Abstract
As part of a study of elicited angiogenesis, hyaluronan (HA)-based hydrogels crosslinked by polyethylene glycol diacrylate (PEGDA) were loaded with combinations of the cytokine growth factors vascular endothelial growth factor (VEGF), angiopoietin-1 (Ang-1), keratinocyte growth factor (KGF) and platelet derived growth factor (PDGF). GF release in vivo was controlled by covalent incorporation of thiol-modified heparin into thiolated HA hydrogels, which were injected into the ear pinnae of mice and allowed to crosslink in situ. GF release in vivo was controlled by covalent incorporation of thiol-modified heparin in the gels. The ears were harvested at 7 or 14 days post implantation, and vascularization evaluated via a Neovascularization Index (NI). The study demonstrates that in situ gelling implants produced no gross inflammation, redness or swelling, and an improved tolerance compared to HA-based dry film implants. All treatments showed significantly more vascularization than either contralateral ears or ears receiving a sham surgery. The maximum response was observed after 14 days in the ears receiving 0.3% Hp, gelatin-containing gels loaded with VEGF+KGF (NI = 3.91). The study revealed injected growth factor-loaded HA-based hydrogels can successfully produce localized controllable vascularization, while minimizing tissue necrosis, polymorphonuclear leukocytes and inflammation. The ability to target and controllably release growth factors can prove a useful tool in specific diseased tissue/organ angiogenesis.
1. INTRODUCTION
Recent approaches to in vivo therapeutic tissue regeneration have often involved one-time delivery of single growth factors. For example, engineered tissue has been implanted in liver, pancreas, muscle and other tissues along with a single cytokine administered either as a liquid aliquot or within the solid implant. It has been shown by many investigators that such therapeutic cytokine delivery can elicit an initial angiogenic response [1–3]. However, long term viability and functionality of the blood vessels formed has not been demonstrated [4].
One important reason for the lack of greater clinical success in producing therapeutically viable capillary networks appears to be the intricacy of the angiogenic process. Development of new capillary vessels from existing microvessels is initiated by the appearance in tissue of peptide growth factors such as vascular endothelial growth factor (VEGF), a potent endothelial mitogen [5]. Subsequent release of proteinase enzymes leads to degradation of the extracellular matrix (ECM) and parent capillary basement membrane, liberating ECM-bound growth factors. Dividing endothelial cells are then able to spread into the extracellular space and form new capillary sprouts. These sprouts grow by continuing endothelial proliferation, eventually forming a lumen as they organize into tubules. New vessels reach maturity when contiguous vessels connect and a new basement membrane is formed [6, 7].
Capillary formation is a central aspect of many physiologic functions, including tissue and organ growth, wound healing, female reproductive function, as well as pathologic tumor formation. Its stages of are known to be regulated by over two dozen stimulatory or inhibitory cytokine growth factors that act in complex, specific sequences [6–10]. Furthermore, various ECM glycoproteins, glycosaminoglycans (GAGs) and proteins, including collagen, have also been shown to have regulatory effects on microvessel growth [11,12]. The cascade of events surrounding the development of mature microvessel networks depends on interactions between all these factors. As a result, one possible way to enhance the angiogenic response to exogenously delivered biomaterials would be to introduce more than one growth factor with the implant.
Chemically modified forms of hyaluronic acid (HA) have been developed to create biocompatible, macroporous hydrogels that have applications in tissue engineering [13] and for drug discovery and evaluation [14]. These HA hydrogels offer a modular [15], clinically-relevant [16], simple, material with which researchers can create customized compositions with specific compliance to recreate a three-dimensional cell culture environment [17]. The only non-sulfated GAG in the ECM of vertebrates, HA consists of repeating disaccharide units (β-1,4-D glucuronic acid—β-1,3- N acetyl-D glucosamine), with an overall molecular weight between 100 and 5000 kDa [18]. HA is a strong inducer of angiogenesis, although this phenomenon depends on molecular weight [19–21]. High molecular weight HA (n-HA) has been shown to inhibit angiogenesis [22], but low molecular weight fragments (o-HA) have the opposite effect, stimulating endothelial cell proliferation and migration [22–25]. Consequently, when HA is used as a delivery vehicle for growth factors, it can be expected to actively participate in tissue responses, rather than serving only as an inert conduit. For example, Peattie et. al. showed in a mouse ear pinna study that delivery of VEGF in HA-based hydrogels produced an angiogenic effect greater than the sum of the effects of HA and VEGF delivered singly [26]. However, although the resulting endothelial proliferation was substantial, mature functional microvessels were not observed.
One of the critical hypotheses underlying our studies is that simultaneous delivery of one growth factor chosen to stimulate an initial angiogenic response and a second selected for later vessel maturation can promote the formation of lasting, mature, perfusable vessel networks. This hypothesis was developed on the basis of the use of the incorporation of small amounts of thiol-modified heparin (Hp) covalently into HA-based gels to control the release of the growth factor bFGF from the gels [27]. Heparin is known to sequester growth factors in the ECM, primarily through electrostatic interactions between N- and O-sulfated residues of heparin and the lysine and arginine residues of the GF [28–32]. Heparin binding is thought to control GF release in vivo and stabilize GFs against thermal denaturation and degradation by ECM proteinases, while retaining their bioactivity. Initial studies of in vivo delivery of two cytokines in a mouse ear pinna model produced microvessels organized into recognizable tubular networks filled with erythrocytes [33, 34]. In addition, small amounts of thiol-modified Heparin (Hp) covalently incorporated in HA-based gels successfully regulated the relative rates at which growth factors are released from the gels in vitro [35] and thereby augmented elicited vessel maturation and functionality [28, 29,36].
In the present study, we continue to test those expectations through implantation of HA-Hp gels containing VEGF along with either keratinocyte growth factor (KGF), angiopoietin 1 (Ang-1) or platelet-derived growth factor (PDGF) in a subcutaneous mouse model. However, based on previous observations, our technique has been modified to deliver the gel components in aqueous form and allow them to crosslink in situ. Here we hypothesize that this approach should not only maintain the strong biologic response elicited by film delivery of these growth factors, but also should result in reduced inflammation in the surgerized ear. As a result, ears implanted with aqueous gels that crosslink primarily in situ would be expected to show an overall improved tolerance compared to ears receiving film implants, with no cases of gross inflammation, redness or swelling, and reduced numbers of polymorphonuclear leukocytes.
2. MATERIALS AND METHODS
2.1 Materials
Fermentation-derived HA (sodium salt, MW = 750 kDa) was a gift from Novozymes Biopolymers A/S (Bagsvaerd, Denmark). 1-ethyl-3-[3-(dimethylamino)propyl]carbodiimide (EDCI), 3,3’-dithiobis(propanoic acid) (DTP) and hydrazine hydrate were from Aldrich Chemical Co. (Milwaukee, WI). Dithiothreitol (DTT) was from Diagnostic Chemicals Limited (Oxford, CT). Gelatin (bovine skin, type B, gel strength approximately 225 Bloom) (Gtn), heparin (sodium salt from porcine mucosa, unfractionated, MW = 15 kDa), Dulbecco’s phosphate-buffered saline (DPBS) and bovine serum albumin (BSA) were purchased from Sigma Chemical Co. (St. Louis, MO). Angiopoietin-1 (Ang-1, human recombinant, 476 amino acid, MW = 66 kDa), bFGF (human recombinant, 154 amino acid, MW = 17.2 kDa), keratinocyte growth factor (KGF/FGF-7, human recombinant, 163 amino acid, MW = 18.9 kDa), platelet-derived growth factor (PDGF-AA, human recombinant, 25 amino acid, MW = 25 kDa) and VEGF (human recombinant, 165 amino acid, MW = 46 kDa) were from R&D Systems, Inc. (Minneapolis, MN). Poly(ethylene glycol)-diacrylate (PEGDA, MW = 3400 Da) was obtained from Nektar Therapeutics (Huntsville, AL). Male Balb/C mice were provided by Simonson Laboratories (Gilroy, CA).
2.2 Chemical syntheses
HA-DTPH, Gtn-DTPH and Hp-DTPH, the thiol-modified forms of hyaluronic acid, gelatin and heparin, were synthesized as previously described [34–36]. In brief, HA (or Gtn or Hp) was modified with 3,3 dithiobis(propanoic hydrazide) (DTP) in the presence of EDCI, then reduced with dithiothreitol (DTT), resulting in HA-DTPH after dialysis. Final thiol substitution percentages were determined using a modified DTNB method. The free thiol contents of HA-DTPH and Hp-DTPH were 42 and 56 thiols per 100 disaccharide units, respectively. The free thiol content of Gtn-DTPH was 0.45 mmol/g, indicating that approximately 37% of the carboxylate groups in Gtn were modified. Subsequently, all materials were lyophilized for storage purposes.
2.3 Hydrogel preparation
Four separate sets of HA-based hydrogels were prepared, HA, HA:Hp, HA/Gtn and HA/Gtn:Hp. For HA/Gtn gels, the total masses of HA-DTPH and Gtn-DTPH were equal. Within each Hp-containing set, gels were fabricated with three different total Hp concentrations, 0%, 0.03% or 0.3% w/w relative to total HA-DTPH or HA-DTPH + Gtn-HTPH. For gelatin-free HA gels, 1.25% (w/v) HA-DTPH and 1.25% (w/v) Hp-DTPH in DPBS solution (pH 7.4) were pre-mixed in the appropriate ratio (i.e. 99.7:0.3 (v/v)). These HA:Hp solutions were then crosslinked by mixing with 4.5% (w/v) PEGDA in DPBS in a volume ratio of 4:1, maintaining solution pH at 7.4 by dropwise addition of Na2HPO4 / NaH2PO4 as needed (Figure 1). For HA/Gtn gels, half of HA-DTPH was replaced with Gtn-DTPH, while the Hp-DTPH proportion remained unchanged.
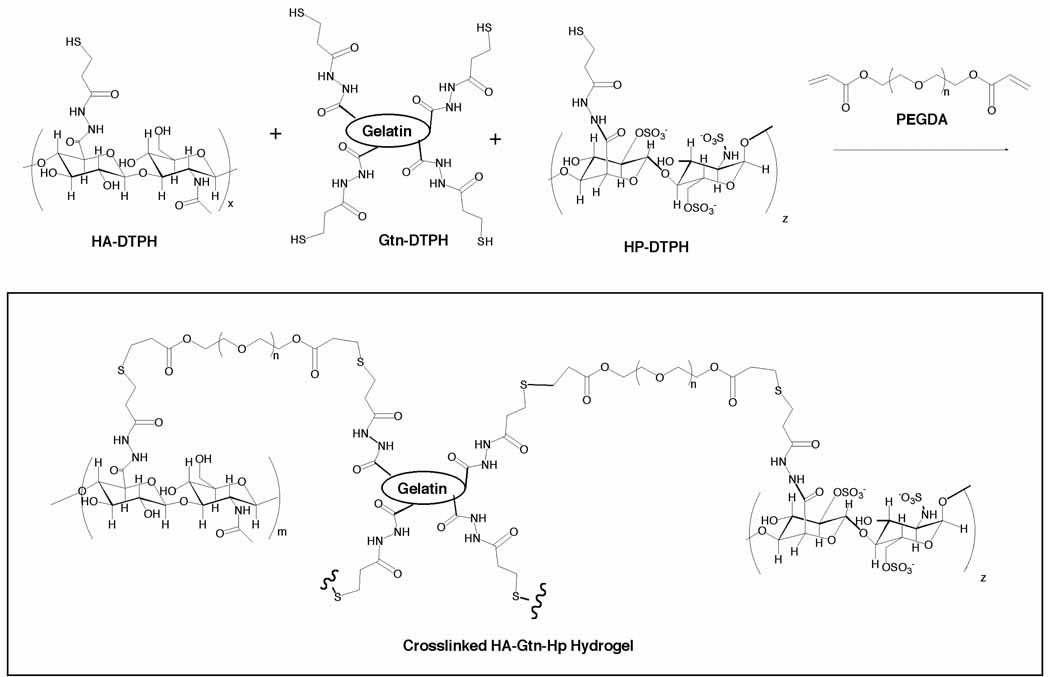
Figure 1.
Chemical structure of crosslinked HA-DTPH, Hp-DTPH and Gtn-DTPH
In the hydrated state, PEGDA-crosslinked HA-DTPH hydrogels have been shown to take on an open, porous structure suitable for storing molecules within the gel [35]. Accordingly, growth factors were non-covalently incorporated by mixing them with the dissolved HA-DTPH solution before crosslinking. Experiments were performed with concentrations that resulted in 25 ng of each growth factor delivered to the animal for non-heparinized gels. However, 100 ng of each was delivered in heparin-containing disks, to compensate for the slower anticipated release.
2.4 Surgical and experimental procedures
The ability of growth factor-loaded HA-DTPH hydrogels to stimulate angiogenesis was tested on live animal models. All procedures were carried out with the full approval of the Oregon State University Institutional Animal Care and Use Committee. Male Balb/C mice aged 6–8 weeks were anesthetized using 2.5% isoflurane with an inhalation anesthesia system (Summit Medical Equipment, Inc., Bend, OR). Once a deep general anesthetic plane had been reached, a shallow 4–5mm incision was created through the superficial skin on the posterior pinna of the right ear. A blunt probe was inserted through the incision, and a 5-mm pocket created under the skin.
Hydrogels were formed at room temperature immediately prior to delivery, placing volumes of each solution in a small, polystyrene dish in a 1:2 ratio of acrylate to thiol functionalities, and mixing with gentle swirling for 2 minutes. Crosslinking of these gels proceeds rapidly, within 10–15 minutes, even at room temperature. A 50 µL aliquot containing 0.25 mg HA-DTPH was therefore removed immediately while the mixture was still liquid, before it could fully crosslink, and delivered to the pocket by pipette. The ear was held in a fixed position until a gelled bolus formed in situ in the pocket, and the incision was allowed to close without sutures. Mice recovered without incident within 5–10 minutes after these brief surgeries, and the incisions healed in 2–3 days.
On days 7 or 14 post implantation, the mice were anesthetized by overdose of isofluorane (5%) and sacrificed by cervical dislocation. Both the surgical (right) and contralateral ears were retrieved and fixed in formalin. The ears were then embedded in paraffin, thin sectioned parallel to the pinna surface, and stained with hemotoxylin and eosin (H&E). Microvessels were counted directly from the slides at 400× magnification after identification marks on each slide were covered so the observer was blind to which treatment group the slide belonged. Ten locations per ear from within the pocket area, were selected at random for quantification resulting in microvessel density representative of that ear.
2.5 Data analysis
A total of 6 animals (n = 6) received implants for each treatment case and each time point. Microvessel density data are accordingly presented as mean ± standard deviation. Due to the number of observers in our laboratory a protocol has been developed to minimize the effects of intra-observer variability. For that purpose, a set of 22 slides from 11 treatment cases were set aside and vessels in them counted by each member of the laboratory. Group averages were then calculated, and a correction factor determined for each individual observer to equate that individual’s counts to the group means. This correction procedure has been applied to all the data presented in this paper. The vessel counts presented here were performed independently by two observers.
To account for the number of vessels present before surgery as well as those induced by the surgical procedure alone, so that the effect of the gel and/or growth factor addition could be identified, a dimensionless Neovascularization Index (NI) was defined as,
| (1) |
where refers to the vessel count from the implanted ear of a particular animal minus that of its contralateral ear, averaged over all the animals in a particular treatment group, represents the same quantity for a sham surgery control case that underwent pocket formation but received no implant, and mean CL is the average count from all contralateral ears over all treatment groups. Thus defined, NI represents the number of additional vessels present post-implant in a treatment group, minus the additional number due to the surgical procedure alone, normalized by the average contralateral count [26]. (Mathematically, NI can be expected to be a Gaussian random variable, since both (treatment—CL) and (sham—CL) are themselves random variables. Calculation of its mean and standard deviation therefore requires appropriately linearly combining the properties of the constituent terms.)
Statistical significance was determined using a two-way ANOVA and post hoc Fisher’s PLSD analysis (StatView 5.0, SAS Institute Inc., Cary, NC), with significance taken at the level p≤ 0.05.
3. RESULTS
Hydrogels for this study were prepared with thiolated derivatives of HA, Gtn and Hp. Prior to crosslinking, the degree of substitution on the HA or HA/Hp was found to be 42%, based on the number of available glucuronate residues modified [34]. Gels formed quickly by the addition crosslinking reaction of thiols to PEGDA at a neutral to slightly basic pH (Figure 1). The resulting disulfide-crosslinked hydrogels are stable and highly bio- and cytocompatible both in vitro and in vivo [34–39]. They can be dehydrated to a film state, which swells reversibly with no degradation when rehydrated in an aqueous solution [37]. In addition, their physico-chemical properties of mechanical strength, ability to degrade in vivo without systemic sequelae and favorable interactions with VEGF and other important growth factors [26] make them ideally suited for delivery of bioactive agents.
In vivo microvessel development in response to implantation of gels that were allowed to undergo in situ gelation was assessed in an ear pinna model for a series of control and experimental conditions, in both the presence and absence of heparin. Non-heparinized cases included (i) an HA gel preloaded with VEGF and KGF (ii) an HA gel preloaded with VEGF and Ang-1 (iii) an HA gel preloaded with VEGF and PDGF (iv) an HA/Gtn gel preloaded with VEGF and KGF (v) an HA/Gtn gel preloaded with VEGF and Ang-1 and (vi) an HA/Gtn gel preloaded with VEGF and PDGF. Heparinized cases included the same GF and HA/Gtn combinations as experiments (i–vi), but with either 0.03% or 0.3% Hp (experiments vii–xviii). There was also a sham surgical case in which a pocket was formed but no implant was delivered (experiment xix). These cases provide controls allowing the neovascular effects attributable to the individual gel components as well as the interaction between preloaded gels and cytokine combinations to be separately identified.
Representative photographic images of tissue microvasculaturization show significant differences that characterized the tissue response to each treatment (Figure 2). However, for all animals, the appearances of microvascular networks in contralateral ear sections were very similar regardless of the treatment of the surgical ear (Figure 2a). No evidence of any systemic response to the implant was observable at either time point for any of the treatment cases. Chondrocytes were widely distributed in this tissue, along with numerous hair follicles and sebaceous and other glands typical of ear pinnae. Contralateral ear sections contained relatively low capillary densities, but the endothelial borders of those capillaries that were present were well defined. Red cells were confined within fully intact, well-developed capillary walls.
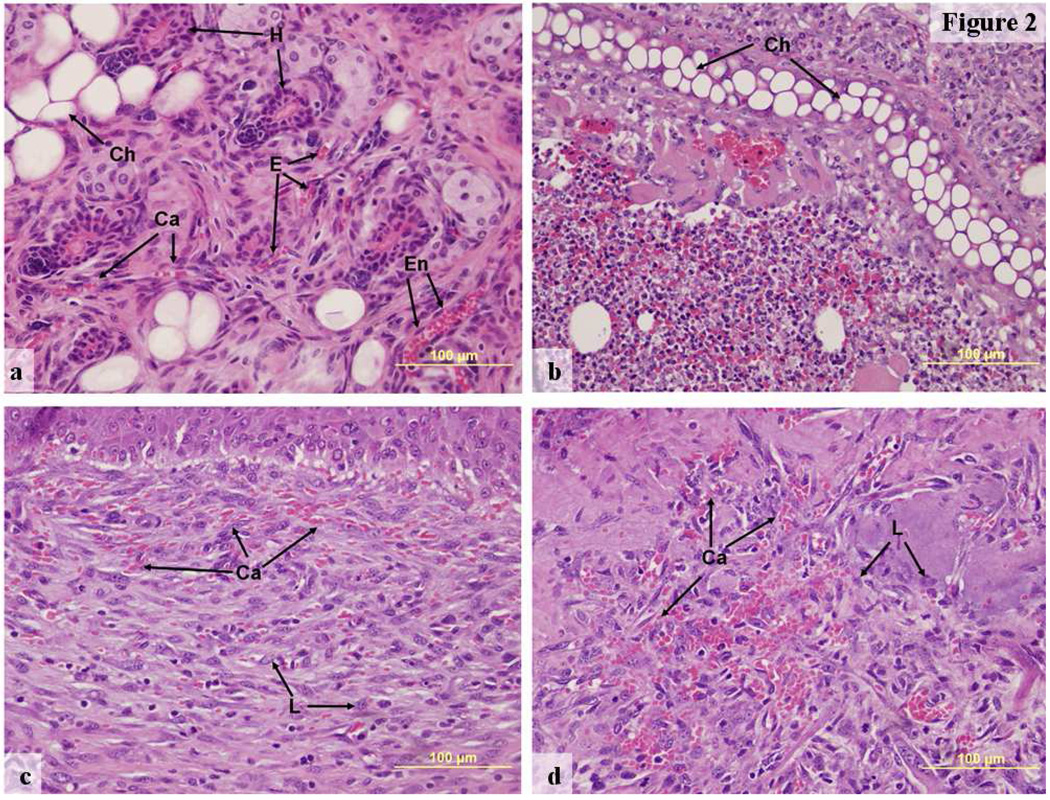
Figure 2.
Hematoxylin and eosin stained representative images of ear tissue for different implant types, 400×, (a) Contralateral ear, day 7 post-surgery, (b) HA:0.03% Hp-VEGF+KGF implant day 7, (c) HA:Gtn-VEGF+PDGF implant day 14, (d) HA:Gtn:0.3% Hp-VEGF+KGF implant day 14.
Ca—capillary, Ch—chrondrocyte lacunae, E—erythrocytes, En—endothelial cell, H—hair follicle, L—polymorphonuclear leukocyte.
In contrast, ears receiving implants showed distinct histological differences from the contralateral ear tissue (Figure 2b–d). Treated ears continued to show the chondrocytes, hair follicles and glands typical of ear pinnas. Many apparently partially formed new microvessels could also be recognized in these ears through their associated erythrocytes. However, the endothelial borders of these vessels were poorly developed and incomplete, particularly at day 7 (Figure 2b, treatment with HA:Hp-VEGF+KGF). As a result, large numbers of extravasated erythrocytes could be found distributed among the identifiable vessels.
As opposed to the highly permeable, incomplete vessels present at day 7, however, by day 14 vessel walls were more mature and much less permeable (Figure 2c and d, treatment with HA/Gtn:VEGF+PDGF and HA/Gtn:Hp-VEGF+KGF respectively). Although the density of microvessels remained high, far fewer extravasated red cells were apparent. Instead, chains of erythrocytes appeared to be confined within microvessels showing well developed borders and obvious endothelial cell nuclei, creating the appearance of hyperfused microvascular lumena.
Most importantly, although polymorphonuclear leukocytes recognizable by their multilobed nuclei were present in all treated ears, the number of such leukocytes was significantly reduced compared to previous experiments with dry film implants [26, 28, 33].
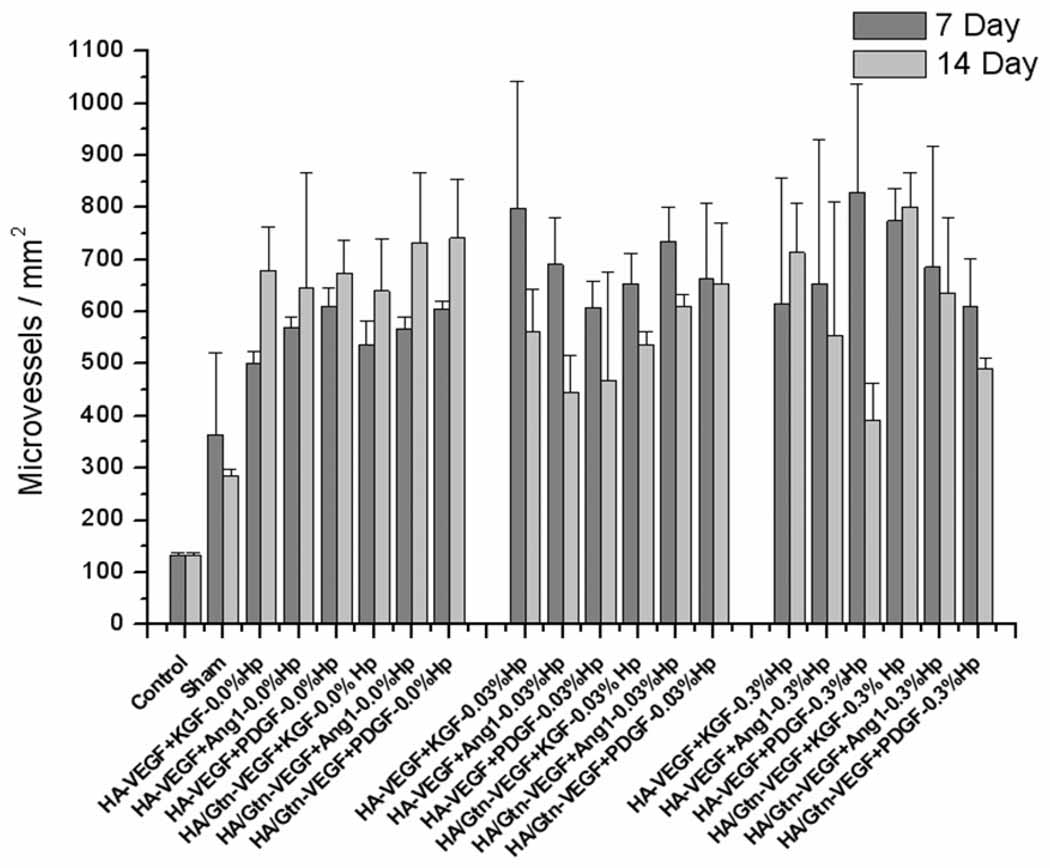
Elicited microvessel density was analyzed initially through direct counting of microvessels in the implanted ears of each treatment group (Figure 3). Neovessel density was quantitatively unchanged at either time in the contralateral ears of all treatment groups (Figure 3, control), indicating that the tissue response to the implants was localized to the region of the implant, with no systemic reaction. Vessel counts in the sham surgical case, in which a pocket was formed but no implant was delivered, were significantly greater than counts in the contralateral controls at both time points (p < 0.01). Further, all treatment cases receiving an implant showed statistically significantly greater microvessel density than the sham surgical case (p < 0.05). At day 7 post-implantation (Figure 3, dark bars), the greatest density of microvessels was found for the treatment case HA:0.3%Hp-VEGF+PDGF (829.84 microvessels/mm2), although several other groups were not statistically significantly different from this case (HA:0.03%Hp-VEGF+KGF, 799.0 microvessels/mm2, and HA/Gtn:0.3%Hp-VEGF+KGF, 775.06 microvessels/mm2). At day 14 (Figure 3, light bars), the treatment group HA/Gtn:0.3%Hp-VEGF+KGF showed the highest microvessel density, (801.01 microvessels/mm2), though again other groups were not statistically different. Ears implanted with this treatment showed microvessels that exhibited defined endothelial borders with few fenestrations, and the tissue showed a normal distribution of chondrocytes, hair follicles and glands (Figure 2d).
Figure 3.
Microvessel density for HA:Gtn:Hp-cytokine loaded treatment groups at days 7 and 14 post-implantation. Control cases represent contralateral ears. Mean ± s.d., n = 6.
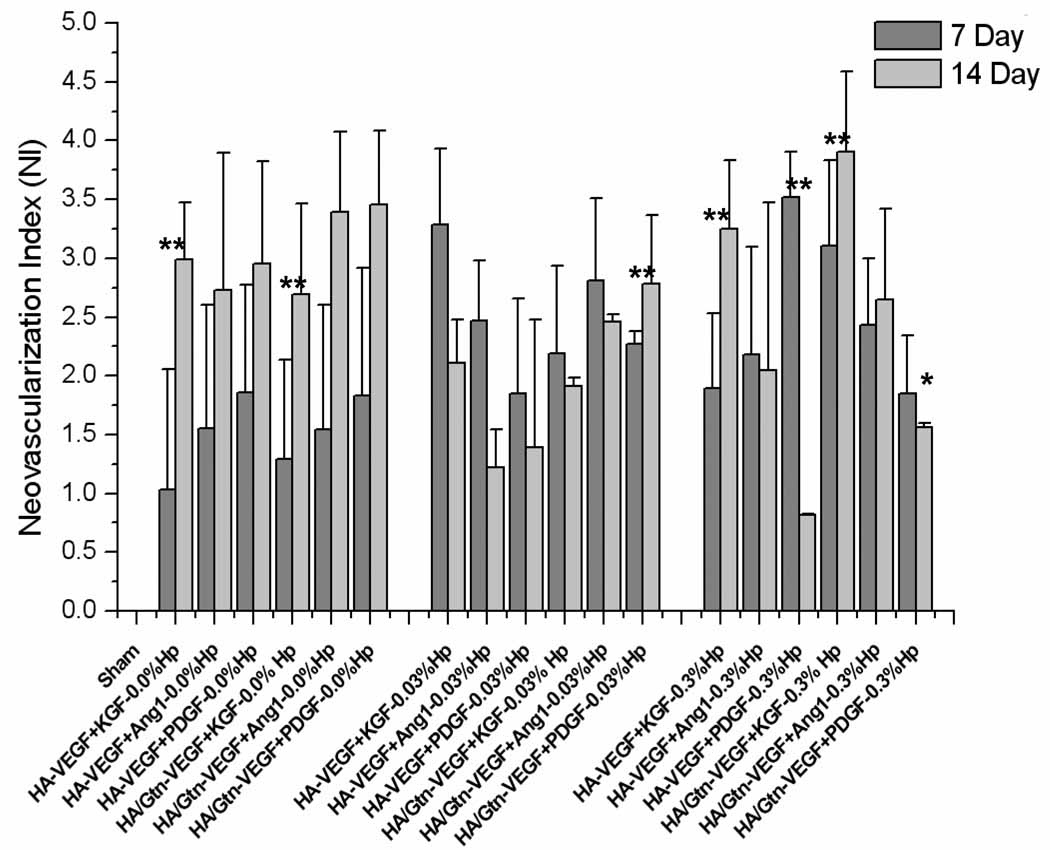
To distinguish the tissue response to different implants from the response to surgical intervention, vessel growth was re-expressed through the neovascularization index (Figure 4). Positive NI values indicate a tissue response greater than that of sham control ears, while a negative value would indicate a lesser response than sham. In spite of the trends seemingly apparent in Figure 3, analysis with NI shows distinct differences in the tissue response between day 7 and day 14 than does the direct counts, particularly for treatment cases with 0.3% Hp (Figure 4). On day 7 post-implantation, the highest NI value was produced by the case HA:0.3%Hp-VEGF+PDGF (NI = 3.52 ± 0.38), followed by HA:0.03%Hp-VEGF+KGF (NI = 3.29 ± 0.65) and HA/Gtn:0.3%Hp-VEGF+KGF (NI = 3.11 ± 0.73). In contrast, at 14 days post-implantation the highest overall NI value was observed for the case HA/Gtn:0.3%Hp–VEGF+KGF (NI = 3.91 ± 0.68), followed by HA/Gtn:VEGF+PDGF (NI = 3.46 ± 0.62) and HA/Gtn:VEGF+Ang-1 (NI = 3.39 ± 0.68).
Figure 4.
Neovascularization index for HA:Gtn:Hp-cytokine loaded treatment groups at days 7 and 14 post-implantation. Sham cases represent surgery without implant placement. NI is defined in the text, Eq. (1). Mean ± s.d., n = 6. * indicates statistical significance for comparison with sham, ** indicates statistical significance for comparison between two individual treatment cases as well as sham (only comparisons discussed in the text are identified).
4. DISCUSSION
The ability of dual cytokine pre-loaded HA hydrogel implants to elicit growth and development of new capillary networks in vivo by providing sustained, localized growth factor delivery is well demonstrated by this experiment. The combined effects of the growth factors, in conjunction with the ability of HA and the growth factors to potentiate each other’s activity [26,40], led to a strong angiogenic response even when the growth factors were delivered in very low ng doses. Furthermore, heparin-regulated delivery of two growth factors chosen to stimulate different stages of microvessel growth produced neovessel beds capable of supporting perfusion without inappropriate permeability or extravasation of red cells by two weeks post-implantation.
Similar effects were successfully achieved in our previous experiments, in which a mature neovascularization response was induced by implantation of dried film samples in the mouse ear [28,29,33]. However, unlike ears implanted with dry film samples, ears implanted with aqueous gels that crosslinked primarily in situ showed no cases of gross inflammation, redness or swelling. Numbers of polymorphonuclear leukocytes were significantly reduced and very sparse. In general, although dry film implants were normally well tolerated with little tissue necrosis, in situ gelling implants were tolerated even more successfully, with reduced or no inflammation, no signs of exudation and with no cases of failure.
The microvessel density data shown in Figure 3 indicate that contralateral ears showed no change in vessel density from day 7 to day 14, which be expected if those ears were unaffected by the implant. Significant growth of new microvessels occurred just in response to surgical intervention, as sham surgical ears showed approximately twice the vessel density of contralateral ears (p < 0.05). In addition, sham surgical ears showed reduced numbers of vessels on day 14 compared to day 7, which would be expected for tissue recovering from surgical manipulation. Most importantly, the data in Figure 3 and Figure 4 indicate that at both time points of the experiment, all ears receiving implants showed significantly higher microvessel counts than did ears receiving only sham surgery.
Further analysis of the tissue angiogenic response via the neovascularization index (Figure 4) shows that many of the treatment cases that appeared to produce very similar results based on the vessel counts (Figure 3) actually produced fundamentally different responses compared to sham surgery when normalized. For example, the case HA/Gtn:VEGF+Ang-1 produced 567 ± 23 microvessels/mm2at day 7 while the case HA:0.03% Hp-VEGF+KGF produced 562 ± 80 microvessels/mm2 at day 14. However the first case, HA/Gtn:VEGF+Ang-1 on day 7, produced a neovascularization index of 1.54 on day 7 whereas the second case, HA:0.03% Hp-VEGF+KGF on day 14, produced an NI of 3.28.
As a result, many treatment cases that appeared to generate similar vessel densities on day 7 and day 14 in fact went through notable changes during that period. For example, the treatment HA:Gtn:0.03% Hp-VEGF+PDGF produced 664 ± 143 microvessels/mm2 at day 7, while for day 14 it showed a similar count of 652 ± 116 microvessels/mm2. However, the NI values at the two time points were significantly different (p < 0.05), 2.27 for the day 7 treatment but 2.79 at day 14. Similarly, the treatment HA:Gtn:0.3% Hp-VEGF+KGF produced a microvessel density of 775 ± 61 microvessels/mm2 at day 7 and 801 ± 67microvessels/mm2 at day 14 post-implantation, which appear to not be significantly different. However, the corresponding NI values were 3.11 at day 7 and 3.91 at day 14, which in fact are again significantly different (p < 0.05).
Analysis with NI brings out distinct differences in the tissue response to the different implant compositions (Figure 4). The lowest value of NI, although it was still significantly greater than the result of sham surgery, was produced by the case HA:0.3% Hp-VEGF+PDGF on day 14 (NI = 0.82 ± 0.01, p < 0.05 for comparison with sham). In contrast, the highest NI value was obtained at day 14 post implantation for the case HA/Gtn:0.3% Hp-VEGF+KGF (NI = 3.91 ± 0.77, p < 0.01 for comparison with sham). Accordingly, an almost 5-fold difference was observed between the least and most effective treatments. Overall, there appeared to be no single growth factor combination that stood out as most effective irrespective of the presence or absence of Gtn or Hp. However, treatments containing the growth factor combination VEGF+KGF produced consistently among the strongest responses, regardless of the gel Gtn or Hp content, which is consistent with our observations in past studies [33].
The angiogenic response to gels containing gelatin was evaluated in this experiment because prior studies have shown that the synthetic ECM created by cocross-linking thiol-modified GAGs with thiol-modified gelatin produces robust, bioresorbable scaffolds that can be implanted to achieve cell and tissue growth in vivo [38, 39, 41]. In the absence of a protein component, cells did not grow sustainably within these scaffolds. However, a 50% HA-DTPH, 50% gelatin mixture provided an optimum mixture for seeding with fibroblasts, chondrocytes, or bone marrow cells to grow healthy tissue in vivo [38, 41]. Previous in vitro measurements of the rate of release of growth factors from these gels showed more rapid release in the presence of gelatin in the gel than in its absence [35]. Presumably this effect is due to the smaller molecular weight of gelatin compared with long-chain HA. As a result, gelatin-containing gels would be expected to present less steric interference, and therefore less hindered diffusion and less resistance to growth factor release, than non gelatin-containing gels.
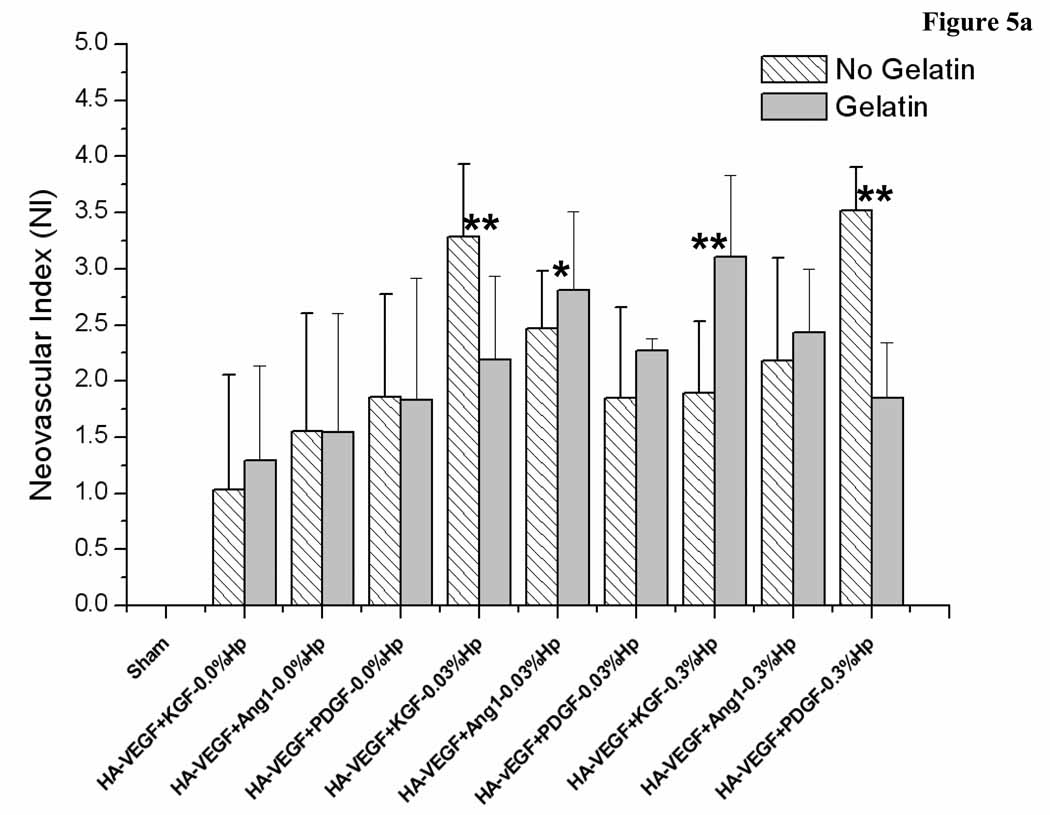
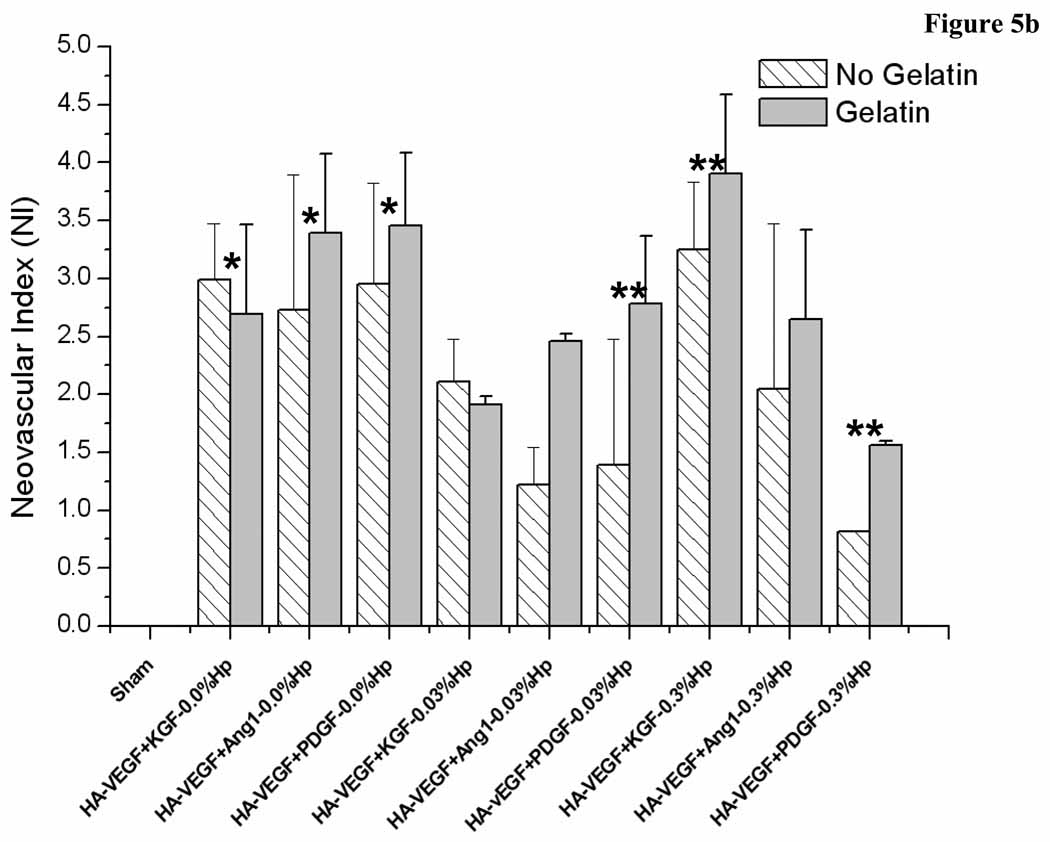
Here the effect of gelatin on the elicited angiogenic response depended on the presence and concentration of heparin in the gel (Figure 5a and b). Gels not containing Hp all produced statistically similar neovascularization responses on day 7 post implantation (Figure 5a). However, all these cases showed an increase in NI from day 7 to day 14 (Figure 5b). In contrast, the presence of both Hp and Gtn led to more complex responses. Inclusion of 0.03% Hp in the gel also resulted in no intra-group differences at day 7, with five of the six treatment cases showing a decrease in NI from day 7 to day 14. Of these five groups, three contained Gtn while two did not. Conversely, of the groups containing 0.3% Hp, there were two significant differences on day 7 due to the presence of Gtn. The case HA:0.3% Hp-VEGF+KGF showed a higher NI when Gtn was incorporated in the gel (p < 0.05), but the case HA:0.3% Hp-VEGF+PDGF produced a lower NI value in the presence of gelatin (p < 0.05). On day 14, only the case HA:0.3% Hp-VEGF+PDGF showed a statistically higher NI in the presence of Gtn. The other cases produced similar values regardless of the presence or absence of Gtn. NI increased from day 7 to day 14 for HA:0.3% Hp-VEGF+KGF, was unchanged for HA:0.3% Hp-VEGF+Ang-1, and decreased for HA:0.3% Hp-VEGF+PDGF (p < 0.05).
Figure 5.
Neovascularization index for (a) HA:Hp-cytokine loaded treatment groups both with and without gelatin at day 7 post-implantation. (b) HA:Hp-cytokine loaded treatment groups both with and without gelatin at day 14 post-implantation. NI is defined in the text, Eq. (1). Mean ± s.d., n = 6. * indicates statistical significance for comparison with sham, ** indicates statistical significance for comparison between two individual treatment cases as well as sham (only comparisons discussed in the text are identified).
Overall, the study results suggest that inclusion of heparin in the gel had a cytokine-dependent influence on elicited microvessel density levels, improving maturation in some cases but inhibiting maturation in others. In the absence of Hp, all cases showed a significant increase in vessel density from day 7 to day 14 (Figure 4, p < 0.05). In contrast, in the presence of Hp, patterns were less clear. For the 0.03% Hp cases, most treatment cases produced no statistically significant change from day 7 to day 14. However, the cases HA:VEGF+KGF and HA:VEGF+Ang-1 showed a decrease in vessel numbers with time (p < 0.05). At 0.3% Hp, VEGF+KGF cases produced a statistically significant increase in vessels from day 7 to day 14, whereas VEGF+Ang-1 cases were unchanged and VEGF+PDGF cases decreased with time. These results are consistent with our previous studies using dry HA:Hp films as delivery vehicle [29], in which delivery of the GF combination VEGF+Ang-1 was also found to produce decreasing NI with time.
The angiogenic response in vivo begins with endothelial cell proliferation, which itself is driven by the appearance in tissue of VEGF. Neovessel development therefore depends on exposure to the growth factors release from the gels. In turn, GF release from the gels occurs in part through diffusion and in part through gel breakdown mediated by tissue-resident HAse. Thus in the absence of Hp, vessel numbers would be expected to increase continuously until the gel is fully broken down, which takes approximately 14 days in the ear. However, when Hp is present in the gels, the interactions between Hp and GFs can be expected to retard GF release and alter the temporal patterns of new vessel formation. Further, Hp interactions depend on the specific GF. In vitro release measurements [36] indicate that heparin-containing gels release different GFs at specific rates unique to the GF. For example, VEGF and KGF were released from the gels in vitro at similar rates. However, the rate of release of Ang-1 was 5–10× less that the rate the rate of release of VEGF. Accordingly, the difference in response to the different delivery cases may in part result simply from differences in the rates of release of the GFs from the gels.
Both the molecular mechanisms by which HA films induce angiogenesis and the mechanisms of interaction between HA, VEGF and KGF, PDGF and Ang-1 are as yet unknown. HA has been shown to be able to potentiate the effects of VEGF both in vitro and in vivo [26,42], although it does not enable all growth factors. In particular, interactions between HA and bFGF show no similar favorable effects. In vivo studies with hydrogels based on adipic dihydrazide derivatives of HA found a maximal synergy for a delivered VEGF dose of 25 ng, with a lesser response that was dose-independent for greater or lesser GF loads [26]. VEGF is the only known cytokine with mitogenic effects confined to endothelial cells, and is known to be a major promoter of both physiologic and pathologic angiogenesis. Its expression correlates with capillary development during embryologic growth, wound healing and the female reproductive cycle, as well as with tumor expansion. Complementary intracellular signaling pathways may be initiated by o-HA and VEGF, or either molecule might up-regulate receptors for the other. Moreover, during wound healing of acute partial- and full-thickness wounds, both chondroitin sulfate (CS) and HA hydrogels promote cell movement in early granulation tissue [43, 44]. In addition, sustained release of bFGF from a hydrogel film composed of co-crosslinked thiolated heparin and thiolated CS significantly improved both the rate and quality of excisional wound repair in a diabetic mouse model [45]. This would seem to be complemented by the ability of VEGF to enhance tissue secretion of pro-angiogenic proteases including uPA, MMP-1 and MMP-2 [46]. Taken together, these observations suggest that the combination of VEGF-induced ECM breakdown and HA-mediated ECM augmentation may favorably condition the extracellular matrix for initiation of an angiogenic response.
While several biopolymer carrier systems for cytokine or drug delivery have been well characterized or are being investigated, currently no other system allows the advantages that HA-based gels offer with regard to the combination of strength, stability, in situ biodegradation without systemic sequelae and ability to interact synergistically with growth factors of interest [26]. For example, similar gels loaded with hepatocyte growth factor elicited strong ingrowth of human bone marrow mesenchymal stem cells in vitro [47]. In addition, to evaluate anticancer agents for treatment of lung cancer, the commercially available, HA-based injectable matrix Heprasil was loaded with VEGF and bFGF in addition to A549 non-small cell lung carcincoma cells, and used to form subcutaneous tumors in nude mice [48]. Incorporation of two GFs significantly augmented tumor growth, similar to GF-containing tumor-derived matrices such as Matrigel. Heprasil loaded with VEGF and bFGF was also used in cell-free “angiogenesis plugs” to attract invading cells during tests of new anti-angiogenic compound [49]. For cell therapy, the HA-heparin-gelatin matrix Heprasil was electrosprayed into an electrospun PCL-collagen scaffold, and this construct enhanced the infiltration of fetal osteoblasts into the construct compared with scaffolds lacking the Heprasil [50]. Most recently, Heprasil was employed as a matrix for injection of murine neural progenitor cells into the infarcted region of a stroke-damaged brain, increasing cell survival and decreasing inflammatory response during to the injected cells [51]
As a result, even very low doses of delivered factors can produce a highly effective desired biologic response. Consequently, HA-based hydrogels offer the opportunity to design “intelligent” implants that participate in, direct and control the tissue response rather than serving only as inert transport vehicles. Regulated delivery of multiple vascular growth factors to generate a controlled, localized angiogenic response is one important application of this biomaterial. More generally, however, the ability to regulate tissue development by controlling the local availability of cytokine combinations will provide a powerful approach to managing a wide number of pathologic conditions. Therapeutic techniques may ultimately be greatly enhanced by the temporal and spatial regulation of signals achievable in vivo in this way.
5. CONCLUSIONS
The ability of dual growth factor-loaded, hyaluronic acid-based hydrogels to stimulate an in vivo angiogenic response has been investigated in a mouse model, using gels allowed to crosslink in situ after implantation. Although previous studies demonstrated that HA-based film implants were well tolerated in this model, in situ gelling implants were tolerated even more successfully, with significantly reduced or no inflammation, no signs of exudation and no cases of failure. Histopathologic evidence showed that the gels were able to successfully sequester and provide localized in vivo release of the growth factors without loss of their biologic effectiveness, as animals receiving implants developed intact microvessel networks with defined borders and no evidence of inappropriate permeability. At both time points, all treatment cases produced significantly greater microvessel density than was found in either contralateral control ears or ears receiving surgery but no implant (p < 0.01). Gels containing both heparin and gelatin produced stronger angiogenic responses than did gels containing only heparin or only gelatin. In general, the largest vascularization responses were consistently produced by gels delivering the growth factor combination VEGF+KGF. The ability to stimulate localized angiogenic responses at controllable rates for extended periods of time via growth factor delivery by heparin-containing, HA-based GAG hydrogels will provide an effective therapeutic strategy for tissue regenerative applications.
Acknowledgments
Financial support for this project was provided by an NIH award (EB004514), and by Oregon State University, and in part by a Centers of Excellence award from the state of Utah. We thank Novozymes for the gift of HA, and Francis Ball for assistance with the vessel counts.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, et al. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin invest. 1994;93:662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeshita S, Pu LQ, Stein LA, Sniderman AD, Bunting S, Ferrara N, et al. Intramuscular administration of vascular endothelial growth factor induces dose-dependent collateral artery augmentation in a rabbit model of chronic limb ischemia. Circulation. 1994;90:11228–11234. [PubMed] [Google Scholar]
- 3.Baumgartner I, Pieczek A, Manor O, Blair R, Kearney M, Walsh K, et al. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97:1114–1123. doi: 10.1161/01.cir.97.12.1114. [DOI] [PubMed] [Google Scholar]
- 4.Simons M, Bonow RO, Chronos NA, Cohen DJ, Giordano FJ, Hammond HK, et al. Clinical trials in coronary angiogenesis: issues, problems, consensus: an expert panel summary. Circulation. 2000;102:E73–E86. doi: 10.1161/01.cir.102.11.e73. [DOI] [PubMed] [Google Scholar]
- 5.Goth MI, Hubina E, Raptis S, Nagy GM, Toth BE. Physiological and pathological angiogenesis in the endocrine system. Microsc Res Tech. 2003;60:98–106. doi: 10.1002/jemt.10248. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 7.Paku S, Paweletz N. First steps of tumor related angiogenesis. Lab Invest. 1991;65:334–336. [PubMed] [Google Scholar]
- 8.Liekens S. The role of growth factors, angiogenic enzymes and apoptosis in neovascularization and tumor growth-collected publications. Verh K Acad Geneeskd Belg. 2002;64:197–224. [PubMed] [Google Scholar]
- 9.Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. 2002;29:10–14. doi: 10.1053/sonc.2002.37264. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N, Gerber H-P, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 11.Nikosia RF, Bonanno E, Smith M, Yurchenco P. Modulation of angiogenesis in vitro by laminin-entactin complex. Dev Biol. 1994;164:197–206. doi: 10.1006/dbio.1994.1191. [DOI] [PubMed] [Google Scholar]
- 12.Ingber DE, Folkmann J. Mechano-chemical switching between growth and differentiation during growth factor-stimulated angiogenesis in vitro: role of the extracellular matrix. J Cell Biol. 1989;109:317–330. doi: 10.1083/jcb.109.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allison D, Grande-Allen K. Hyaluronan: a powerful tissue engineering tool. Tissue Eng. 2006;12(8):2131–2140. doi: 10.1089/ten.2006.12.2131. [DOI] [PubMed] [Google Scholar]
- 14.Prestwich GD. Evaluating drug toxicity and efficacy in three dimensions: using synthetic extracellular matrices in drug discovery. Acc Chem Res. 2008;41:139–148. doi: 10.1021/ar7000827. [DOI] [PubMed] [Google Scholar]
- 15.Serban MA, Prestwich GD. Making modular extracellular matrices: solutions for the puzzle. Methods. 2008;45:93–98. doi: 10.1016/j.ymeth.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prestwich GD. Engineering a clinically-useful matrix for cell therapy. Organogenesis. 2008;4:42–47. doi: 10.4161/org.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prestwich GD. Simplifying the extracellular matrix for 3-D cell culture and tissue engineering: a pragmatic approach. J Cell Biochem. 2007;101:1370–1383. doi: 10.1002/jcb.21386. [DOI] [PubMed] [Google Scholar]
- 18.Knudson CB, Knudson W. Cartilage proteoglycans. Semin Cell Dev Biol. 2001;12:69–78. doi: 10.1006/scdb.2000.0243. [DOI] [PubMed] [Google Scholar]
- 19.Forrester JV, Balasz EA. Inhibition of phagocytosis by high molecular weight hyaluronate. Immunology. 1980;40:435–446. [PMC free article] [PubMed] [Google Scholar]
- 20.Kujawa MJ, Carrino DA, Caplan AI. Substrate-bonded Hyaluronic acid exhibits a size-dependent stimulation of chondrogenic differentiation of stage 24 limb mesenchymal cells in culture. Dev Biol. 1986;144:519–528. doi: 10.1016/0012-1606(86)90215-0. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg RL, Toole BP. Hyaluronate inhibition of cell proliferation. Arthritis Rheum. 1987;30:769–778. doi: 10.1002/art.1780300707. [DOI] [PubMed] [Google Scholar]
- 22.Rooney P, Kumar S, Ponting J, Wang M. The role of hyaluronan in tumor neovascularization. Int J Cancer. 1995;60:632–636. doi: 10.1002/ijc.2910600511. [DOI] [PubMed] [Google Scholar]
- 23.West DC, Kumar S. The effects of hyaluronate and its oligosaccharides on endothelial cell proliferation and monolayer integrity. Exp Cell Res. 1989;183:179–196. doi: 10.1016/0014-4827(89)90428-x. [DOI] [PubMed] [Google Scholar]
- 24.Sattar A, Kumar S, West DC. Does hyaluranan have a role in endothelial cell proliferation in the synovium? Semin Arthritis Rheum. 1992;22:37–43. doi: 10.1016/0049-0172(92)90047-h. [DOI] [PubMed] [Google Scholar]
- 25.Sattar A, Rooney P, Kumar S, Pye D, West DC, Scott I, et al. Application of angiogenic oligosaccharides of hyaluronan increase blood vessel numbers in skin. J Invest Dermatol. 1994;103:573–579. doi: 10.1111/1523-1747.ep12396880. [DOI] [PubMed] [Google Scholar]
- 26.Peattie RA, Nayate AP, Firpo MA, Shelby J, Fisher RJ, Prestwich GD. Stimulation of in vivo angiogenesis by cytokine-loaded hyaluronic acid hydrogel implants. Biomaterials. 2004;25:2789–2798. doi: 10.1016/j.biomaterials.2003.09.054. [DOI] [PubMed] [Google Scholar]
- 27.Cai S, Liu Y, Shu XZ, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26(30):6054–6067. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 28.Pike DB, Cai S, Pomraning KR, Firpo MA, Fisher RJ, Shu XZ, et al. Heparin-regulated release of growth factors in vitro and angiogenic response in vivo to implanted hyaluronan hydrogels containing VEGF and bFGF. Biomaterials. 2006;27:5242–5251. doi: 10.1016/j.biomaterials.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Riley CM, Fuegy PW, Firpo MA, Shu XZ, Prestwich GD, Peattie RA. Stimulation of in vivo angiogenesis using dual growth factor-loaded crosslinked glycosaminoglycan hydrogels. Biomaterials. 2006;27:5935–5943. doi: 10.1016/j.biomaterials.2006.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wissink MJB, Beernink R, Poot AA, Engbers GHM, Beugeling T, van Aken WG, et al. Improved endothelialization of vascular grafts by local release of growth factor from heparinized collagen matrices. J Control Rel. 2000;64:103–114. doi: 10.1016/s0168-3659(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 31.Cleland JL, Duenas ET, Park A, Daugherty A, Kahn J, Kowalski J, et al. Development of poly-(D,L-lactide-coglycolide) microsphere formulations containing recombinant human vascular endothelial growth factor to promote local angiogenesis. J Control Rel. 2001;72:13–24. doi: 10.1016/s0168-3659(01)00258-9. [DOI] [PubMed] [Google Scholar]
- 32.Godspodarowicz D, Cheng J. Heparin protects basic and acidic FGF from inactivation. J Cell Physiol. 1986;128:475–484. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- 33.Peattie RA, Rieke ER, Hewett EM, Fisher RJ, Shu XZ, Prestwich GD. Dual growth factor-induced angiogenesis in vivo using hyaluronan hydrogel implants. Biomaterials. 2006;27:1868–1875. doi: 10.1016/j.biomaterials.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 34.Shu XZ, Liu Y, Luo Y, Roberts MC, Prestwich GD. Disulfide cross-linked hyaluronan hydrogels. Biomacromolecules. 2002;3:1304–1311. doi: 10.1021/bm025603c. [DOI] [PubMed] [Google Scholar]
- 35.Peattie RA, Yu B, Cai S, Firpo MA, Pike DB, Fisher RJ, et al. Effect of gelatin on heparin regulation of cytokine release from hyaluronan-based hydrogels. Drug Delivery. 2008;15:363–371. doi: 10.1080/10717540802035442. [DOI] [PubMed] [Google Scholar]
- 36.Hosack LW, Firpo MA, Scott AJ, Prestwich GD, Peattie RA. Microvascular maturity elicited in tissue treated with cytokine-loaded hyaluronan-based hydrogels. Biomaterials. 2008;15:2336–2347. doi: 10.1016/j.biomaterials.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shu XZ, Liu Y, Palumbo FS, Luo Y, Prestwich GD. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials. 2004;25:1339–1348. doi: 10.1016/j.biomaterials.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 38.Shu XZ, Ahmad S, Liu Y, Prestwich GD. Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering. J Biomed Mat Res A. 2006;79:902–912. doi: 10.1002/jbm.a.30831. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Shu XZ, Prestwich GD. Biocompatibility and stability of disulfide-crosslinked hyaluronan films. Biomaterials. 2005;26:4737–4746. doi: 10.1016/j.biomaterials.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Montesano R, Kumar S, Orci L, Pepper MS. Synergistic effect of hyaluronan oligosaccharides and vascular endothelial growth factor on angiogenesis in vitro. Lab Invest. 1996;75:249–262. [PubMed] [Google Scholar]
- 41.Uemura A, Ogawa M, Hirashima M, Fujiwara T, Koyama S, Takagi H, et al. Recombinant angiopoietin-1 restores higher-order architecture of growing blood vessels in mice in the absence of mural cells. J Clin Invest. 2002;110:1619–1628. doi: 10.1172/JCI15621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raman R, Venkataraman G, Ernst S, Sasisekharan V, Saasisekharan R. Structural specificity of heparin binding in the fibroblast growth factor family of proteins. Proc Natl Acad Sci. 2003;100:2357–2362. doi: 10.1073/pnas.0437842100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirker KR, Luo Y, Nielson JH, Shelby J, Prestwich GD. Glycosaminoglycan hydrogel films as bio-interactive dressings for wound healing. Biomaterials. 2002;23:3661–3671. doi: 10.1016/s0142-9612(02)00100-x. [DOI] [PubMed] [Google Scholar]
- 44.Kirker KR, Luo Y, Morris SE, Shelby J, Prestwich GD. Glycosaminoglycan hydrogel films as supplemental wound dressings for donor sites. J Burn Care Rehabil. 2004;25:276–286. doi: 10.1097/01.bcr.0000124790.69026.3d. [DOI] [PubMed] [Google Scholar]
- 45.Liu Y, Cai S, Shu XZ, Shelby J, Prestwich GD. Sustained release of basic fibroblast growth factor from a crosslinked glycosaminoglycan hydrogel promotes wound healing in genetically diabetic mice. Wound Repair Regen. 2007;15:245–251. doi: 10.1111/j.1524-475X.2007.00211.x. [DOI] [PubMed] [Google Scholar]
- 46.Yabushita H, Shimazu M, Noguchi M, Kishida T, Narumiya H, Sawaguchi K, et al. Vascular endothelial growth factor activating matrix metalloproteinase in ascitic fluid during peritoneal dissemination of ovarian cancer. Oncol Rep. 2003;10:89–95. [PubMed] [Google Scholar]
- 47.Zhao J, Zhang N, Prestwich GD, Wen X. Recruitment of endogenous stem cells for tissue repair. Macromol Biosci. 2008;8:836–842. doi: 10.1002/mabi.200700334. [DOI] [PubMed] [Google Scholar]
- 48.Xu X, Prestwich GD. Inhibition of tumor growth and angiogenesis by a lysophosphatidic acid antagonist in a engineered three-dimensional lung cancer xenograft model. Cancer. 2009 doi: 10.1002/cncr.24907. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu X, Yang G, Zhang H, Prestwich GD. Evaluating dual activity LPA pan-antagonist/autotaxin inhibitors as anti-cancer agents in vivo using engineered human tumors. Prostagland Other Lipid Med. 2009;89:140–146. doi: 10.1016/j.prostaglandins.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ekapurta AK, Prestwich GD, Cool S, Hutmacher DW. Electrospinning of scaffolds-the challenge to engineer cell-permeable constructs. Biomacromolecules. 2008;9:2097–2103. doi: 10.1021/bm800565u. [DOI] [PubMed] [Google Scholar]
- 51.Zhong J, Chan A, Morad L, HKornblum H, Fan G, Carmichael S. Hydrogel matrix to support stem cell survival after brain transplantation in stroke. Neurorehab. Neural Repair. 2010 doi: 10.1177/1545968310361958. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]