Abstract
Environmental cues that once predicted reward can restore extinguished behavior directed toward that reward. This process may be modeled by the Pavlovian-instrumental transfer (PIT) paradigm where a previously learned Pavlovian conditioned stimulus (CS) elicits a representation of the reward associated with that CS, prompts motivation towards the absent reward, and triggers an instrumental action. We recorded in the medial and orbital prefrontal cortex (mPFC and OFC) and dorsal striatum of freely moving rats during PIT and found that a Pavlovian CS, as compared to neutral or no stimuli, amplified the phasic neuronal responses to instrumental nosepokes ("transfer" event). In mPFC and OFC, but not the dorsal striatum, representation of the transfer event correlated with the strength of PIT behavior. Neurons in all three regions showed CS-selective amplification of Pavlovian approaches toward the reward delivery site. Whereas striatal neurons represented transfer and approach behavior through mostly segregated neuronal subsets, overlapping subsets represented these events in the mPFC and OFC. These findings suggest that parallel phasic activation of mPFC and OFC neuronal subsets participates in the transfer from Pavlovian incentives to instrumental actions.
Keywords: Pavlovian Conditioning, Instrumental Conditioning, Cue-triggered Motivation, Orbitofrontal cortex, Freely Moving Rat
Introduction
Pavlovian-instrumental transfer (PIT) is a process through which goal-directed behavior is influenced by cues previously associated with rewards (Estes, 1948; Balleine and Dickinson, 1998). PIT involves two fundamentally distinct modes of associative learning: (1) Pavlovian conditioning in which a relationship is established between an environmental cue, referred to as a conditioned stimulus (CS), and a reinforcer, and (2) instrumental conditioning in which an association is established between an action and a reinforcing outcome. During PIT, presence of a Pavlovian CS influences an independently-acquired instrumental response (Rescorla and Solomon, 1967; Lovibond, 1983). Hence, there is a “transfer” between a CS and an action which had been independently associated with a common reinforcer. This transfer process also occurs after animals have gone through extinction of instrumental responding. In this transfer process, the CS triggers instrumental action towards an absent reinforcer and, thus, models conditions where behavior is driven by the memory of, and motivation for, reward. Recent research suggests that PIT is susceptible to usurpation by drugs of abuse (Wyvell and Berridge, 2000; Cardinal and Everitt, 2004), and may be a key component of the maladaptive learning associated with addiction (Everitt et al., 2001; Kelley, 2004). Accordingly, PIT has been suggested as a mechanism through which drug-related cues may evoke reinstatement of drug seeking behavior and cause relapse (Dickinson et al., 2000; Volkow and Fowler, 2000; Cardinal and Everitt, 2004).
The neuronal basis of PIT is not well understood. Lesion studies have suggested that regions associated with affective processing such as amygdala and nucleus accumbens are necessary for behavioral expression of PIT (Corbit et al., 2001; Hall et al., 2001; de Borchgrave et al., 2002; Holland and Gallagher, 2003). There is little evidence that these regions directly integrate Pavlovian and instrumental incentive processes during PIT. These regions, however, act within a distributed network, which notably includes subregions of prefrontal cortex (PFC) such as medial and orbital PFC (mPFC and OFC, respectively), to integrate affective stimuli with executive commands (Christakou et al., 2004; Kelley, 2004; Pasupathy and Miller, 2005; Saddoris et al., 2005; Stalnaker et al., 2007). Emerging evidence suggest that mPFC and OFC encode distinct components of both Pavlovian and instrumental processes (Gallagher et al., 1999; Chudasama and Robbins, 2003; Homayoun and Moghaddam, 2008a; Ostlund and Balleine, 2007) leading us to hypothesize they play a role in integration of Pavlovian and instrumental processes during PIT. To test this hypothesis, we performed ensemble unit recording in behaving rats and reasoned that if these regions are involved in encoding for PIT, then 1) their neuronal activity should show preferential encoding of instrumental response only during presentation of Pavlovian conditioned stimulus, 2) this neuronal activity should correlate with behavioral PIT, and 3) disruption of this neuronal activity such as that observed after blockade of N-methyl D-aspartate (NMDA) receptors, should be associated with disruption of PIT. We also compared cortical representations with that in the dorsal striatum, a region implicated in maintenance of action-outcome contingencies (Baldwin et al., 2000; Yin et al., 2005).
Materials and Methods
Subjects
Adult male Sprague-Dawley rats (n=19, 320–380 g) were used. Animals were individually housed on a 12-h light/dark cycle (lights on at 0700 h) and placed on a restricted food diet of 15 g per day. Experimental procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Electrophysiology
Under halothane anesthesia, one or two arrays of eight Teflon-insulated stainless steel wires (NB Labs, Denison, Texas), spanning 0.25×0.7 mm in a 2×4 pattern were implanted in the mPFC (AP, +2.2–3.2, ML 0.5–0.8, DV –3.5, N = 14), OFC (AP +2.2–3.2, ML 3.2–3.5, DV –5.5, N = 12) or DS (AP +0.5–1.5, ML 1.9–2.2, DV –4.5, N = 7). Among 14 rats used in the non-drug experiments, 7 had implants in mPFC and DS, 2 had implants in mPFC and OFC, and 5 had implants only in OFC. Two rats had misplaced electrodes (targeting mPFC and DS) and were excluded. The five rats used in NMDA antagonist experiments had implants in mPFC and OFC. Coordinates were according to the atlas of Paxinos and Watson (Paxinos and Watson, 1998). After 5 days of recovery from surgery, animals were handled and habituated in an automated operant conditioning box (Coulbourn Instruments, Allentown, PA). During habituation, animals were connected to a unity-gain field effect transistor (FET) headstage (NB Labs) using lightweight cabling that passed through a commutator and allowed unrestricted movement. After the initial training on Pavlovian and instrumental tasks (see below), animals were tested on a single PIT session, during which extracellular neuronal activity was recorded. Methodological details for recording and single unit isolation were the same as reported previously (Homayoun et al., 2004; Homayoun and Moghaddam, 2006). In brief, a multiple channel amplifier with 1000× gain and 220–5900 Hz band pass filter (Plexon Inc., Dallas, Texas) was used for recording simultaneous ensemble unit activity from up to 16 channels. The amplified signal from each electrode was digitized (40 kHz sampling rate) using Recorder software (Plexon Inc.) and saved for off-line spike sorting. A minimum waveform amplitude threshold of two standard deviations higher than the noise amplitude was used for initial saving of the waveform segments during recording. Using Off-Line Sorter software (Plexon Inc.), spikes were sorted through a combination of automatic and manual sorting techniques previously described (Homayoun et al., 2004). Only discrete clusters of units with characteristic and temporally stable waveforms, and a minimum absolute refractory period of 1.1 ms in their interspike interval histogram, were accepted as single units. In addition, four different statistical measures, including parametric F statistics of multivariate analysis of variance, J3 and pseudoF statistics, and Davies-Bouldin validity index were used, on a case-by-case basis, to assess the quality of separation between identified clusters and the effects of incremental increases or decreases on the number of units isolated from a channel (Nicolelis et al., 2003). Neurons were further classified based on their firing characteristics during a baseline (1–3 min) pre-task period. In the mPFC and OFC, the isolated single units were classified as regular firing (RF, spontaneous firing rate < 10Hz, putative pyramidal neurons) or fast firing (FF, spontaneous firing rate >10Hz, tonic firing pattern in autocorrelogram, narrow spike width, putative interneurons) units. Using dual criteria of high frequency and narrow spike width for separation of putative FF units was based on previously established criteria (McCormick et al., 1985; Tierney et al., 2004). In the DS, isolated units were classified as putative pyramidal neurons (Spontaneous firing rate < 8 Hz, some interspike intervals higher than 2s and lack of central valley in auto-correlogram, medium spiny neurons) or putative fast firing units (spontaneous firing rate >10 Hz, absence of interspike intervals higher than 1sec, putative interneurons), based on the criteria used by Barnes et al. (Barnes et al., 2005).
Behavioral Paradigm
Operant chambers (Coulbourn Instruments, Allentown, PA) equipped with an illuminating house light, nosepoke modules, an illuminated food magazine and a food delivery system were used for behavioral training. Nosepoke modules were illuminated by light emitting diodes; the food magazine, located on the opposite wall of the chamber, was illuminated by a white incandescent bulb. Head entries into nosepoke module or food magazine were detected by photosensors. A PC-based controller and Graphic State data acquisition software (Coulbourn Instruments, Allentown, PA) was used to control the chamber and collect behavioral data. Digital event markers were used to synchronize the behavioral events with electrophysiological recordings.
Animals were habituated in a 10 min session 2 −3 days after surgery. The habituation session was divided into two 5 min segments. In the first segment, the animals were given 5 min of free exploration of the operant chamber with house light on and 5 food pellets (dextrose pellets, 45 mg, Bio-Serv, Frenchtown, NJ) left in the food trough. During the second 5 min, food pellets were intermittently dropped into the food trough. Subsequently, animals underwent a training regimen that consisted of 2 days of Pavlovian conditioning, 5 days of instrumental conditioning, and 1 day of instrumental extinction. This regimen was an adaptation from previous studies (Dickinson et al., 2000; Hall et al., 2001; Holland and Gallagher, 2003), aimed at maximizing the expression of PIT, and modified to accelerate the speed of training in order to maximize the time during which high quality electrophysiological recordings could be obtained from the implanted electrodes. The paradigm used in this study was a general PIT paradigm, which involves a single CS and outcome, as opposed to outcome-specific versions of PIT in which distinct outcomes are associated with specific responses and cues (Balleine and Dickinson, 1998). Training started with 2 days of Pavlovian conditioning. During each session, a conditioned stimulus (CS), consisting of a 30 sec pulsating tone (100 ms on-100 ms off), was presented 16–18 times. Each CS was associated with the delivery of five food pellets, given at 5 sec intervals with the first pellet delivered 5 sec after CS onset. The food trough light turned on simultaneously with the delivery of the first food pellet and remained on until the animal made a head entry into the food magazine. The first magazine entry after the pellet delivery was considered the time of reward consumption. The inter-stimulus interval between subsequent CS was 90 sec. The approach latency, defined as the interval between the onset of the tone and the first pellet consumption, was recorded as a measure of Pavlovian conditioning. In addition, the number of head entries into the food trough during the 30 sec CS presentation was measured. At the end of the 2nd Pavlovian conditioning session, a neutral stimuli (NS), consisting of a different tone (50 ms on-4000 ms off, 30 sec duration interspersed with 30 sec intervals), was presented 5 times. The NS was once more presented at the end of the 4th instrumental session to ensure its familiarity during PIT testing. Training continued with 5 days of Instrumental conditioning. Each daily session (40 min on day 1; 45 min on days 2–5) consisted of a fixed ratio (FR) 1 continuous reinforcement schedule, during which nosepoke into the illuminated left nosepoke module (instrumental response) resulted in the delivery of a food pellet into the food magazine and illumination of the food trough light. The food trough light remained on until head entry (food consumption) which initiated a new cycle (nosepoke module illumination). During the first instrumental session, instrumental responses into the nosepoke module immediately led to the food delivery and magazine entry immediately started the next cycle. Afterwards, variable and incrementally increasing intervals (1–2 sec, 1–3 sec, 2–4 sec, and 3–5 sec on days 2, 3, 4 and 5, respectively) were used between the nosepoke and food delivery, and intertrial intervals alternated between 3 or 6 seconds. This schedule was based on previous evidence that using longer and more variable response-reinforcer intervals during training enhances the expression of PIT (Holland and Gallagher, 2003). In addition to the active nosepoke module, the animals had the option to respond on two non-active modules which did not lead to any reinforcement. As the final step before PIT testing, the animals underwent a single session of instrumental extinction. During this 10-min session, poking into the illuminated (active) nosepoke module was not rewarded with food, and as a result, the nosepoke response rate dramatically dropped within session (p < 0.01, comparison between the first and last 2 min blocks). The extinction session was included as some previous researchers have suggested that partial extinction of instrumental responding prior to PIT testing can increase the expression of PIT (Dickinson et al., 2000; Hall et al., 2001; Murschall and Hauber, 2006).
PIT was tested under extinction condition, removing the primary reinforcement by eliminating reward. This condition also minimized the impact of secondary reinforcement because the Pavlovian cues were presented non-continent on instrumental response thus serving to elicit these responses rather than reinforcing them as would occur in a conditioned reinforcement paradigm. (Wyvell and Berridge, 2000). This design allowed us to determine the neural substrates of incentive cue-evoked transfer as compared to behavior associated with a neutral or no cue. The 30 min PIT session consisted of 5 blocks of 6 min length; each block included 2 min epochs during which either CS, NS or an intertrial interval (ITI, with no stimulus) was presented (Figure 1A). The epochs were presented in a pseudorandom order and no food pellet was delivered at any time during the session. The 2min length of epochs was used to maximize the behavioral expression of PIT. The number of nosepokes during each epoch was recorded as the measure of PIT. The number of food magazine entries were also recorded as an index of Pavlovian approach behavior, as suggested by previous literature (Holland and Gallagher, 2003; Pecinna et al., 2006). To ensure that the electrophysiological analysis of this event was not affected by the possibility of occasional instances of instrumental response-approach sequences, we excluded those magazine entries that were preceded by an immediate nosepoke (e.g. an interval of less than 10sec between nosepoke and magazine entry). Only a small fraction of magazine entries (average 8.2%) were removed in the latter analysis, indicating their limited impact on the final analysis. It should be emphasized that during the training period, Pavlovian and instrumental contingencies were acquired independently and therefore PIT behavior was not a result of habitual pursuit of a stimulus-response association. The possibility that food trough light may have acted as a secondary CS may be a concern but this effect, if any, was not significant as Pavlovian tone continued to act as a potent CS during PIT session (see Results). A subset of animals (N = 5) were pre-treated with a systemic injection of the NMDA receptor antagonist MK801 (0.1 mg/kg, Sigma-RBI, St. Louis, Missouri) prior to PIT session. The goal of this experiment was to assess the effect of MK801, an intervention previously shown to disrupt baseline PFC activity (Jackson et al., 2004), on PIT behavior and its neuronal correlates.
Figure 1. Behavioral task and histology.
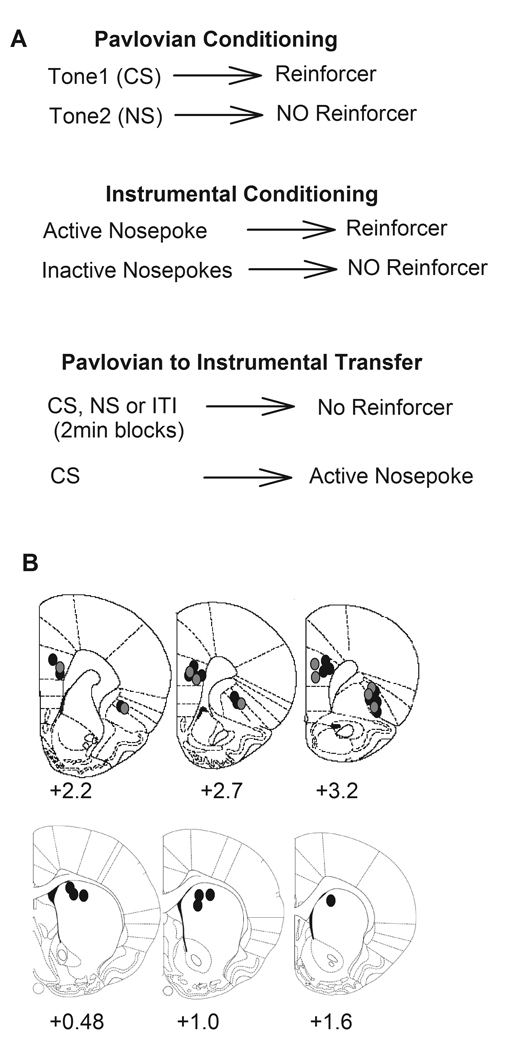
A) Schematic diagram of Pavlovian to instrumental transfer (PIT) paradigm. Animals were trained separately on Pavlovian and instrumental conditioning tasks and PIT was tested under extinction, during which presentation of Pavlovian conditioned stimulus (CS, tone) triggered instrumental action (nosepokes). B) Placement of electrode arrays (0.7 × 0.25 mm, center of each array shown) in the mPFC (prelimbic), lateral OFC and dorsal striatum. Numbers under each coronal section indicate the distance anterior to bregma (Paxinos and Watson, 1998). Placements from the NMDA antagonist experiment are shown in gray.
Data Analysis
Behavior
PIT was assessed by comparing rate of nosepokes among three behavioral epochs of CS, NS and ITI using one-way ANOVA with Bonferroni post hoc test for significance (p < 0.05). This between-session analysis was verified using a within-session measure of PIT, based on the difference in the number of CS-related and CS–unrelated nosepokes in each animal. This difference score was calculated separately for NS and ITI epochs, with a positive value indicating the transfer. The initial Pavlovian training was assessed by comparing the latency of approach to food magazine after CS during early (start of day 1), middle (start of day 2) and late (end of day2) blocks of CS presentation. For each 2min block, the latency of response to the first CS within that block was assessed. The food magazine approach latencies were also compared between early (after Pavlovian training day 2) and late (after instrumental training day 4) blocks of NS presentation.
The instrumental training was assessed by comparing the number of active and inactive nosepokes within first 10min blocks of each training session (day 1–5). The extinction behavior was assessed by comparing the number of responses on the active nosepoke module, in the absence of reward, during early, middle and last 2-min blocks of extinction session. We repeated the analysis of behavioral training and PIT for subsets of the animals, including those with simultaneous mPFC/DS or mPFC/OFC recordings and those with MK801 pre-treatment, to ensure that these subsets received similar training and expressed PIT at comparable levels.
Electrophysiology
Electrophysiological data were analyzed using NeuroExplorer (Plexon) and Matlab (Mathworks, Natick, MA). Firing rates during the 2-min behavioral epochs were compared using one-way ANOVA with Bonferroni post-hoc test (p < 0.05). Peri-event rasters and firing rate time histograms (50 ms bins, smoothed by a Gaussian filter with bin width of 3) were used to analyze the phasic neuronal responses to significant behavioral events (instrumental nosepoke, magazine entry). The window of analysis for assessing neuronal correlates of each behavioral event consisted of −2 sec to +1 sec around instrumental nosepoke or food magazine entry. Peri-event time histograms were normalized using the local baseline consisting of 1 sec immediately preceding the window of analysis. We also used an alternative method of normalization based on pre-CS firing instead of pre-event baseline. This latter analysis led to similar results. We, therefore, only include results based on pre-event baseline as this method is more sensitive to detection of phasic responses. A phasic response was considered significant if a minimum of four consecutive histogram bins (within window of analysis) exceeded the 95% confidence intervals of the local baseline. The main patterns of phasic neuronal responses to CS-associated nosepoke and magazine entry were classified as either an increase, decrease or no change in firing. The final classification was verified by obtaining a significant ANOVA with time as repeated measure and response type as factor. The distribution of neurons with each response type was compared between epochs using Chi-square test (p < 0.05). The average phasic activity of neurons in each region, separated by response direction, was compared between epochs using two-way ANOVA with time as repeated measure. The magnitude of each phasic response was calculated according to a method adapted from Ghitza et al. (2006) using the ratio of A / (A+B), with A being the peak change (excitatory or inhibitory) in firing rate within the corresponding window of analysis and B being the firing rate during the local baseline window. Thus, a magnitude value between 0–0.5 indicates a phasic decrease and a value between 0.5–1 indicates a phasic increase. Only neurons with a significant phasic response to event of interest (CS-associated instrumental nosepoke or Pavlovian approach behavior) were included in this analysis. The phasic response magnitude was then standardized by calculating the absolute value of its difference from 0.5 (Ghitza et al., 2006). This standardized ratio ranged between 0 (minimum change) and 0.5 (maximum change) and allowed for assessment of both excitatory and inhibitory phasic responses irrespective of directionality. One-way ANOVA (or t-test) was used to compare the average standardized magnitude of phasic responses to each task event in each region. Pearson correlation coefficient was used to assess correlation between the magnitude of phasic neuronal response to nosepokes and PIT behavior within individual sessions (for all subjects). Individual neurons were further classified based on their phasic response profiles. To this end, the standardized magnitude of phasic response of each neuron during CS epoch was separately compared with its responses during NS and ITI epochs. Units displaying at least 25% enhancement in their response magnitude during CS compared to both NS and ITI were classified as event-discriminative units. The overlap of encoding for instrumental nosepoke and magazine entry was assessed by comparing the proportion of units encoding for magazine entry among neuronal subsets classified by their nosepoke response profile (CS-epoch selective responder, non-selective responder, non-responder). This distribution was compared between subsets of neurons using Chi-square test (p < 0.05).
Histology
Animals were anesthetized with chloral hydrate and intracardially perfused with saline followed by 10% buffered formalin. Fixed brains were cut at 150 µm intervals and sections were stained with cresyl violet to probe electrode placements. Under a light microscope, tips of recording electrodes were confirmed to be in the mPFC (prelimbic), lateral OFC, or dorsomedial Striatum (Figure 1B), as defined by the atlas of Paxinos and Watson (1998).
Results
Behavior
During Pavlovian phase of training, the magazine approach latency after CS presentation progressively decreased, indicating Pavlovian conditioning (Figure 2A, one-way ANOVA, F(2,54) = 102.5, p < 0.001). In contrast, the response latency after neutral stimulus (NS) significantly increased from early to late trials. Pavlovian training was also associated with an increase in the number of head entries into the food trough during 30 sec CS presentation (23.36 early block vs. 48.78 late block, p < 0.05). During instrumental phase of training, the number of active nosepoke responses increased and inactive nosepoke responses decreased from day 1 to 5 (Figure 2B, active, F(4,90) = 29.05, p < 0.001, inactive, F(4,90) = 25.48, p < 0.001). This training was followed by a brief extinction session, during which the instrumental responding was extinguished in the absence of reinforcer (Figure 2C, F (2,54) = 53.08, p < 0.001). Note that this extinction was incomplete and animals continued to show minimal response at the end of the extinction session. A breakdown of behavioral training data showed that subsets of animals (simultaneous mPFC/OFC or mPFC/DS recordings or MK801 pre-treated) underwent comparable training (Supplementary Figure 1).
Figure 2. Pavlovian and Instrumental training.
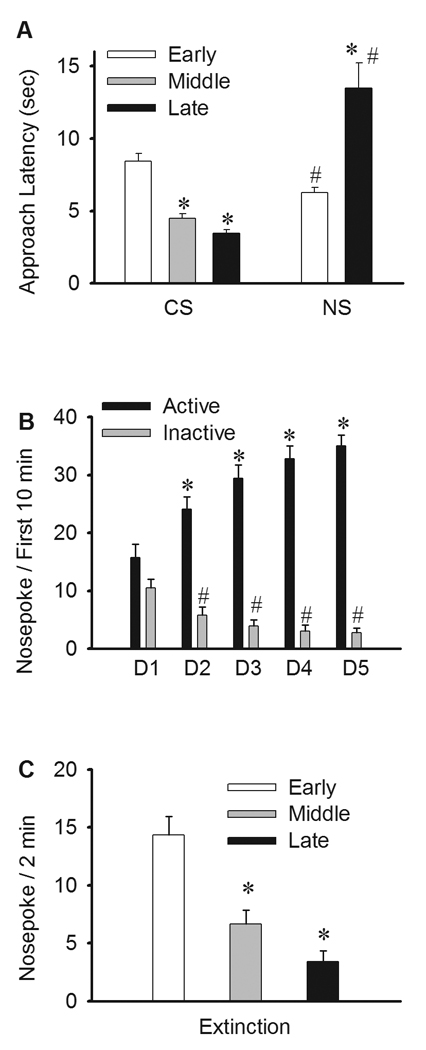
A) Pavlovian training. The latency of food magazine approach after CS was compared during early (first 5 blocks on day 1), middle (first 5 blocks on day2) and late (last 5 blocks on day2) blocks of Pavlovian training. For each 2min block, the approach latency after first CS was measured. The approach latency significantly decreased after CS. In contrast, the approach latency after NS significantly increased from first to second block (see Methods). * p < 0.05 compared to early block. # p < 0.05 compared to corresponding CS block. B) Instrumental training. The number of nosepokes into active (rewarded) and inactive (non-rewarded) nosepoke modules during first 10min of each training day were compared. * p < 0.05 compared to day1. # p < 0.05 compared to active responses on the same day. C) Instrumental Extinction. The number of responses into previously active nosepoke module was compared between early, middle and late 2-min blocks within the 10 min extinction session. * p < 0.05 compared to early block.
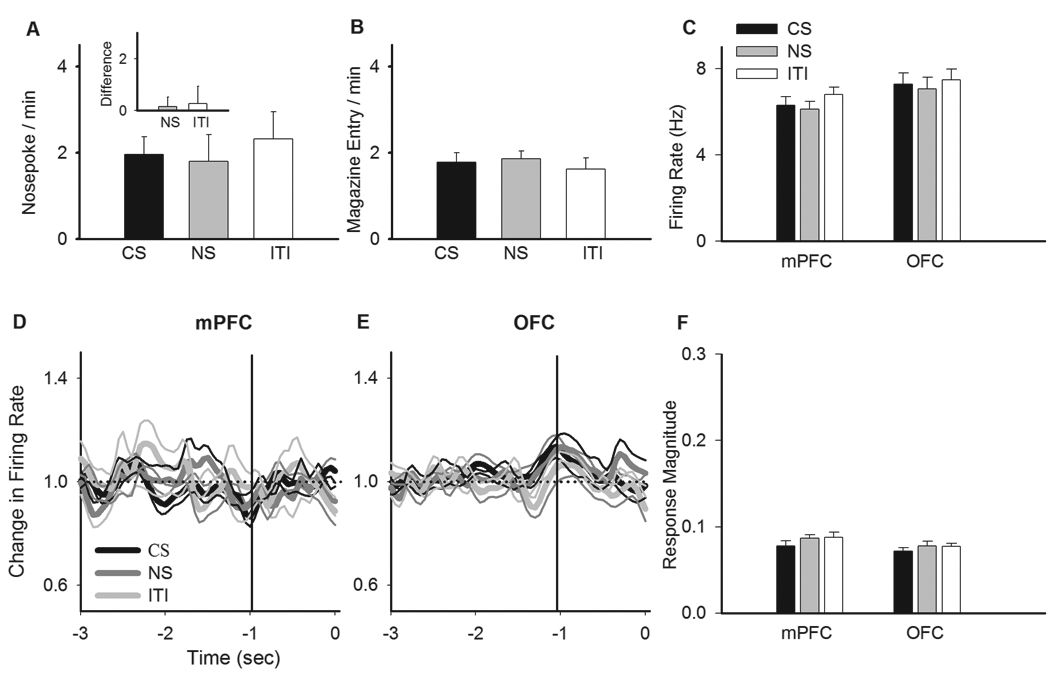
The PIT was tested in the absence of reinforcer using intermittent presentations of Pavlovian CS, the NS and an intertrial interval (ITI). The rate of instrumental nosepokes during CS block, as compared to NS and ITI blocks, was recorded as a measure of PIT. The CS evoked a significantly higher rate of instrumental nosepokes as compared to both NS and ITI (Figure 3A, one-way ANOVA, F(2,39) = 14.04, Bonferroni post hoc test, p < 0.05). This finding was further confirmed by assessing the differential transfer scores, calculated by the difference between nosepoke rates during CS and non-CS (NS or ITI) epochs per individual sessions. Positive transfer scores, based on either NS or ITI, verified the selective excitatory effect of CS on execution of instrumental action (Figure 3A inset). In addition, the CS also elicited Pavlovian approach behavior as manifested by a significant increase in the number of food magazine entries compared to NS and ITI epochs (Figure 3B, F (2,39) = 4.26, p < 0.05).
Figure 3. PIT behavior and population firing rates.
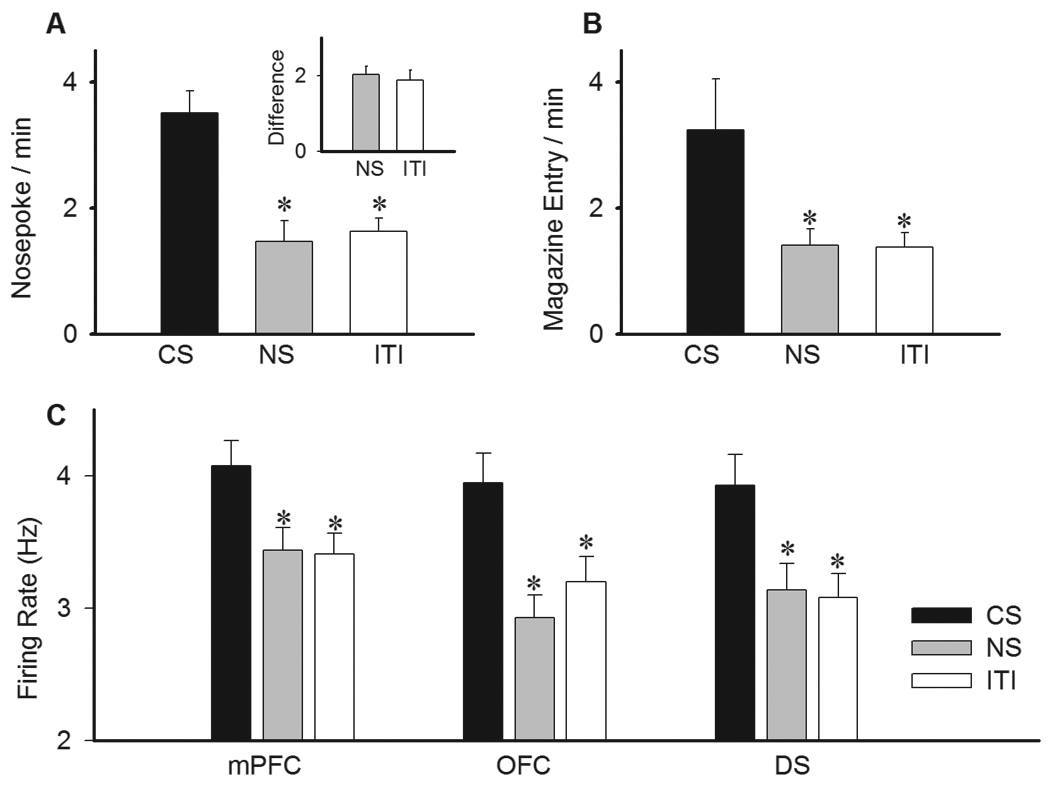
A) The effect of CS presentation on instrumental nosepoke responses as compared to neutral stimulus (NS) or inter-trial interval (ITI) epochs. Each bar depicts the mean ± S.E.M. of nosepoke rate during the corresponding epoch. Inset) The same measure has been transformed to the within-subject difference score, expressed by the increase in nosepokes elicited by CS compared to either NS or ITI. A value greater than zero indicates transfer. B) Comparison of food magazine approach behavior (magazine entry) during presentations of CS, NS and ITI. C) Average firing rate of neurons in medial prefrontal cortex (mPFC), orbitofrontal cortex (OFC) and dorsal striatum (DS) during different epochs of PIT session. * p < 0.05 compared to CS epoch.
Neuronal Activity
In drug-free experiments, 475 single units were recorded during 14 PIT sessions (from 14 rats). This population was classified as either RF or FF neurons in the mPFC (RF, N = 184, FF, N = 2) and OFC (RF, N = 134, FF, N = 5), and as putative projection neurons or putative interneurons in DS (N = 134 and 16, respectively). The subsequent analyses focus on the main neuronal population in each region, cortical putative pyramidal neurons (RF units) and striatal putative projection neurons (examples of isolated single unit waveforms shown in Supplementary Figure 2). In addition, a total of 116 single units (including 64 mPFC and 52 OFC putative pyramidal neurons) were recorded during experiments where animals were treated with the NMDA antagonist MK801.
We first examined the effect of CS presentation epochs on population neuronal activity in the mPFC, OFC, and dorsal striatum. Average firing rates in all three regions significantly increased during periods of CS, compared to both NS and ITI epochs (Figure 3C, one-way ANOVA, mPFC, F(2,549) = 4.62, p < 0.05, OFC, F(2,399) = 5.15, p < 0.01, DS, F(2,399) = 7.17, p < 0.001). This initial analysis of population activity, based on 2-min blocks of CS, suggested that mPFC, OFC and DS neuron may reflect the increased level of behavioral activity during CS epochs of PIT paradigm. However, a closer examination of neuronal responses revealed that these neurons did not maintain a persistent firing rate increase, i.e. starting immediately after CS and sustained at a constant rate for the entire 2 min period, as would have been expected if neural representation of PIT consisted of a task-insensitive elevated firing. Instead, a majority of neurons in each region showed phasic firing changes during discrete task events such as instrumental responding and food magazine approach behavior. Thus, further analysis mostly focused on these events.
Few neurons showed an immediate phasic response (within 2 sec) to the presentation of CS (< 2–5% of units in each region, supplementary Figure 3), as would have been expected if they represented its sensory qualities. This lack of immediate cue-related activity indicates that, in the setting of general PIT paradigm where the CS had not been associated with distinct outcome-specific responses, cortical neurons do not signify the sensory aspects of CS.
Next, we examined the phasic response of each neuron to CS-associated instrumental nosepokes using normalized firing rate histograms while limiting the analysis to a short peri-event window (see Methods). Significant phasic responses to CS-associated nosepokes were classified based on their direction as either an increased or a decreased response. The average firing of all neurons with a phasic increase, decrease or no change in response to nosepoke is depicted in Figure 4A (two-way ANOVA with time as repeated measure, type, F(2, 449) = 278.32, p < 0.001, time, F(79, 35471) = 331.08, p < 0.001, type × time interaction, F(158, 35471) = 44.33, p < 0.001). The distribution of response types were significantly different among regions, with predominantly inhibitory responses in the mPFC and DS but mostly excitatory responses in OFC (Figure 4B, Chi-Square test, χ2 = 102.72, p < 0.001). The phasic encoding of instrumental nosepokes may entail both motoric and non-motoric aspects of instrumental responding. However, the motoric components of representation, within the short peri-event window, are expected to remain stable across behavioral epochs while the non-motoric components, such as incentive, must be exclusively associated with CS. To distinguish between these components, we used normalized peri-event rate histograms to compare the neuronal correlates of nosepoke-related excitations and inhibitions between CS, NS and ITI epochs (Figure 4C–E). The predominant response patterns in each region, including mPFC and DS inhibitions and OFC excitation, were significantly potentiated during CS compared to both NS and ITI epochs. Two-way ANOVA with time as repeated measure (limited to a −1 to +0.5 sec peri-event window) revealed that the following types of peri-nosepoke responses were significantly different during CS compared to NS and ITI epochs: mPFC inhibitions (vs. NS, epoch, F(1, 146) = 2.73, p < 0.05, time, F(79, 11534) = 8.02, p < 0.01, epoch × time interaction, F(79, 11534) = 1.81, p < 0.01, vs. ITI, epoch, F(1, 146) = 7.38, p < 0.01, time, F(79, 11534) = 44.92, p < 0.001, epoch × time interaction, F(79, 11534) = 1.77, p < 0.001), OFC excitations (vs. NS, epoch, F(1, 110) = 6.34, p < 0.05, time, F(79, 8690) = 32.44, p < 0.001, epoch × time interaction, F(79, 8690) = 4.26, p < 0.001, vs. ITI, epoch, F(1, 110) = 4.92, p < 0.05, time, F(79, 8690) = 50.2, p < 0.001, epoch × time interaction, F(79, 8690) = 1.70, p < 0.001), OFC inhibitions (only significant vs. ITI, epoch, F(1, 30) = 5.78, p < 0.05, time, F(79, 2370) = 16.41, p < 0.001, epoch × time interaction, F(79, 2370) = 1.40, p < 0.05), and DS inhibitions (vs. NS, epoch, F(1, 158) = 5.76, p < 0.05, time, F(79, 12482) = 13.04, p < 0.01, epoch × time interaction, F(79, 12482) = 2.03, p < 0.01, vs. ITI, epoch, F(1, 158) = 27.25, p < 0.001, time, F(79, 12482) = 43.50, p < 0.001, epoch × time interaction, F(79, 12482) = 7.68, p < 0.001). This analysis indicates that CS-associated instrumental nosepokes were primarily represented by phasic mPFC and DS inhibitions and OFC excitations. In addition to responses at the population level, individual units at each region displayed discriminative encoding of nosepokes during CS versus NS and ITI epochs (Figure 5A–F, examples of single neurons, Supplementary Figure 4, examples of peri-event raster and histograms for individual neurons). Using a standardized index of phasic response magnitude that allowed comparison of inhibitory and excitatory responses irrespective of directionality (see Methods), we compared the magnitude of phasic neuronal responses to instrumental nosepokes during different behavioral epochs. This analysis revealed that CS was associated with a stronger phasic encoding of instrumental nosepokes in all three regions (Figure 5G, one-way ANOVA with Bonferroni post hoc test, CS compared to both NS and ITI epochs, mPFC, F(2, 270) = 59.95, p < 0.001; OFC, (2, 213) = 6.47, p < 0.01, DS, (2, 270) = 45.57, p < 0.001). A within-session analysis identified neurons that selectively fired for CS epoch instrumental nosepokes in their individual firing. Figure 5H depicts the proportion of these stimulus-selective nosepoke-responder units in each region.
Figure 4. Population phasic correlates of instrumental nosepoke responses.
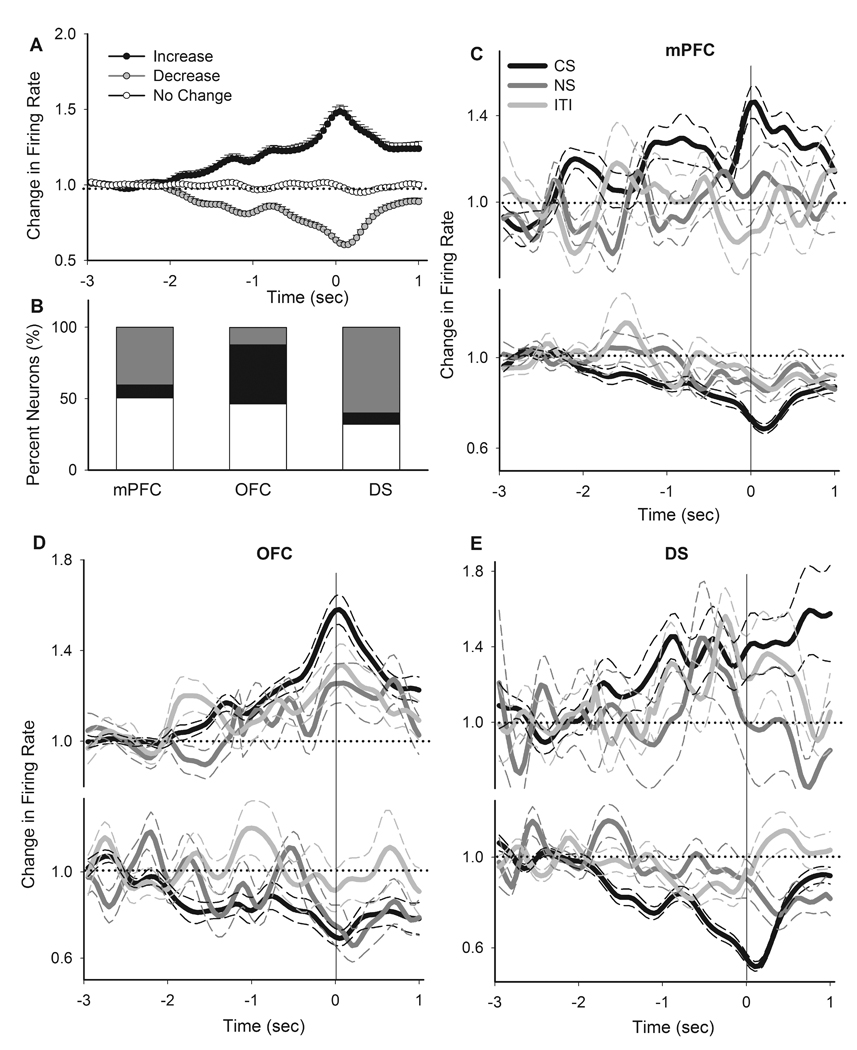
A) Average phasic activity of all neurons with an increase, decrease or no change response during CS nosepokes. Each line depicts the mean ± S.E.M. of the normalized peri-event time histogram of all units with the corresponding response type (50 ms bin). Zero indicates the time of nosepoke. Dotted line indicates no change from baseline (−3 sec to −2 sec pre-event). B) The distribution of different types of responses to nosepoke per region. Color codes as in section A. C–E) Comparison of peri-nosepoke time histograms of all neurons, which had an increase (upper panels) or decrease (lower panels) phasic response to CS nosepokes, during different epochs of PIT session (CS, NS, ITI). Dotted line indicates no change from baseline. Solid line indicates the time of nosepoke. Thick and thin color lines indicate the mean and SEM, respectively. Responses are separately compared within mPFC (C), OFC (D) and DS (E).
Figure 5. Individual neuronal correlates of instrumental nosepokes.
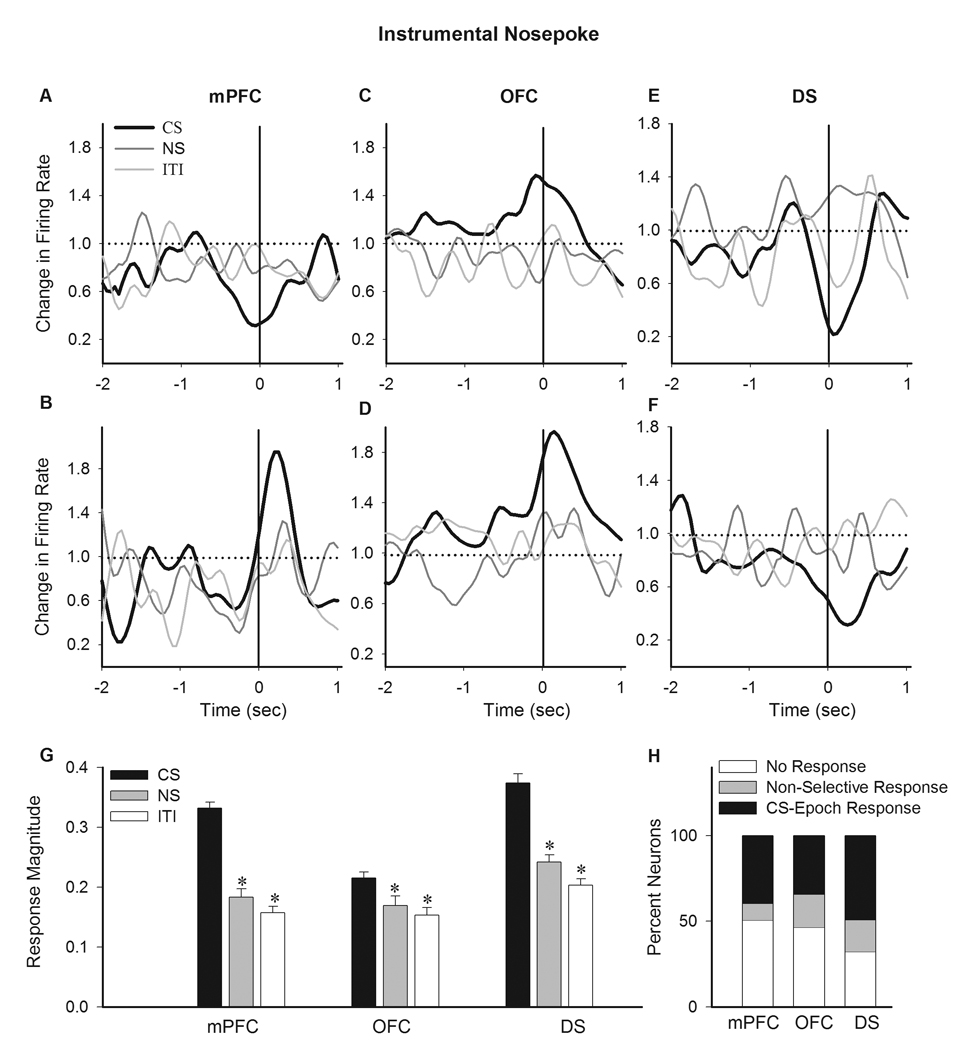
A–E) Examples of individual neurons exhibiting a CS selective phasic response to instrumental nosepoke. Each section depicts the normalized peri-nosepoke time histograms (50 ms bins, smoothed by a Gaussian filter with bin width of 5) of two individual neurons in mPFC (A–B), OFC (C–D) and DS (E–F). The correlates of the same behavior (nosepoke) during CS (thick black line), NS and ITI (thin gray lines) epochs have been compared within each panel. Dotted line indicates no change from baseline. Solid line indicates the time of nosepoke. G) Average magnitude of phasic peri-nosepoke responses are compared within each region between different epochs. Response magnitudes were standardized to reflect both excitatory and inhibitory responses irrespective of directionality, ranging between 0 (no change) to 0.5 (maximum change) (see Methods). * p < 0.05 compared to CS epoch. H) The distribution of neurons per region based on the profile of their individual responses to nosepokes. Units were classified based on displaying no response, non-selective response or CS epoch selective response to nosepokes.
To evaluate correlation between this nosepoke-related neuronal activity and PIT behavior, we compared the phasic neuronal responses as the animals progressed from early to late blocks of PIT session. Individual neurons showed a stronger response to early versus late CS-associated nosepokes within the same session (examples in Figure 6A–C). Moreover, the magnitude of cortical, but not striatal, phasic responses showed a strong correlation with PIT behavior (Figure 6D–E, correlations with CS-associated behavioral responses across the 2-min blocks within individual sessions, Figure 6F, correlation coefficients for all sessions).
Figure 6. Correlation between PIT behavior and neuronal correlates of instrumental transfers.
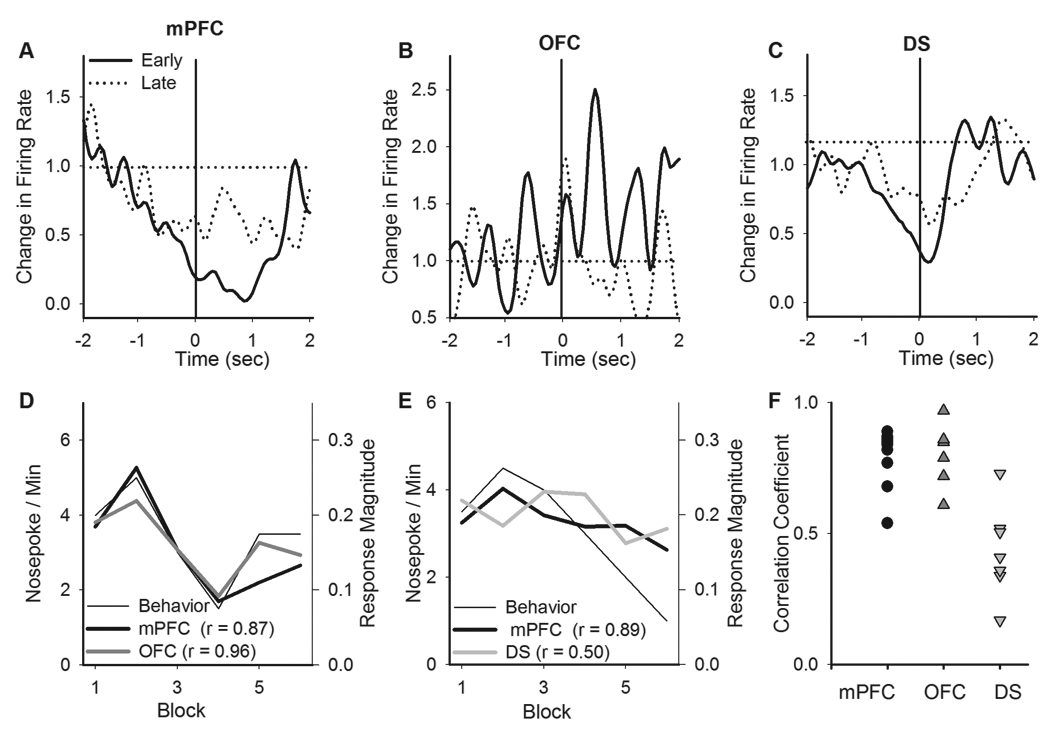
A–C) Examples of the phasic response of individual neurons to CS-associated instrumental nosepokes during early and late blocks of the same PIT session. The neuronal correlates of the instrumental transfers decreased in these examples. D–E) Examples of correlation between the magnitude of phasic neuronal responses to instrumental nosepokes and the rate of nosepokes across 2-min CS blocks within individual PIT sessions. In shown examples mPFC and OFC neurons showed strong correlation with behavior while in case of DS the correlation was weaker. F) A plot of region-behavior correlation coefficients for all sessions. Each dot represents the coefficient for correlation between the phasic response of neurons in a region and the corresponding PIT behavior within a single session. Note that mPFC and OFC demonstrated strong correlation with behavior while DS showed weak correlation.
Next, we asked whether the corticostriatal neuronal subsets engaged during the instrumental response were also involved in encoding for the Pavlovian approach behavior. To address this question, we examined the phasic neuronal correlates of CS-associated food magazine entries, a behavior thought to primarily reflect Pavlovian incentives (Holland and Gallagher, 2003; Pecinna et al., 2006). To limit the less likely confound that some food magazine entries may occur as part of a habitual instrumental response-approach sequence, we excluded from this analysis the minority of magazine entries (8.2%) that occurred immediately (< 10 sec) after a nosepoke entry. This analysis was consistent with previous studies in finding that the majority of cue-associated Pavlovian approach behaviors are temporally distanced from instrumental responses (Pecinna et al., 2006). A substantial subgroup of units in each region displayed phasic encoding for Pavlovian (CS epoch) approach behavior. Figure 7A depicts the average peri-magazine entry activity of all neurons based on their phasic response direction (two-way ANOVA with time as repeated measure, type, F(2, 449) = 108.96, p < 0.001, time, F(79, 35471) = 261.32, p < 0.001, type × time interaction, F(158, 35471) = 28.74, p < 0.001). The distribution of magazine entry responses was different between regions, with predominant inhibitions in the mPFC and OFC, and equally frequent excitations and inhibitions in DS (Figure 7B, Chi-Square test, χ2 = 21.0, p < 0.001). All three regions displayed a pattern of selective amplification of their peri-magazine responses during CS epochs. Two-way ANOVA with time as repeated measure revealed that peri-magazine responses were significantly different during CS compared to NS and ITI epochs (mPFC excitations, vs. NS, epoch, F(1, 32) = 4.47, p < 0.05, time, F(79, 2528) = 5.037, p < 0.001, epoch × time interaction, F(79, 2528) = 1.61, p < 0.05, vs. ITI, epoch, F(1, 32) = 30.17, p < 0.001, time, F(79, 2528) = 5.72, p < 0.001, epoch × time interaction, F(79, 2528) = 2.99, p < 0.001, mPFC inhibitions, vs. NS, epoch, F(1, 104) = 15.3, p < 0.001, time, F(79, 8216) = 21.3, p < 0.001, epoch × time interaction, F(79, 8216) = 4.46, p < 0.01, vs. ITI, epoch, F(1, 104) = 9.42, p < 0.005, time, F(79, 8216) = 5.02, p < 0.001, epoch × time interaction, F(79, 8216) = 1.86, p < 0.001, OFC excitations, vs. NS, epoch, F(1, 30) = 26.3, p < 0.001, time, F(79, 2370) = 9.21, p < 0.001, epoch × time interaction, F(79, 2370) = 2.26, p < 0.001, vs. ITI, epoch, F(1, 30) = 7.9, p < 0.05, time, F(79, 2370) = 7.16, p < 0.001, epoch × time interaction, F(79, 2370) = 1.66, p < 0.01, OFC inhibitions, vs. NS, epoch, F(1, 60) = 6.92, p < 0.05, time, F(79, 4740) = 15.43, p < 0.001, epoch × time interaction, F(79, 4740) = 3.02, p < 0.001, vs. ITI, epoch, F(1, 60) = 12.29, p < 0.001, time, F(79, 4740) = 14.3, p < 0.001, epoch × time interaction, F(79, 4740) = 3.68, p < 0.05, DS excitations, vs. NS, epoch, F(1, 68) = 41.54, p < 0.001,time, F(79, 5372) = 5.92, p < 0.001, epoch × time interaction, F(79, 5372) = 4.46, p < 0.001, vs. ITI, epoch, F(1, 68) = 40.13, p < 0.001, time, F(79, 5372) = 6.56, p < 0.001, epoch × time interaction, F(79, 5372) = 3.99, p < 0.001, DS inhibitions, vs. NS, epoch, F(1, 70) = 4.31, p < 0.05, time, F(79, 5530) = 2.75, p < 0.001, epoch × time interaction, F(79, 5530) = 2.03, p < 0.05, vs. ITI, epoch, F(1, 70) = 4.08, p < 0.05, time, F(79, 5530) = 11.79, p < 0.001, epoch × time interaction, F(79, 5530) = 3.09, p < 0.001).
Figure 7. Population phasic correlates of Pavlovian approach behavior.
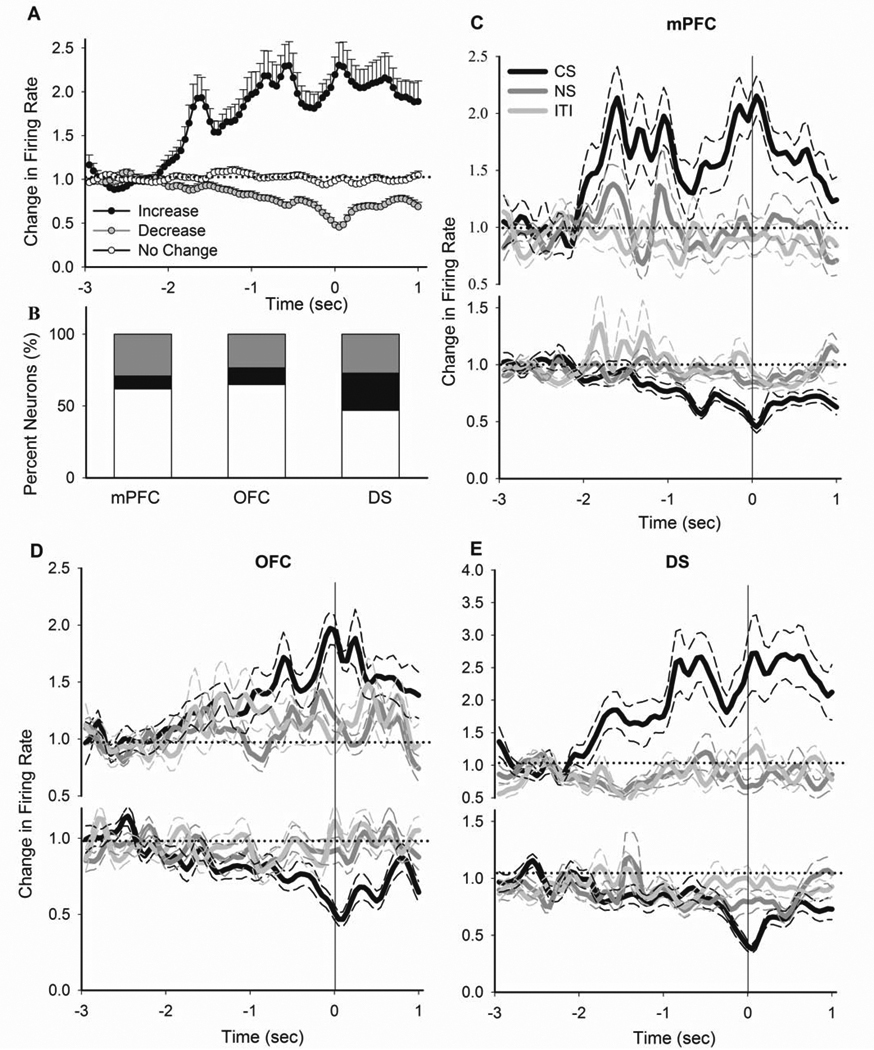
A) Average phasic activity of all neurons with an increase, decrease or no change response during Pavlovian approach behavior (CS magazine entries not immediately preceded by a nosepoke, see Methods). B) The distribution of different types of responses to magazine entry per region. C–E) Comparison of peri-entry time histograms of all neurons, which had an increase (upper panels) or decrease (lower panels) phasic response to CS magazine entries, during different epochs of PIT session. Responses are separately compared within mPFC (C), OFC (D) and DS (E). All conventions are as in Figure 4.
This population-level CS epoch selectivity was also evident at the single unit level (Figure 8A–F, examples of single units). In all regions, the phasic magnitude of CS-associated magazine entries was significantly stronger than those associated with NS or ITI epochs (Figure 8G, one-way ANOVA with Bonferroni post hoc test, mPFC, F(2, 207) = 27.85, p < 0.001; OFC, (2, 138) = 10.08, p < 0.01, DS, (2, 210) = 10.18, p < 0.001). This selectivity was mainly driven by a subgroup of units that discriminated in their encoding of magazine entry based on the stimulus presented (Figure 8H, distribution per region).
Figure 8. Individual neuronal correlates of Pavlovian Approach behavior.
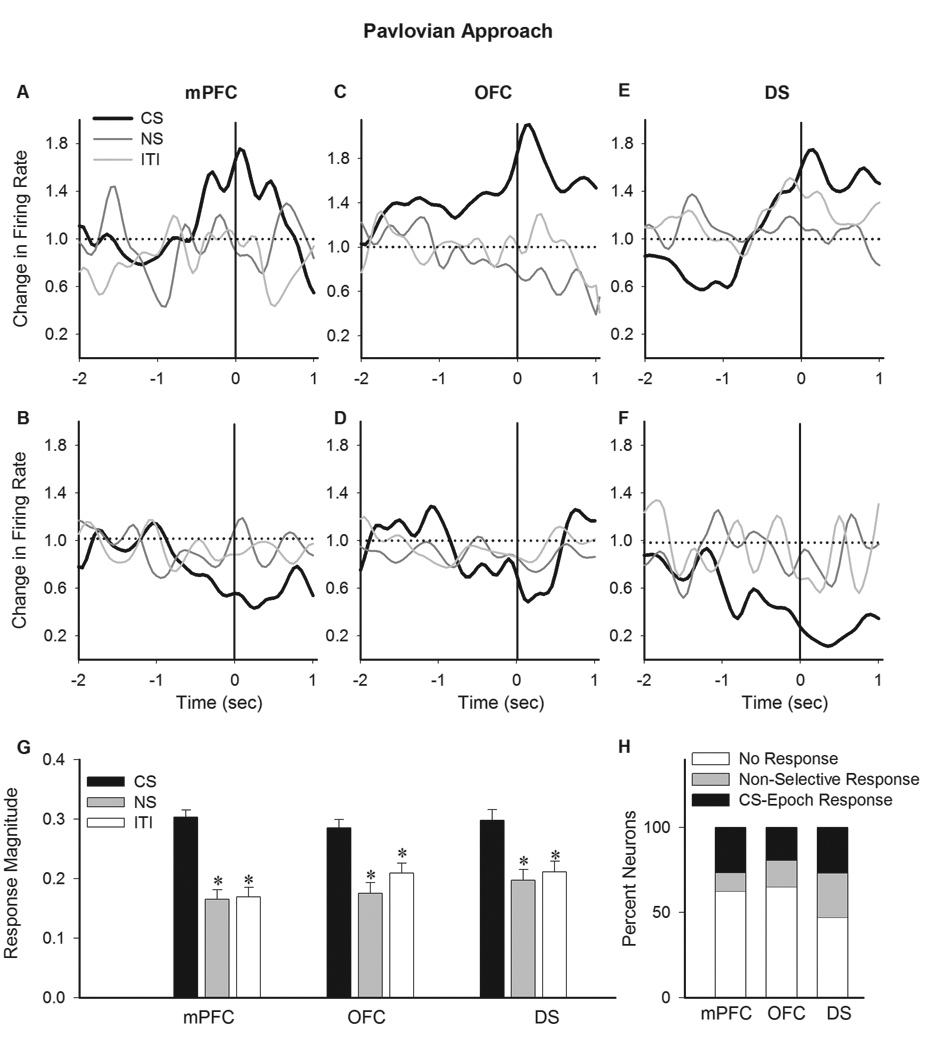
A–E) Examples of individual neurons exhibiting a CS selective phasic response to Pavlovian magazine entries. Each section depicts the normalized peri-entry time histograms of two individual neurons (upper and lower panels) in mPFC (A–B), OFC (C–D) and DS (E–F). The correlates of the same behavior (magazine entry) during CS (thick black line), NS and ITI (thin gray lines) epochs have been compared within each panel. G) Average magnitude of phasic peri-magazine entry responses are compared in each region between different epochs. * p < 0.05 compared to CS. H) The distribution of neurons per region based on the profile of their individual responses to magazine entry. Units were classified based on displaying no response, non-selective response or CS epoch selective response to magazine entry. All conventions are as in Figure 5.
We then asked whether overlapping populations of neurons represented the Pavlovian and instrumental processes. Thus, we compared the proportion of neurons encoding for Pavlovian approach (Pavlovian responder) among subsets of neurons divided by their instrumental representations (as shown in Figure 5H): A) CS-epoch nosepoke responder (transfer responder) B) non-selective nosepoke responder and C) nosepoke non-responders. In the mPFC (Figure 9A) and OFC (Figure 9B), the majority of transfer responder neurons also represented the Pavlovian approach (56% and 54%, respectively). This was significantly higher than the proportion of Pavlovian responders in the other two subsets (Chi-Square test between subsets in each region, mPFC, χ2 = 17.65, p < 0.001, OFC, χ2 = 11.96, p < 0.01). Notably, comparison of the phasic responses to nosepoke and approach by individual cortical neurons which represented both events revealed significant differences in latency and amplitude of responses, suggesting that these subset of neurons carry distinct, rather than similar, representations for these events (Supplementary Figure 5). A different pattern of overlap was observed in DS; the Pavlovian responders predominantly overlapped with nosepoke non-responders and non-selective responders as compared to transfer responders (Figure 9C, χ2 = 7.62, p < 0.05). In addition, we also performed this analysis for only those Pavlovian responders that had a selective response to CS (CS-epoch Pavlovian responders, as shown in Figure 8H), with similar results suggestive of significant overlap between CS-selective subsets in mPFC and OFC, and segregation in DS (Supplementary Figure 6). Notably, the neurons with and without overlapping representations of two behaviors did not differ in their baseline firing rates (data not shown).
Figure 9.
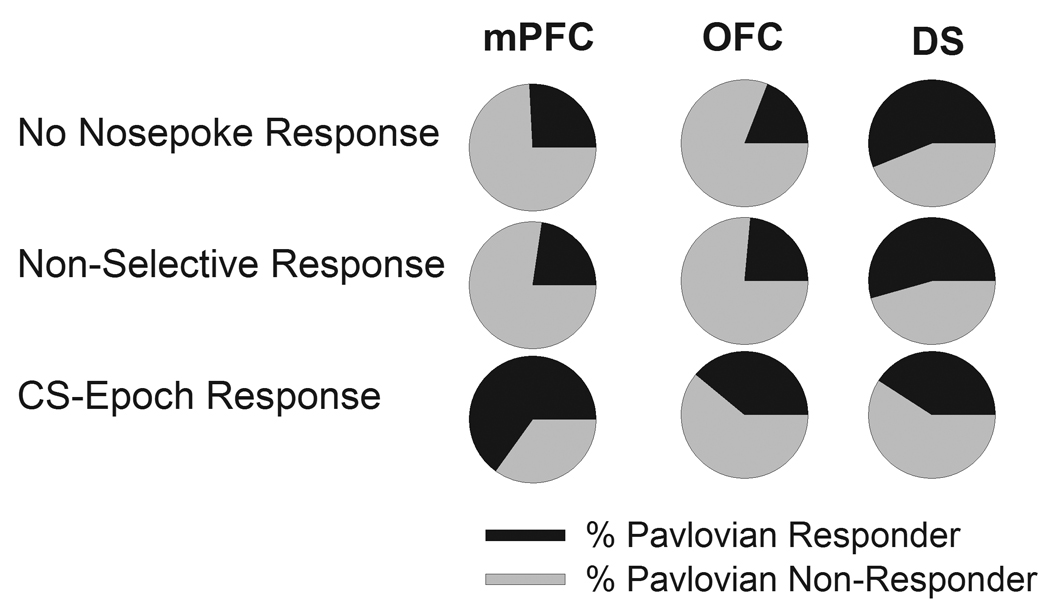
Overlap in distribution of neurons encoding for instrumental nosepoke and Pavlovian approach (magazine entry). Neurons in each region have been divided into three subsets based on their phasic response to instrumental nosepoke as nosepoke non-responder, non-selective nosepoke responder and CS-epoch nosepoke responder (as shown in Figure 5H). The proportion of units encoding for Pavlovian approach (Figure 8H, all responders) is shown in black. In mPFC and OFC, the proportion of Pavlovian responders was significantly higher among CS-epoch nosepoke responders compared to the other two subsets. In striatum, the proportion was significantly lower in CS-epoch nosepoke responder subset compared to the two other subsets.
Finally, we examined whether disruption of cortical activity would interrupt behavioral and neuronal correlates of PIT. We used an NMDA receptor antagonist (MK801) for these experiments because this treatment disrupts mPFC (Jackson et al., 2004) and OFC (Homayoun and Moghaddam, 2008b) activity in awake animals, and selectively disrupts PFC-dependent tasks such as working memory and set-shifting (Moghaddam and Jackson, 2003). This treatment disrupted PIT behavior (Figure 10A, nosepoke responses, Figure 10B, magazine entries, p > 0.05), while, consistent with previous observations (Jackson et al., 2004), increased mPFC and OFC basal firing rates. This increase was not selective for CS epoch (Figure 10C) and was associated with diminished phasic firing responses to nosepokes and absence of a preferential response during CS epochs (Figure 10 D–E, average phasic responses to nosepokes in each region per epoch, Figure 10F, average magnitude of significant phasic responses to nosepoke, p > 0.05). Thus, blocking NMDA receptors, while hyperactivating baseline firing of mPFC and OFC neurons, specifically abolished the phasic CS-associated nosepoke responses at the same time that it disrupted the PIT behavior.
Figure 10.
Effect of systemic MK801 on neuronal correlates of instrumental transfers. Animals were pre-treated with the NMDA receptor antagonist MK801 (0.1 mg/kg) 30 min prior to PIT test session. A–B) MK801 abolished the preferential CS-associated nosepoke and magazine entries. The animals continued to respond at baseline level but the behavioral transfer did not occur. C) MK801 increased the tonic firing rate of mPFC and OFC neurons regardless of the associated context (CS, NS or ITI). Conventions are as in Figure 3. D–E) MK801 diminished the phasic neuronal responses to instrumental nosepoke. While the mPFC and OFC neurons were tonically hyper-activated by MK801, they failed to show a preferential CS-associated response to nosepokes. Conventions as in figure 4. F) Comparison of the magnitude of phasic neuronal responses to instrumental nosepokes across epochs. Note the decrease in the amplitude of phasic responses compared to Figure 5G.
Discussion
PIT presents an interaction between Pavlovian and instrumental incentive processes in which previously learned Pavlovian cues elicit a representation of reward and then evoke a previously extinguished instrumental action directed toward the same reward. In this first demonstration of neuronal correlates of PIT, we find that neuronal representations of the key behavioral events, i.e. instrumental nosepokes and magazine approach, were amplified in the presence of Pavlovian CS. These representations were integrated within the mPFC and OFC but remained segregated in the striatum. In addition, phasic responses in PFC regions were correlated with the strength of PIT behavior, and their disruption by the NMDA receptor antagonist was associated with impaired PIT behavior. Together, these findings suggest that parallel phasic activity of neuronal subsets in mPFC and OFC may encode the transfer from Pavlovian incentives to instrumental actions.
Phasic event-specific responses during PIT
In general, PIT is considered a conditioned incentive process in which a CS brings online a representation of Pavlovian association, prompts motivation towards the desired - but absent - reward, and triggers the execution of a goal-directed action (Rescorla and Solomon, 1967; Dickinson, 1994; Wyvell and Berridge, 2000). In the general form of PIT used here, the Pavlovian CS activates a conditioned motivational state that is transferred to instrumental responding. This paradigm is distinct from the outcome-specific form of PIT, where the transfer from specific CS-reward associations to specific action-outcome associations occurs, and may model real life situations where motivational cues can lead to non-specific reinforcing actions. Here, we focused on three key events in PIT paradigm: CS presentation, CS-associated instrumental response (transfer events), and, CS- associated magazine entry (Pavlovian approach behavior).
Tonic firing during the CS epoch was increased compared to NS or ITI epochs. This CS-selective general activation suggests that these regions differentiated between CS and other epochs. A more detailed analysis, however, revealed that few neurons showed an immediate phasic response to the CS onset, indicating a lack of cue-locked immediate responses. This is in contrast to findings of cue-locked responses in paradigms wherein distinct cues are associated with specific responses/outcomes. This interesting distinction is in agreement with the idea that PFC subregions do not directly represent the sensory qualities of the stimuli per se but rather encode for them based on the context and relevance of their associations. In terms of neural circuitry of PIT, this finding implies that the initial response to the sensory aspects of CS may arise from other brain regions, such as amygdala, and then be relayed to the PFC.
A large number of cortical, and to a lesser extent striatal, neurons represented the instrumental nosepokes, and increased their responses during CS epochs. The representation of instrumental actions by prefrontal neurons has been previously established by several groups (Peters et al., 2005; Mulder et al., 2003). These representations were shown to attenuate with extinction (Mulder at al., 2003). Similarly, the ITI-epoch nosepoke responses indicate the baseline level of encoding for an extinguished instrumental action. In PIT paradigm, the CS-associated instrumental responses are the core feature of PIT, where the motivational state evoked by CS “transfers” to instrumental incentives that reinstate an extinguished instrumental action (Dickinson, 1994; Cardinal et al., 2002). Thus, the observed amplification of the neuronal responses to the same event (instrumental nosepoke) during CS epochs is likely a neural representation of incentive processes that drive the PIT behavior. The CS-associated amplification of nosepoke representations did not reflect the motoric aspects of the task, which were similar during all epochs, or the stimulus effects on attention, which were shared by CS and NS. It is also unlikely that this selective amplification was a result of a general arousal effect of CS because CS did not cause an immediate effect on neuronal activity, had minimal effects on the rate of response on non-active (not previously rewarded) nosepokes and did not increase nosepoke rates in ITI or NS epochs as would have been expected if it caused a prolonged arousal effect.
If the CS-associated amplification of phasic peri-nosepoke responses is in fact a neuronal representation of PIT process, it would be predicted that this signal should correlate with strength of PIT behavior, and that its disruption should be associated with disruption of PIT behavior. The mPFC and OFC neurons fulfilled both predictions in that 1) their CS-selective phasic responses showed strong correlation with PIT behavior, and that 2) the disruption of their phasic encoding by an NMDA receptor antagonist was associated with disruption in PIT behavior. While the NMDA antagonist increased the basal firing rates of most PFC neurons (Jackson et al., 2004), it disrupted phasic CS-associated representation of instrumental nosepokes in association with PIT behavior impairment.
A subset of neurons in each region represented the food magazine entries with a notable fraction discriminating the frequent CS-associated entries from their occasional non-CS-associated counterparts. Pavlovian approach behavior is believed to be a direct result of Pavlovian incentive processes activated by CS and directed towards the expected reinforcer (Cardinal et al., 2002; Holland and Gallagher, 2003). We utilized this behavior to identify the neural substrates for representation of Pavlovian incentives. Since entries during CS and non-CS epochs only differ in their motivational significance, our data indicates that PFC subregions and dorsal striatum may participate in the representation of the Pavlovian incentive for reward.
OFC and mPFC integrate their representations of PIT
Our data indicate that mPFC and OFC participate in neuronal encoding for the general form of PIT. This is consistent with findings of mPFC and OFC involvement in encoding components of Pavlovian and instrumental processes (Gallagher et al., 1999; Chudasama and Robbins, 2003; Homayoun and Moghaddam, 2008a; Ostlund and Balleine, 2007). Lesion studies, however, have failed to establish a clear role for PFC in PIT process (Cardinal et al., 2002; Chudasama and Robbins, 2003; Corbit and Balleine, 2003). This discrepancy may reflect methodological differences, as electrophysiological methods allow detection of transient dynamics of discrete events. Furthermore, mPFC and OFC may act as components of a distributed network that controls PIT. PFC damage, therefore, may be compensated by other structures in network, sparing PIT. Regardless, the present findings demonstrate that OFC and mPFC participate in selective encoding of instrumental transfers in a way that shows strong within-session correlation with PIT behavior. Contrary to suggestions that mPFC and OFC exclusively encode the instrumental and Pavlovian processes, respectively (Ostlund and Balleine, 2007), the two cortical regions displayed simultaneous phasic encoding for both processes. Both cortical regions represented the Pavlovian approach and instrumental action through the same neuronal subsets, suggesting that they participated in integrating Pavlovian and instrumental incentives. This integration involved distinct operations as mPFC and OFC displayed predominantly inhibitory and excitatory phasic responses to the same events, respectively.
The excitatory response of lateral OFC neurons may signify a positive motivational signal associated with the expected reward (Tremblay and Schultz, 1999; Schoenbaum et al., 2003). While both general and outcome-specific incentives are thought to contribute to general form of PIT, our findings that neurons did not show a phasic response to CS, and that they represented the magazine entry and instrumental events through distinct representations, suggests that general, rather than outcome-specific, incentive processes were primarily involved in the current PIT paradigm, and in its encoding by OFC (Burke et al., 2008). In contrast, the prelimbic mPFC neurons exhibited a predominantly inhibitory pre-nosepoke response that was reminiscent of their response to the same event in animals well-trained in instrumental responding (Homayoun and Moghaddam, 2006; Homayoun and Moghaddam, 2008a). The re-emergence of this inhibitory response during CS nosepokes, as opposed to degraded encoding of ITI nosepokes, suggests that mPFC re-activated its representation of instrumental action under the influence of the Pavlovian incentives that were represented by the same neuronal subsets. This integration of Pavlovian and instrumental processes, where cue-evoked incentives recruit instrumental representations, may provide a mechanism for the prelimbic mPFC, to execute motivational control over goal-directed behavior. These results may also be considered in the context of emerging differences between mPFC subregions in motivational incentive processing. For example, infralimbic region has been linked to habitual responding whereas prelimbic region is primarily associated with action-outcome association (Killcross and Coutureau, 2003; Hitchcott et al., 2007).
DS was distinct from cortical regions in that it maintained mostly segregated representations of Pavlovian approaches and instrumental nosepokes. Moreover, the CS-selective encoding in DS showed a weaker correlation with PIT behavior. These findings suggest that DS is an unlikely site for integration of Pavlovian incentives with instrumental responding. Instead, DS may provide a substrate for functional segregation of Pavlovian and instrumental components, allowing selective modulation of Pavlovian motivation versus instrumental action.
Functional implications
The findings that the same mPFC and OFC neuronal subsets maintain representations of both Pavlovian and instrumental processes have implications for understanding the neuronal basis of disorders of affect and motivation. One example is addiction. Repeated exposure to drugs of abuse alters the functions of OFC and mPFC (Bolla et al., 2003; Ersche et al., 2005; Goldstein et al., 2007b). It is possible that altered cortical encoding of PIT may serve as a mechanism for making addicts susceptible to influence of drug cues on behavior. Interestingly, long-term amphetamine exposure, an animal model shown to potentiate PIT (Wyvell and Berridge, 2001), amplifies tonic cortical responses, with OFC activated and mPFC inhibited (Homayoun and Moghaddam, 2006), in the same direction as their phasic responses here. Similar divergent activation patterns have been observed in the OFC and dorsolateral PFC (primate equivalent of rodent mPFC) of drug abusers during cue-induced craving (Volkow et al., 2004; Goldstein et al., 2007a). Another example is schizophrenia where aberrant stimulus-response associations may precipitate psychotic and affective symptoms (Kapur, 2003; Homayoun and Moghaddam, 2008a). The disruption of cortical encoding of PIT by MK801, a drug used to model aspects of schizophrenia, supports this view. Together, these findings suggest that parallel neuronal encoding in mPFC and OFC support PIT and may play an important role in disorders such as addiction and schizophrenia that involve aberrant influence of external cues on behavior.
Supplementary Material
Acknowledgement
This study was supported by the National Institute of Mental Health, Pittsburgh Life Sciences Green House, and Tourette Syndrome Association.
References
- Baldwin AE, Holahan MR, Sadeghian K, Kelley AE. N-methyl-D-aspartate receptor-dependent plasticity within a distributed corticostriatal network mediates appetitive instrumental learning. Behav. Neurosci. 2000;114:84–98. doi: 10.1037//0735-7044.114.1.84. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Barnes T, Kubota Y, Hu D, Jin D, Graybiel A. Activity of striatal neurons reflects dynamic encopding and recoding of procedural memories. Nature. 2005;437:1158–1161. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- Bolla K, Eldreth D, London E, Kiehl K, Mouratidis M, Contoreggi C, Matochik J, Kurianl V, Cadet J, Kimes A, Funderburk F, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal R, Everitt B. Neural and psychological mechanisms underlying appetitive learning: links to drug addiction. Cur. Op. Neurobiol. 2004;14:156–162. doi: 10.1016/j.conb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Cardinal R, Parkinson J, Hall J, Everitt B. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci. Biobehav. Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Christakou A, Robbins T, Everitt B. Prefrontal cortical-ventral striatal interactions involved in affective modulation of attentional performance: implications for corticostriatal circuit function. J. Neurosci. 2004;24:773–780. doi: 10.1523/JNEUROSCI.0949-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Robbins T. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J. Neurosci. 2003;23:8771–8780. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit L, Balleine B. The role of prelimbic cortex in instrumental conditioning. Behav. Brain Res. 2003;146:145–157. doi: 10.1016/j.bbr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Corbit L, Muir J, Balleine B. The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. J. Neurosci. 2001;21:3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Borchgrave R, Rawlins J, Dickinson A, Balleine B. Effects of cytotoxic nucleus accumbens lesions on instrumental conditioning in rats. Exp. Brain Res. 2002;144:50–68. doi: 10.1007/s00221-002-1031-y. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Smith J, Mirenowicz J. Dissociation of Pavlovian and instrumental incentive learning under dopamine antagonists. Beh. Neurosci. 2000;114:468–483. doi: 10.1037//0735-7044.114.3.468. [DOI] [PubMed] [Google Scholar]
- Dickinson A, Balleine B. Motivational control of goal-directed action. Animal Learn. Behav. 1994;22:1–8. [Google Scholar]
- Ersche K, Fletcher P, Lewis S, Clark L, Stocks-Gee G, London M, Deakin J, Robbins T, Sahakian B. Abnormal frontal activations related to decision making in current and former amphetamine- and opiate-dependent individuals. Psychopharmacology. 2005;180:612–623. doi: 10.1007/s00213-005-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes W. Discriminative conditioning. II. Effects of a Pavlovian conditioned stimulus upon a subsequently established operant response. J. Exp. Physiol. 1948;38:173–177. doi: 10.1037/h0057525. [DOI] [PubMed] [Google Scholar]
- Everitt B, Dickinson A, Robbins T. The neuropsychological basis of addictive behaviour. Brain Res. Brain Res. Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Gallagher M, McMahan R, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J. Neurosci. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza U, Prokopenko V, West M, Fabbricatore A. Higher magnitude accumbal phasic firing changes among core neurons exhibiting firing increases during cocaine self-administration. Neuroscience. 2006;137:1075–1085. doi: 10.1016/j.neuroscience.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Tomasi D, Rajaram S, Cottone LA, Zhang L, Maloney T, Telang F, Alia-Klein N, Volkow ND. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience. 2007a;144:1153–1159. doi: 10.1016/j.neuroscience.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Tomasi D, Zhang L, Cottone LA, Maloney T, Telang F, Caparelli EC, Chang L, Ernst T, Samaras D, Squires NK, Volkow ND. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am. J Psych. 2007b;164:43–51. doi: 10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Parkinson J, Connor T, Dickinson A, Everitt B. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur. J. Neurosci. 2001;13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- Hitchcott PK, Quinn JJ, Taylor JR. Bidirectional modulation of goal-directed actions by prefrontal cortical dopamine. Cereb. Cortex. 2007;17:2820–2827. doi: 10.1093/cercor/bhm010. [DOI] [PubMed] [Google Scholar]
- Holland P, Gallagher M. Double dissociation of the effects of lesions of basolateral and central amygdala on conditioned stimulus-potentiated feeding and Pavlovian-instrumental transfer. Eur. J. Neurosci. 2003;17:1680–1694. doi: 10.1046/j.1460-9568.2003.02585.x. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Progression of cellular adaptations in medial prefrontal and orbitofrontal cortex in response to repeated amphetamine. J. Neurosci. 2006;26:8025–8039. doi: 10.1523/JNEUROSCI.0842-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Divergent plasticity of prefrontal cortex networks. Neuropsychopharmacology. 2008a;33:42–55. doi: 10.1038/sj.npp.1301554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Orbitofrontal cortex neurons as a common cellular target for different classes of antipsychotic drugs. Proc. Nat. Acad. Sci. USA. 2008b;105:18041–18046. doi: 10.1073/pnas.0806669105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Jackson ME, Moghaddam B. Activation of metabotropic glutamate 2/3 (mGlu2/3) receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats. Journal of Neurophysiology. 2004;93:1989–2001. doi: 10.1152/jn.00875.2004. [DOI] [PubMed] [Google Scholar]
- Jackson M, Homayoun H, Moghaddam B. NMDA receptor hypofunction produces concomitant firing rate potentiation and burst activity reduction in the prefrontal cortex. Proc. Nat. Acad. Sci. USA. 2004;101:6391–6396. doi: 10.1073/pnas.0308455101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am. J. Psych. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kelley A. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb. Cortex. 2003;13:400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- Lovibond P. Facilitation of instrumental behavior by a Pavlovian appetitive conditioned stimulus. J. Exp.Psychol.:Animal Behav. Proc. 1983;9:225–247. [PubMed] [Google Scholar]
- McCormick DA, Connors BW, Lighthall JW, Prince DA. Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J. Neurophysiol. 1985;54:782–806. doi: 10.1152/jn.1985.54.4.782. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Jackson ME. Glutamatergic animal models of schizophrenia. Ann. N. Y. Acad. Sci. 2003;1003:131–137. doi: 10.1196/annals.1300.065. [DOI] [PubMed] [Google Scholar]
- Mulder AB, Nordquist RE, Orgut O, Pennartz CM. Learning-related changes in response patterns of prefrontal neurons during instrumental conditioning. Behav. Brain Res. 2003;146:77–88. doi: 10.1016/j.bbr.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Nicolelis M, Dimitrov D, Carmena J, Crist R, Lehew G, Kralik J, Wise S. Chronic, multisite, multielectrode recordings in macaque monkeys. Proc. Nat. Acad. Sci. USA. 2003;100:11041–11046. doi: 10.1073/pnas.1934665100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund S, Balleine B. Orbitofrontal cortex mediates outcome encoding in Pavlovian but not instrumental conditioning. J. Neurosci. 2007;27:4819–4825. doi: 10.1523/JNEUROSCI.5443-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasupathy A, Miller E. Different time course of learning-related acitvity in the prefrontal cortex and striatum. Nature. 2005;433:873–876. doi: 10.1038/nature03287. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Pecinna S, Schulkin J, Berridge K. Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol. 2006;4:8. doi: 10.1186/1741-7007-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters Y, O'Donnell P, Carelli R. Prefrontal cortical cell firing during maintenance, extinction, and reinstatement of goal-directed behavior for natural reward. Synapse. 2005;56:74–83. doi: 10.1002/syn.20129. [DOI] [PubMed] [Google Scholar]
- Rescorla R, Solomon R. Two-process learning theory: Relationships between Pavlovian conditioning and instrumental learning. Psychol. Rev. 1967;74:151–172. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- Saddoris M, Gallagher M, Schoenbaum G. Rapid associative encoding in basolateral amygdala depends on connections with orbitofrontal cortex. Neuron. 2005;46:321–331. doi: 10.1016/j.neuron.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Stalnaker T, Franz T, Singh T, Schoenbaum G. Basolateral amygdala lesions abolish orbitofrontal-dependent reversal impairments. Neuron. 2007;54:51–58. doi: 10.1016/j.neuron.2007.02.014. [DOI] [PubMed] [Google Scholar]
- Tierney P, Degenetais E, Thierry AM, Glowinski J, Gioanni Y. Influence of the hippocampus on interneurons of the rat prefrontal cortex. Eur. J. Neurosci. 2004;20:514–524. doi: 10.1111/j.1460-9568.2004.03501.x. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398:704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb. Cor. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: brain circuits and treatment strategies. Neuropharmacology. 2004;1(47 Suppl):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Wyvell C, Berridge K. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J. Neurosci. 2000;20:8122–8130. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell C, Berridge K. Incentive sensitization by previous amphetamine exposure: increased cue-triggered “wanting” for sucrose reward. J. Neurosci. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Ostlund S, Knowlton B, Balleine B. The role of the dorsomedial striatum in instrumental conditioning. Eur. J. Neurosci. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


