c0. [DOI] [PubMed] [Google Scholar]
- 34.Muthuchamy M, Pieples K, Rethinasamy P, Hoit B, Grupp IL, Boivin GP, et al. Mouse model of a familial hypertrophic cardiomyopathy mutation in α-tropomyosin manifests cardiac dysfunction. Circ Res. 1999;85:47–56. doi: 10.1161/01.res.85.1.47. [DOI] [PubMed] [Google Scholar]
- 35.Prabhakar R, Petrashevskaya N, Schwartz A, Aronow B, Boivin GP, Molkentin JD, Wieczorek DF. A mouse model of familial hypertrophic cardiomyopathy caused by a α-tropomyosin mutation. Mol Cell Biochem. 2003;251:33–42. [PubMed] [Google Scholar]
- 36.Evans CC, Pena JR, Phillips RM, Muthuchamy M, Wieczorek DF, Solaro RJ, Wolska BM. Altered hemodynamics in transgenic mice harboring mutant tropomyosin linked to hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2000;279:H2414–H2423. doi: 10.1152/ajpheart.2000.279.5.H2414. [DOI] [PubMed] [Google Scholar]
- 37.Michele DE, Gomez CA, Hong KE, Westfall MV, Metzger JM. Cardiac dysfunction in hypertrophic cardiomyopathy mutant tropomyosin mice is transgene-dependent, hypertrophy-independent, and improved by β-blockade. Circ Res. 2002;91:255–262. doi: 10.1161/01.res.0000027530.58419.82. [DOI] [PubMed] [Google Scholar]
- 38.Jagatheesan G, Rajan S, Petrashevskaya N, Schwartz, Boivin GP, Arteaga GM, Solaro RJ, et al. Rescue of tropomyosin-induced familial hypertrophic cardiomyopathy mice by transgenesis. Am J Physiol Heart Circ Physiol. 2007;293:H949–H958. doi: 10.1152/ajpheart.01341.2006. [DOI] [PubMed] [Google Scholar]
- 39.del Monte F, Hajjar RJ. Targeting calcium cycling proteins in heart failure through gene transfer. J Physiol. 2003;546:49–61. doi: 10.1113/jphysiol.2002.026732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penia J, Goldspink P, Prabhakar R, del Monte F, Hajjar R, Wieczorek DF, Wolska B. Neonatal gene transfer of SERCA2a improves the response to β-adrenergic stimulation in the α-tropomyosin (Glu180Gly) mouse model of familial hypertrophic cardiomyopathy (Abstract). AHA Scientific Conference on Molecular Mechanisms of Growth, Death, and Regeneration in the Myocardium; 2003. [Google Scholar]
- 41.Penia J, Urboniene D, Goldspink P, Kranias E, Wieczorek DF, Wolska B. Phospholamban knockout prevents the development of hypertrophy and cardiac dysfunction in a FHC α-tropomyosin (Glu180Gly) mouse model (Abstract). AHA Scientific Conference on Molecular Mechanisms of Growth, Death, and Regeneration in the Myocardium; 2003. [Google Scholar]
- 42.Coutu P, Bennett CN, Favre EG, Day SM, Metzger JM. Parvalbumin corrects slowed relaxation in adult cardiac myocytes expressing hypertrophic cardiomyopathy-linked alpha-tropomyosin mutations. Circ Res. 2004;94:1235–1241. doi: 10.1161/01.RES.0000126923.46786.FD. [DOI] [PubMed] [Google Scholar]
- 43.Sussman MA, Lim HW, Gude N, Taigen T, Olson EN, Robbins J, et al. Prevention of cardiac hypertrophy in mice by calcineurin inhibition. Science. 1998;281:1690–1693. doi: 10.1126/science.281.5383.1690. [DOI] [PubMed] [Google Scholar]
- 44.Larkin MA, Blackshields G, Brown NP, Chednna R, McGettigan PA, McWilliam H, et al. Clustal W and clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]