Abstract
Background
The establishment of effective treatment of neonatal anemia using recombinant human erythropoietin (r-HuEPO) requires a thorough understanding of the physiology and mechanism of EPO’s pharmacologic effect. The purpose of the present preclinical study in sheep was to elucidate the stimulatory effect of EPO on erythroid progenitors and their differentiation into reticulocytes useful in predicting optimal r-HuEPO dosing.
Methods
Five young adult sheep each underwent two phlebotomies spaced 4–6 weeks apart in which their hemoglobin levels were reduced from 12 g/dL to 3–4 g/dL. Endogenous EPO levels and reticulocyte counts produced in response to anemia were sampled throughout the study and analyzed using a pharmacokinetic/pharmacodynamic (PK/PD) model.
Results
The phlebotomy-induced drop in hemoglobin resulted in a increase in EPO levels, which reached a maximum of 764 ± 55 mU/mL (mean ± %CV) in 0.5–2.6 days. The reticulocyte counts increased from baseline values of 76.9 × 103 ± 67/μL to 619 × 103 ± 30/μL in 8 days. The PK/PD analysis indicated an increased maturation time for the reticulocytes (4.88 ± 35 days) and demonstrated that the Emax model for EPO’s activation of the progenitors did not show significant effect saturation at the endogenous EPO levels reached.
Conclusions
In extrapolating from the animal pilot experiment, the present study provides a case for the use of higher r-HuEPO doses in human studies to determine if higher doses are more effective in treatment of neonatal anemia to reduce, and in some less severe cases, eliminate, the need for blood transfusions.
Keywords: anemia of prematurity, erythropoietin, pharmacokinetic/pharmacodynamic analysis, progenitor activation, reticulocyte
Erythropoietin (EPO) is the principal hormone regulating red blood cell (RBC) production and is primarily produced in the kidney.1 EPO receptors (EPOR) have been detected in the brain, retina, heart, skeletal muscles, kidneys and endothelial cells, 2–4 suggesting other functions of EPO in addition to its erythropoietic properties. Recombinant human EPO (r-HuEPO) is most widely used as an effective treatment modality for anemic patients with insufficient EPO production, for example in end-stage renal disease, and for the treatment of anemia associated with cancer chemotherapy.
Anemia of prematurity, commonly observed during the first few weeks after birth in very low-birthweight infants, is generally treated with RBC transfusions. 5,6 Approximately 70–80% of the transfusions are given during the first 4 weeks7 when the postnatal hemoglobin (Hb) drop begins to occur.8–10. The anemia is exaggerated by iatrogenic phlebotomy losses, decreased RBC survival and reduced EPO levels.10–14 The markedly low EPO concentrations for the degree of anemia seen in premature infants have been assumed to reflect decreased EPO production. 15,16 There is, however, experimental evidence of expanded plasma volume and increased EPO clearance compared to adults, which could be a plausible explanation for the reduced EPO concentrations.17–20 In addition to these decreased levels, bone marrow erythroid hypoplasia21 and also the sensitivity of fetal and neonatal progenitors to EPO in vitro22,23 being comparable to those of adults provide a rationale for the use of r-HuEPO as a potential therapy for anemic neonates. But controlled trials of r-HuEPO have resulted in contradictory views regarding its use. While some studies have shown that it is beneficial in reducing RBC transfusions,11,24–28 others have seen little to no significant difference between control and r-HuEPO treatment groups. 29–33
Recombinant human EPO was primarily developed for treatment of anemia in adults. Accordingly, in its drug development, the recommended dosing was determined only for this patient population. As is often the case, optimal dosing for neonates was not established in the drug development stage. Investigations of the treatment of neonatal anemia with r-HuEPO in clinical trials have largely been based on adult per kg dosing, which proved to be less efficacious in infants. The progressive developmental decrease in the clearance of r-HuEPO from fetus to adult indicates that doses higher than those used for adults are required for the effective treatment of neonatal anemia. Moreover, the dynamic changes in the erythroid receptor pool in response to anemia and prior exposure to EPO emphasize the need for proper optimization of the timing and dosing of r-HuEPO, which can be obtained through controlled pharmacokinetic/pharmacodynamic (PK/PD) experiments.
The main purpose of this study was to make predictions about the efficacy of EPO at higher doses. This is done by investigating the PK/PD relationship between EPO and its pharmacologic effect of activation and differentiation of the erythroid precursors to reticulocytes using the sheep/lamb animal model. The analysis provides insight into the maximum achievable activation by EPO, thereby providing information on the peak EPO concentrations and maximal EPO dosing that can be attained without any significant saturation of the EPOR pool that would result in diminished efficacy. The endogenous EPO levels observed in the preclinical phlebotomy experiments were generally below the saturable limits and comparable to the levels seen in clinical dosing situations, thus lending support to the administration of higher r-HuEPO doses to treat anemia of prematurity.
Methods
Animals
All surgical and experimental procedures received prior approval by the local institutional animal care review committee. Five healthy young adult sheep were selected. The sheep is particularly well suited for the study of erythropoiesis, Hb switching, and EPO production, because the sites of erythropoiesis, and the types of Hb produced at different stages, most closely resemble the situation in humans. 34 The animals were 2–4 months old and weighed 21.3 ± 16 kg (mean ± %CV) at the beginning of the experiments. The animals were housed in an indoor, light- and temperature-controlled environment. All animals were in good health. Jugular venous catheters were placed under anesthesia using pentobarbital. i.v. ampicillin (1 g) was administered daily for the first 3 days following surgery.
Assays
Plasma reticulocyte counts, Hb and EPO concentrations were measured in 1–4 blood samples (0.5 mL per sample) per day drawn throughout the 5–10 week study period. On an average, around 35–40 blood samples were drawn from each animal during the study period. Plasma EPO was measured in triplicate using a double antibody radioimmunoassay (RIA) as previously described35 (lower level of quantification is 1 mU/mL). All samples were measured in the same assay set-up to reduce assay variability. Hb was measured spectrophotometrically using a CO-Oximeter (IL482, Watham, MA, USA) and the number of reticulocytes was determined by flow cytometry (FACScan, Becton-Dickinson, San Jose, CA, USA). 36
Study protocol
An increase in endogenous EPO was induced by controlled phlebotomy performed using the jugular venous catheter. Animals were bled to Hb levels of 3–4 g/dL. To maintain a constant blood volume during the procedure, equal volumes of 0.9% NaCl solution were infused for each volume of blood removed. Each animal underwent two phlebotomies performed 4–6 weeks apart wherein the Hb levels were reduced to 3–4 g/dL after each phlebotomy. No iron supplementation other than in the animal’s feed was given. To minimize RBC loss due to frequent blood sampling, blood was centrifuged, the plasma removed, and the RBC re-infused.
Pharmacokinetic/pharmacodynamic analysis
A mathematical model has been developed based on linear systems analysis (LSA) principles of convolution37,38 to resolve the underlying kinetic relationship between EPO levels, CEPO(t) and the absolute reticulocyte counts, R(t) using a PD Emax link model. The absolute reticulocyte counts are used rather than the relative percentage because they provide a more meaningful and real representation of the changes in the reticulocyte response, especially when the RBC count is dynamically changing during the animal’s recovery from the phlebotomy.
The PD transduction function relating the rate of erythroid progenitor activation, fac, and the EPO concentration can be described by the Emax model and is given by:
| (1) |
The Emax model (Eqn 1) enables the estimation of the maximum progenitor activation rate (Emax) that can be achieved and also C50, which is the EPO concentration that results in half of this maximum activation rate. Emax provides a measure of the size of the receptor pool while C50 gives the receptor binding property of EPO-EPOR. The progenitor activation rate versus time profiles, fac(t), is determined from the PD function (Eqn 1) and the EPO plasma profile, CEPO(t).
Applying LSA principles, 37,38 the reticulocyte counts can be determined from the progenitor activation rate through a convolution operation as follows:
| (2) |
The convolution integral (Eqn 2) is used to represent the response, R(t) to an arbitrary input, fac(t) where the unit impulse response (UIR(t)) is the response normalized to a unit input (impulse) and ‘*’ denotes the convolution operator. In the present analysis the response, R(t), is the reticulocyte counts; fac(t) is the progenitor activation rate, and UIR(t) is the reticulocyte disposition function, or single cell response, which describes the time course for the appearance and disappearance of a single reticulocyte from the circulation. UIR(t) is given by:
| (3) |
where
| (4) |
Here, ‘a’ and ‘b’ are the reticulocyte disposition parameters. The time it takes for a new reticulocyte to enter the bloodstream, relative to the progenitor cell activation, is given by ‘a’, and the difference ‘b-a’ is the time taken for that reticulocyte to mature into an RBC, that is, the maturation time. More appropriately, b-a should be denoted the residence time of the reticulocyte in the circulation. It should be noted that b-a is only an apparent maturation time because ‘a’ denotes the time when the reticulocyte first appears in the circulation and not the time for the progenitor cell to differentiate into a reticulocyte, which occurs in the bone marrow.
Equations 1–3 were fitted to the individual data sets to obtain estimates of C50, Emax, and a and b. The individual data were also fitted using the linear PD transduction function given here.
| (5) |
Estimate of the Emax/C50 ratio (Eqn 5) is informative because it provides a valuable measure of the efficacy of EPO in inducing the proliferation and differentiation of the precursors into reticulocytes. The larger the Emax/C50 ratio, the more efficacious is EPO. Akaike information criteria (AIC) 39 were used in the model selection between the non-linear Emax and the linear PD transduction functions in the individual fits. All data were also simultaneously fitted where the C50 parameter was shared between the fits. In the simultaneous fitting, the Emax (Eqn 1) and the reticulocyte disposition parameters, a and b (Eqs 1,2) were not shared, but separately estimated for the individual animals.
Data analysis
The progenitor activation rate (Eqn 1) and, subsequently, the reticulocyte concentration (Eqns 2,3) for the individual and the simultaneous estimations were determined using WINFUNFIT, a computer program for general non-linear regression, which is a development from the earlier version, FUNFIT.40 Special end-constrained cubic splines 41 fitted to the EPO data were used to represent non-parametrically the EPO profile (CEPO(t) in Eqn 1). The response fittings and convolution codes were written in FORTRAN 90/95 and compiled using the Compaq Visual FORTRAN Professional Edition 6.6.B.
Results
EPO and reticulocyte response to phlebotomy
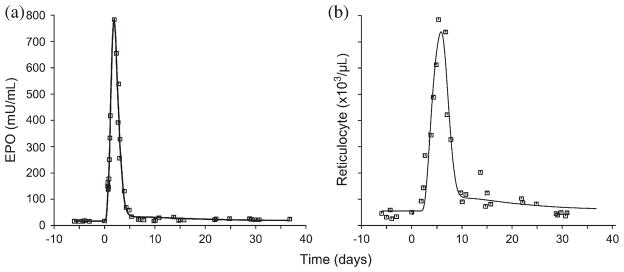
Representative plot showing the non-parametric cubic spline estimation of the EPO data and the fitted reticulocyte response is shown in Figure 1. The pre-phlebotomy, baseline values of EPO concentration and the reticulocyte counts were 20.2 ± 44 mU/mL and 76.9 × 103 ± 67/μL, respectively.
Fig. 1.
Non-parametric(cubic spline) estimation of (a) erythropoietin (EPO) vs time profile and (b) model-fitted reticulocyte response vs time profiles, resulting from phlebotomy-induced anemia.
The phlebotomy-induced anemia (Hb < 4 g/dL) was followed by a rapid increase in plasma EPO concentrations within a few hours, with mean peak concentration of 764 ± 55 mU/mL at 0.5–2.6 days after phlebotomy. The stimulated EPO levels were maintained only over a few days and a rapid drop consistently started as early as 2–4 days following the phlebotomy. The progressive phlebotomy-induced increase in the reticulocyte count was observed 2–5 days following the start of phlebotomy, with maximum mean stimulated counts of 619 × 103 ± 30/μL at 4–8 days. The increased reticulocyte count is sustained for only a few days and returned to baseline values in approximately 8–12 days.
Pharmacokinetic/pharmacodynamic analysis
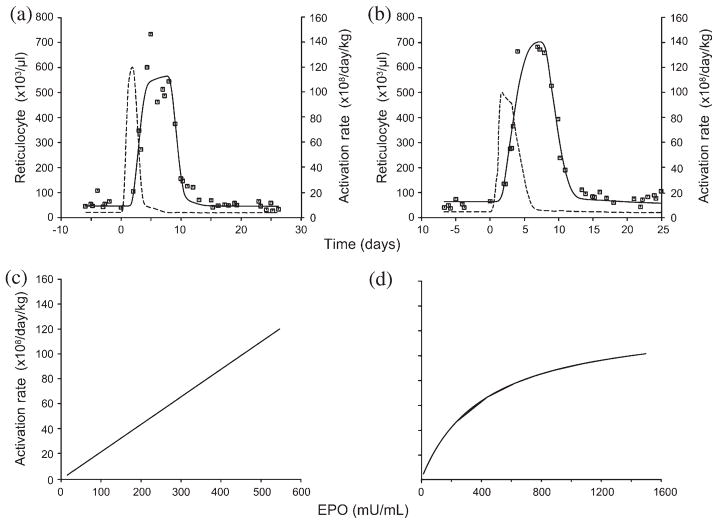
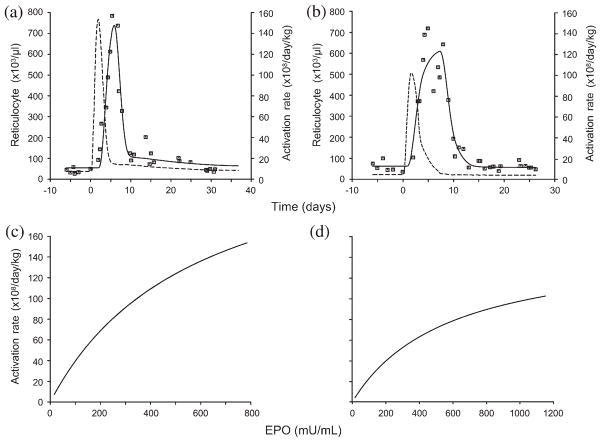
The individual fittings (r = 0.91–0.99) and the simultaneous fittings (r = 0.90–0.98) by the proposed PD stimulatory model are in excellent agreement with the data. Representative plots of the individual fit to the reticulocyte data and the PD function are shown in Figure 2. Illustrative plots for the simultaneous fits to the phlebotomy data where C50 is shared are shown in Figure 3.
Fig. 2.
Representative plots showing (a,b) model-fitted reticulocyte response to the individual data (––, model-estimated reticulocyte responses; - - -, activation rates) and (c,d) their corresponding pharmacodynamic (PD) transduction functions. The PD model was selected based on Akaike information criteria. EPO, erythropoietin.
Fig. 3.
Representative plots of (a,b) simultaneously fitted reticulocyte responses (––, estimated reticulocyte responses; - - -, activation rates) and (c,d) the corresponding PD transduction functions relating the erythropoietin (EPO) levels and the progenitor activation rates.
The summary of the results of the PK/PD model for the individual fits and the simultaneous fits are presented in Tables 1,2, respectively. The mean estimated values for a and b-a from Table 1 (Table 2) are 0.971 ± 62 days (0.889 ± 64 days) and 4.71 ± 39 days (4.88 ± 35 days), respectively. This indicates that the reticulocyte enters the systemic circulation 0.971 days (0.889 days) following progenitor cell activation and it takes 4.71 days (4.88 days) days (i.e. b-a) for the reticulocyte to mature into an RBC. The mean estimated values for Emax and C50 are 267 × 108 ± 107 reticulocytes/day per kg and 526 ± 80 mU/mL for the individual fits (n = 7 for the non-linear model) and 273 × 108 ± 73 reticulocytes/day per kg) and 576 mU/mL for the simultaneous estimations, showing no significant difference in the mean values between individual and simultaneous fittings (P > 0.05). The mean estimated value for Emax/C50 for the linear PD transduction model is 42.4 × 108 ± 64 reticulocytes/day per kg per mU/mL. The large %CV in Emax indicates a high degree of variability in the EPOR mass among animals.
Table 1.
PK/PD parameters from the individual fits
| Phlebotomy | a (days) | b-a (days) | Emax (×108/day/kg) | C50 (mU/mL) | Emax/C50 (×108/day/kg)/(mU/mL) | r‡ |
|---|---|---|---|---|---|---|
| 1† | 0.593 | 6.49 | — | — | 32.0 | 0.925 |
| 2 | 1.75 | 1.41 | 895 | 662 | 135 | 0.946 |
| 3 | 0.564 | 6.31 | 131 | 421 | 31.0 | 0.982 |
| 4 | 1.59 | 3.31 | 124 | 150 | 82.9 | 0.988 |
| 5 | 0.328 | 4.33 | 129 | 287 | 45.0 | 0.954 |
| 6† | 1.00 | 3.11 | — | — | 73.1 | 0.985 |
| 7† | 1.18 | 6.32 | — | — | 22.1 | 0.969 |
| 8 | 1.83 | 3.38 | 238 | 430 | 55.4 | 0.966 |
| 9 | 0.742 | 6.47 | 269 | 1413 | 19.1 | 0.960 |
| 10 | 0.132 | 5.99 | 83.7 | 321 | 26.1 | 0.908 |
| Mean | 0.971 | 4.71 | 267 | 526 | 52.2 | 0.958 |
| % CV | 62 | 39 | 107 | 80 | 70 | 3 |
Linear PD model chosen using Akaike information criteria;
correlation coefficient for the fitted PK/PD model.
PD, pharmacodynamic; PK, pharmacokinetic.
Table 2.
PK/PD parameters from the simultaneous fits
| Phlebotomy | a (days) | b-a (days) | Emax (×108/day/kg) | Emax/C50 (×108/day/kg)/(mU/mL) | r† |
|---|---|---|---|---|---|
| 1 | 0.740 | 5.63 | 255 | 44.3 | 0.913 |
| 2 | 1.69 | 1.53 | 725 | 126 | 0.947 |
| 3 | 0.552 | 6.42 | 149 | 25.9 | 0.983 |
| 4 | 1.10 | 4.70 | 152 | 26.4 | 0.982 |
| 5 | 0.135 | 4.82 | 188 | 32.7 | 0.952 |
| 6 | 1.04 | 2.91 | 531 | 92.3 | 0.984 |
| 7 | 1.21 | 6.03 | 210 | 36.4 | 0.968 |
| 8 | 1.71 | 3.60 | 266 | 46.2 | 0.965 |
| 9 | 0.680 | 6.36 | 154 | 26.8 | 0.955 |
| 10 | 0.040 | 6.81 | 104 | 18.1 | 0.904 |
| Mean | 0.889 | 4.88 | 273 | 47.5 | 0.955 |
| % CV | 64 | 35 | 73 | 73 | 3 |
Correlation coefficient for the fitted PK/PD model; C50 for the simultaneous estimations = 576 mU/mL.
PD, pharmacodynamic; PK, pharmacokinetic.
Discussion
The primary focus of this paper is to investigate the PD of EPO’s stimulation of the erythroid precursors and their differentiation into reticulocytes to predict if higher than normal clinical dosings (50–300 U/kg) of EPO may be efficacious in the treatment of neonatal anemia. The present analysis provides estimates of the efficacious concentration range for EPO that does not result in significant saturation of the EPOR mass in the EPO binding and activation of the EPOR on progenitor cells. This information is useful in avoiding any drug wastage by optimizing r-HuEPO doses so as to not create saturation and resulting diminished efficacy. In the present experiments phlebotomy-induced anemia stimulates the production of endogenous EPO as a feedback mechanism in response to the body’s need for more RBC and better tissue oxygen delivery. The natural response to endogenous EPO as demonstrated in these experiments provides insight into the physiology of erythropoiesis and EPO’s complex PK/PD behavior.
The model using phlebotomy-induced anemia was preferred over the traditional empirical dose–response studies because it provides a more detailed and mechanistic analysis. There is a complex interplay between the endogenous and exogenous EPO and it is therefore essential to have a comprehensive knowledge of the PK/PD of endogenous EPO in order to optimize r-HuEPO doses. Such understanding is best gained from ‘natural experiments’, absent of exogenous drug, where the biological system is observed under dynamic disease state conditions, as in the present case.
Although developmental differences exist between neonatal and adult sheep with respect to the production site of EPO, the present study revolved primarily around the PD effect of stimulating the progenitors rather than its production. It has been shown in earlier studies that there is increased EPO clearance in neonates as compared to adults, 17–20 and there is also experimental evidence that the bone marrow contributes significantly to the elimination of EPO in vivo via the EPOR. 42 These together suggest that neonatal sheep might have a larger EPOR pool compared to adults and may therefore be capable of activating more progenitors than adult sheep. Thus, although analysis of the PK/PD was done using adult sheep, other evidence suggests that crude extrapolations to neonates are reasonable and merit testing. But, as with any extrapolation, caution needs to be exercised because the basic premise might be false due to the lack of a direct measure of the EPOR pool in vivo and due to species differences between sheep and human. The model of phlebotomy-induced anemia in which there is a rapid onset of anemia might also not translate directly to the anemia of prematurity, which develops slowly, because the physiology and morphology, the EPOR pool, iron availability and utilization might be different. But it should be recognized that exogenous, s.c. r-HuEPO doses will create EPO plasma profiles similar to that created by the present phlebotomy experiments. Thus, the present PK/PD conditions are similar to what may be experienced in therapeutic EPO dosing situations.
Pharmacokinetic/pharmacodynamic analysis
The PD investigation makes use of the Emax stimulatory model, which is a commonly applied transduction function in PK/PD modeling approaches. The Emax transduction function for EPO’s stimulatory effect is consistent with a limited capacity for EPO-EPOR binding at higher EPO concentrations. The proposed PK model makes use of LSA principles of convolution and deconvolution, which have found wide application in PK/PD analysis in determining the response to a known drug input or in evaluating drug input rates when their response is measured. 37,38 The LSA approach is an objective, analytic approach that makes minimal assumptions about the system under study and is used in the present study to determine the reticulocyte response due to EPO progenitor activation using a sheep model under a phlebotomy-induced anemic state.
Reticulocyte disposition parameters
The reticulocyte count is a surrogate marker of the erythropoietic activity, which has been used in providing information regarding the patient response to r-HuEPO therapy,43 bone marrow suppression following chemotherapy,44 bone marrow engraftment following marrow transplantation, 45,46 and the diagnosis and treatment of anemia. 47 Reticulocyte changes are observed initially in the erythropoietic process and are therefore the early indicators of erythropoiesis and the bone marrow activity. The present study determines the reticulocyte response to anemia based on the LSA approach of convolution (Eqn 2).
The estimated mean values for a and b-a from the individual fits are 0.971±62 days and 4.71±39 days, respectively. The mean reticulocyte disposition parameters (a and b-a) estimated from the simultaneous fits to the data from all the phlebotomy studies are 0.889±64 days and 4.88±35 days, respectively. These values are comparable to the estimates (0.470 days and 4.98 days) obtained from a previous parametric analysis in sheep. 48 The average reticulocyte maturation time (b-a) under the stimulated state is larger than the maturation time under non-stimulated normal conditions, which is believed to be 1–2 days.49–51 The increased number of immature reticulocytes (‘shift’ reticulocytes) in the blood 50,52 due to the severe, anemia-induced stress erythropoiesis may be responsible for these longer maturations times. It has been suggested that EPO accelerates the release of immature reticulocytes from the bone marrow.53 Some studies have shown that the shift reticulocytes take 1–3 days longer than the normal reticulocytes to lose their reticulum. 50,54 The present results are consistent with the longer maturation times observed under anemic conditions.
Emax and C50
The Emax model enables the estimation of the maximum attainable precursor activation rate, Emax, and also C50, which is the EPO concentration that results in half of this maximal activation rate. In some of the individual fitting scenarios, the EPO levels were much lower than C50, and the PD function parameters, Emax and C50, could not be separately estimated. In those cases the linear PD model was chosen based on the AIC39 PD model selection criterion. In three out of the 10 phlebotomy experiments, the linear PD model was preferred based on the AIC model selection. The mean peak EPO concentrations were 393 ± 44 mU/mL, for which the linear PD was the preferred model, compared to higher EPO levels of 923 ± 43 mU/mL in the non-linear PD model.
The mean estimated value for the Emax/C50 ratio was 52.2×108 ±70 reticulocytes/kg per day per mU/mL for the individual fits (47.5×108 ±73 reticulocytes/kg per day per mU/mL for the simultaneous estimations). This parameter determines the daily rate of reticulocyte production per kilogram bodyweight of the subject relative to the EPO concentration. In the linear range, there is a proportionate increase in the reticulocyte production rate with the EPO concentrations. At higher EPO levels, in the non-linear range, there is a diminishing return in the reticulocyte production rate with increasing EPO concentrations. Identifying the concentration range resulting in the non-linearity (degree of saturation) is thus important to consider in optimizing the EPO dosing.
The maximum reticulocyte production rate capacity of EPO may be quantified by the Emax parameter, with an average value of 267 × 108 ± 107 reticulocytes/kg per day for the simultaneous fits (273 × 108 ± 73 reticulocytes/kg per day for the individual estimations). The high %CV of 107 for the individual prediction (n = 7) indicates a large variability in the size of the EPOR pool between animals. The estimated C50 was 576 mU/mL for the simultaneous fits, which is not statistically different (P > 0.05) from the mean value of 526 ± 80 mU/mL obtained from the individual predictions using the Emax stimulatory model. C50 provides a measure of the EPO-EPOR binding property and was accordingly shared in the simultaneous estimations due to the underlying assumption that the receptor binding affinity is a constant within the same species.
Treating neonatal anemia with r-HuEPO
Numerous studies have investigated the efficacy of r-HuEPO in treating anemia of prematurity since the early controlled trials, which used doses effective in treating adult anemia. 30,31 These preliminary trials used low doses and were not efficacious in reducing transfusion need in preterm infants. In the wake of experimental evidence documenting expanded plasma volume and higher EPO clearance in neonates compared to adults, doses higher than those proven effective in treating adult anemia have been administered. Subsequent trials using higher r-HuEPO doses were able to stimulate significant erythropoiesis and also demonstrate modest yet significant reductions in RBC transfusions. 11,24–28,55,56 Despite these encouraging results, there are still conflicting reports on the utility of r-HuEPO in that the efficacy in increasing reticulocyte counts and stimulating bone marrow activity could not be translated to reduced transfusion requirements. 29,32,33 These differences are likely due to the variable transfusions practices, diversity in patient demographics and inconsistencies in the treatment protocol.
There is a need to better understand the regulatory mechanism of EPO activation and EPO’s complex PK/PD for more efficacious treatment. Dose of r-HuEPO used in the treatment of adult anemic patients are generally between 50 and 500 U/kg administered either i.v. or s.c. The PK studies performed on healthy subjects have used varying s.c. doses from 50 to 300 U/kg, resulting in peak EPO concentrations of approximately 36 ± 26 mU/mL (mean ± SD) to 288 ± 77 mU/mL, respectively,57,58 which are consistently lower than the concentrations (764 ± 55 mU/mL) encountered in the present phlebotomy experiments. The i.v. doses from 50 to 300 U/kg given to healthy volunteers produced maximum concentrations of approximately 950–7332 ± 1314 mU/mL, respectively,57,58 which are above the peak concentrations we encountered in the present phlebotomy experiments. Similar peak levels were observed from PK studies conducted in anemic patients. 59–61 Thus these levels are, according to our predictions (Table 1), resulting in a significant degree of PD saturation, and may explain why i.v. administration is less efficacious than s.c. administration.
Most importantly, mean peak EPO levels after i.v. and s.c. administration of 200 U/kg to preterm infants were 711±166.4 mU/mL and 137.2±68.6 mU/mL, respectively.19 Both of these levels are either comparable or lower than the present peak levels (764±55 mU/mL). High s.c. dose of 300 U/kg in neonates produced median maximum concentrations of 363 mU/mL (range, 173–500 mU/mL); and even higher dose of 500 U/kg resulted in peak concentrations in the range 155–475 mU/mL.19,62 Again all these maximum concentrations in neonates are comparable to the peak levels in the present phlebotomies, for which the PD parameters (i.e. C50 Tables 1,2) did not indicate any real significant efficacy saturation, thus providing a case for use of higher dosing in treatment of neonatal anemia.
The present PD model did not indicate significant saturation in the erythropoietic effect of EPO at levels observed in the s.c. PK studies in neonates that used therapeutic doses 19 and larger than normal therapeutic doses. 19,62 The endogenous EPO concentrations resulting from the present phlebotomy experiments were to a large extent within the linear range of the PD function. Although the Emax model was selected in 70% of the cases based on the AIC, the PD relationship was only slightly non-linear in the majority of cases. This is an important and encouraging observation from a therapeutic stand point because this provides a rationale for testing even higher doses of r-HuEPO for treating anemia of prematurity. Also, the more rapid elimination of EPO in neonates offers sound rationale for administering these increased doses. These predictions and extrapolations from our preclinical studies, however, need to be confirmed in future studies.
Acknowledgments
The recombinant human EPO used in the EPO RIA was a gift from Dr H. Kinoshita of Chugai Pharmaceutical Company (Tokyo, Japan). The rabbit EPO antiserum used in the EPO RIA was a generous gift from Gisela K. Clemens, PhD (University of California, Lawrence Berkeley National Laboratories, Berkeley, CA, USA). The authors gratefully acknowledge the technical help from Lance S. Lowe. The authors thank Dr. Robert T Cook and the personnel (Barbara Stewart, Cathy Byers, Kathy McLatchie, Beth Greif and Lisa Alberty) of the Iowa City VAMC Pathology and Laboratory Medicine Service for their assistance in performing the flow cytometric measurements of reticulocytes. This work is supported by the United States Public Health Service National Institute of health Grants PO1 HL46925 and R21 GM57367, and by the Veterans Administration Medical Center, Iowa City, Iowa.
References
- 1.Fisher JW. Erythropoietin: Physiology and pharmacology update. Exp Biol Med. 2003;228:1–14. doi: 10.1177/153537020322800101. [DOI] [PubMed] [Google Scholar]
- 2.Digicaylioglu M, Bichet S, Marti HH, et al. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc Natl Acad Sci USA. 1995;92:3717–20. doi: 10.1073/pnas.92.9.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juul SE, Yachnis AT, Christensen RD. Tissue distribution of erythropoietin and erythropoietin receptor in the developing human fetus. Early Hum Dev. 1998;52:235–49. doi: 10.1016/s0378-3782(98)00030-9. [DOI] [PubMed] [Google Scholar]
- 4.Rossert J, Eckardt KU. Erythropoietin receptors: Their role beyond erythropoiesis. Nephrol Dial Transplant. 2005;20:1025–8. doi: 10.1093/ndt/gfh800. [DOI] [PubMed] [Google Scholar]
- 5.Maier RF, Obladen M, Messinger D, Wardrop CA. Factors related to transfusion in very low birthweight infants treated with erythropoietin. Arch Dis Child Fetal Neonatal Ed. 1996;74:F182–6. doi: 10.1136/fn.74.3.f182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown MS, Berman ER, Luckey D. Prediction of the need for transfusion during anemia of prematurity. J Pediatr. 1990;116:773–8. doi: 10.1016/s0022-3476(05)82670-8. [DOI] [PubMed] [Google Scholar]
- 7.Widness JA, Seward VJ, Kromer IJ, Burmeister LF, Bell EF, Strauss RG. Changing patterns of red blood cell transfusion in very low birth weight infants. J Pediatr. 1996;129:680–87. doi: 10.1016/s0022-3476(96)70150-6. [DOI] [PubMed] [Google Scholar]
- 8.Shannon K. Recombinant erythropoietin in anemia of prematurity: Five years later. [comment] Pediatrics. 1993;92:614–17. [PubMed] [Google Scholar]
- 9.Strauss RG. Erythropoietin and neonatal anemia. N Engl J Med. 1994;330:1227–8. doi: 10.1056/NEJM199404283301709. [DOI] [PubMed] [Google Scholar]
- 10.Shannon KM. Anemia of prematurity: Progress and prospects. Am J Pediatr Hematol Oncol. 1990;12:14–20. doi: 10.1097/00043426-199021000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Carnielli V, Montini G, Da Riol R, Dall’Amico R, Cantarutti F. Effect of high doses of human recombinant erythropoietin on the need for blood transfusions in preterm infants. J Pediatr. 1992;121:98–102. doi: 10.1016/s0022-3476(05)82552-1. [DOI] [PubMed] [Google Scholar]
- 12.Obladen M, Sachsenweger M, Stahnke M. Blood sampling in very low birth weight infants receiving different levels of intensive care. Eur J Pediatr. 1988;147:399–404. doi: 10.1007/BF00496419. [DOI] [PubMed] [Google Scholar]
- 13.Brown MS, Garcia JF, Phibbs RH, Dallman PR. Decreased response of plasma immunoreactive erythropoietin to ‘available oxygen’ in anemia of prematurity. J Pediatr. 1984;105:793–8. doi: 10.1016/s0022-3476(84)80309-1. [DOI] [PubMed] [Google Scholar]
- 14.Stockman JA, III, Graeber JE, Clark DA, McClellan K, Garcia JF, Kavey RE. Anemia of prematurity: Determinants of the erythropoietin response. J Pediatr. 1984;105:786–92. doi: 10.1016/s0022-3476(84)80308-x. [DOI] [PubMed] [Google Scholar]
- 15.Christensen RD. Recombinant erythropoietic growth factors as an alternative to erythrocyte transfusion for patients with ‘anemia of prematurity’. Pediatrics. 1989;83:793–6. [PubMed] [Google Scholar]
- 16.Dallman PR. Erythropoietin and the anemia of prematurity. J Pediatr. 1984;105:756–7. doi: 10.1016/s0022-3476(84)80296-6. [DOI] [PubMed] [Google Scholar]
- 17.Widness JA, Veng-Pedersen P, Peters C, Pereira LM, Schmidt RL, Lowe LS. Erythropoietin pharmacokinetics in premature infants: Developmental, nonlinearity, and treatment effects. J Appl Physiol. 1996;80:140–48. doi: 10.1152/jappl.1996.80.1.140. [DOI] [PubMed] [Google Scholar]
- 18.Widness JA, Veng-Pedersen P, Modi NB, Schmidt RL, Chestnut DH. Developmental differences in erythropoietin pharmacokinetics: Increased clearance and distribution in fetal and neonatal sheep. J Pharmacol Exp Ther. 1992;261:977–84. [PubMed] [Google Scholar]
- 19.Brown MS, Jones MA, Ohls RK, Christensen RD. Single-dose pharmacokinetics of recombinant human erythropoietin in pre-term infants after intravenous and subcutaneous administration. J Pediatr. 1993;122:655–7. doi: 10.1016/s0022-3476(05)83559-0. [DOI] [PubMed] [Google Scholar]
- 20.Kling PJ, Widness JA, Guillery EN, Veng-Pedersen P, Peters C, DeAlarcon PA. Pharmacokinetics and pharmacodynamics of erythropoietin during therapy in an infant with renal failure. J Pediatr. 1992;121(5 Pt 1):822–5. doi: 10.1016/s0022-3476(05)81923-7. [DOI] [PubMed] [Google Scholar]
- 21.Gairdner D, Marks J, Roscoe JD. Blood formation in infancy. IV The early anemia of prematurity. Arch Dis Child. 1955;30:203–11. doi: 10.1136/adc.30.151.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shannon KM, Naylor GS, Torkildson JC, et al. Circulating erythroid progenitors in the anemia of prematurity. N Engl J Med. 1987;317:728–33. doi: 10.1056/NEJM198709173171203. [DOI] [PubMed] [Google Scholar]
- 23.Rhondeau SM, Christensen RD, Ross MP, Rothstein G, Simmons MA. Responsiveness to recombinant human erythropoietin of marrow erythroid progenitors from infants with the ‘anemia of prematurity’. J Pediatr. 1988;112:935–40. doi: 10.1016/s0022-3476(88)80223-3. [DOI] [PubMed] [Google Scholar]
- 24.Arif B, Ferhan K. Recombinant human erythropoietin therapy in low-birthweight preterm infants: A prospective controlled study. Pediatr Int. 2005;47:67–71. doi: 10.1111/j.1442-200x.2005.02007.x. [DOI] [PubMed] [Google Scholar]
- 25.Maier RF, Obladen M, Scigalla P, et al. The effect of epoetin beta (recombinant human erythropoietin) on the need for transfusion in very-low-birth-weight infants. European Multicentre Erythropoietin Study Group. N Engl J Med. 1994;330:1173–8. doi: 10.1056/NEJM199404283301701. [DOI] [PubMed] [Google Scholar]
- 26.Shannon KM, Keith JF, III, Mentzer WC, et al. Recombinant human erythropoietin stimulates erythropoiesis and reduces erythrocyte transfusions in very low birth weight preterm infants. Pediatrics. 1995;95:1–8. [PubMed] [Google Scholar]
- 27.Ohls RK, Osborne KA, Christensen RD. Efficacy and cost analysis of treating very low birth weight infants with erythropoietin during their first two weeks of life: A randomized, placebo-controlled trial. J Pediatr. 1995;126:421–6. doi: 10.1016/s0022-3476(95)70462-0. [DOI] [PubMed] [Google Scholar]
- 28.Meyer MP, Meyer JH, Commerford A, et al. Recombinant human erythropoietin in the treatment of the anemia of prematurity: Results of a double-blind, placebo-controlled study. Pediatrics. 1994;93(6 Pt 1):918–23. [PubMed] [Google Scholar]
- 29.Ohls RK, Ehrenkranz RA, Wright LL, et al. Effects of early erythropoietin therapy on the transfusion requirements of preterm infants below 1250 grams birth weight: A multicenter, randomized, controlled trial. Pediatrics. 2001;108:934–42. doi: 10.1542/peds.108.4.934. [DOI] [PubMed] [Google Scholar]
- 30.Shannon KM, Mentzer WC, Abels RI, et al. Recombinant human erythropoietin in the anemia of prematurity: Results of a placebo-controlled pilot study. J Pediatr. 1991;118:949–55. doi: 10.1016/s0022-3476(05)82217-6. [DOI] [PubMed] [Google Scholar]
- 31.Obladen M, Maier R, Segerer H, et al. Efficacy and safety of recombinant human erythropoietin to prevent the anaemias of prematurity. European Randomized Multicenter Trial. Contrib. Nephrol. 1991;88:314–26. doi: 10.1159/000419541. [DOI] [PubMed] [Google Scholar]
- 32.Halperin DS, Wacker P, Lacourt G, et al. Effects of recombinant human erythropoietin in infants with the anemia of prematurity: A pilot study. J Pediatr. 1990;116:779–86. doi: 10.1016/s0022-3476(05)82671-x. [DOI] [PubMed] [Google Scholar]
- 33.Avent M, Cory BJ, Galpin J, et al. A comparison of high versus low dose recombinant human erythropoietin versus blood transfusion in the management of anaemia of prematurity in a developing country. J Trop Pediatr. 2002;48:227–33. doi: 10.1093/tropej/48.4.227. [DOI] [PubMed] [Google Scholar]
- 34.Wintour EM, Butkus A, Clemons G, Moritz K. Erythropoiesis and hemoglobin switching in the fetus and neonate. Proc Aust Physiol Pharmacol Soc. 1991;22:44–52. [Google Scholar]
- 35.Widness JA, Schmidt RL, Veng-Pedersen P, Modi NB, Sawyer ST. A sensitive and specific erythropoietin immunoprecipitation assay: Application to pharmacokinetic studies. J Lab Clin Med. 1992;119:285–94. [PubMed] [Google Scholar]
- 36.Peters C, Georgieff MK, de Alarcon PA, et al. Effect of chronic erythropoietin administration on plasma iron in newborn lambs. Biol Neonate. 1996;70:218–28. doi: 10.1159/000244368. [DOI] [PubMed] [Google Scholar]
- 37.Cutler DJ. Linear systems analysis in pharmacokinetics. J Pharmacokinet Biopharm. 1978;6:265–82. doi: 10.1007/BF01312266. [DOI] [PubMed] [Google Scholar]
- 38.Veng-Pedersen P. Noncompartmentally-based pharmacokinetic modeling. Adv Drug Deliv Rev. 2001;48:265–300. doi: 10.1016/s0169-409x(01)00119-3. [DOI] [PubMed] [Google Scholar]
- 39.Akaike H. Automatic control: A new look at the statistical model identification. IEEE Trans Contr Theory. 1974;19:716–23. [Google Scholar]
- 40.Veng-Pedersen P. Curve fitting and modelling in pharmacokinetics and some practical experiences with NONLIN and a new program FUNFIT. J Pharmacokinet Biopharm. 1977;5:513–31. doi: 10.1007/BF01061732. [DOI] [PubMed] [Google Scholar]
- 41.Neelakantan S, Veng-Pedersen P. Determination of drug absorption rate in time-variant disposition by direct deconvolution using beta clearance correction and end-constrained non-parametric regression. Biopharm Drug Dispos. 2005;26:353–70. doi: 10.1002/bdd.468. [DOI] [PubMed] [Google Scholar]
- 42.Chapel S, Veng-Pedersen P, Hohl RJ, Schmidt RL, McGuire EM, Widness JA. Changes in erythropoietin pharmacokinetics following Busulfan-induced bone marrow ablation in sheep: Evidence for bone marrow as a major erythropoietin elimination pathway. J Pharmacol Exp Ther. 2001;298:820–24. [PubMed] [Google Scholar]
- 43.Riley RS, Ben-Ezra JM, Tidwell A, Romagnoli G. Reticulocyte analysis by flow cytometry and other techniques. Hematol Oncol Clin North Am. 2002;16:373–420. doi: 10.1016/s0889-8588(02)00005-9. [DOI] [PubMed] [Google Scholar]
- 44.Aulesa C, Ortega JJ, Ju JM, et al. Flow cytometric reticulocyte quantification in the evaluation of hematologic recovery. Spanish Multicentric Study Group for Hematopoietic Recovery. Eur J Haematol. 1994;53:293–7. [PubMed] [Google Scholar]
- 45.Batjer J, Riddell K, Fritsma G. Predicting bone marrow transplant engraftment by automated flow cytometric reticulocyte analysis. Lab Med. 1994;25:22–6. [Google Scholar]
- 46.Noronha JF, De Souza CA, Vigorito AC, et al. Immature reticulocytes as an early predictor of engraftment in autologous and allogeneic bone marrow transplantation. Clin Lab Haematol. 2003;25:47–54. doi: 10.1046/j.1365-2257.2003.00486.x. [DOI] [PubMed] [Google Scholar]
- 47.Pradella M, Cavill I, d’Onofrio G. Assessing erythropoiesis and the effect of erythropoietin therapy in renal disease by reticulocyte counting. Clin Lab Haematol. 1996;18(Suppl 1):35–7. [PubMed] [Google Scholar]
- 48.Chapel SH, Veng-Pedersen P, Schmidt RL, Widness JA. A pharmacodynamic analysis of erythropoietin-stimulated reticulocyte response in phlebotomized sheep. J Pharmacol Exp Ther. 2000;295:346–51. [PubMed] [Google Scholar]
- 49.Finch CA, Harker LA, Cook JD. Kinetics of the formed elements of human blood. Blood. 1977;50:699–707. [PubMed] [Google Scholar]
- 50.Hillman RS, Finch CA. Erythropoiesis: Normal and abnormal. Semin Hematol. 1967;4:327–36. [PubMed] [Google Scholar]
- 51.Brugnara C. Reticulocyte cellular indices: A new approach in the diagnosis of anemias and monitoring of erythropoietic function. Crit Rev Clin Lab Sci. 2000;37:93–130. doi: 10.1080/10408360091174196. [DOI] [PubMed] [Google Scholar]
- 52.Seip M. Reticulocyte studies. Acta Med Scand. 1953;146(Suppl 282):1–164. [Google Scholar]
- 53.Jelkmann W. Erythropoietin: Structure, control of production, and function. Physiol Rev. 1992;72:449–89. doi: 10.1152/physrev.1992.72.2.449. [DOI] [PubMed] [Google Scholar]
- 54.Heath CW, Daland GA. The life of reticulocytes. Experiments on their maturation. Arch Intern Med. 1930;46:533–51. [Google Scholar]
- 55.Haiden N, Cardona F, Schwindt J, et al. Changes in thrombopoiesis and platelet reactivity in extremely low birth weight infants undergoing erythropoietin therapy for treatment of anemia of prematurity. Thromb Haemost. 2005;93:118–23. doi: 10.1160/TH04-02-0093. [DOI] [PubMed] [Google Scholar]
- 56.Reiter PD, Rosenberg AA, Valuck R, Novak K. Effect of short-term erythropoietin therapy in anemic premature infants. J Perinatol. 2005;25:125–9. doi: 10.1038/sj.jp.7211220. [DOI] [PubMed] [Google Scholar]
- 57.Salmonson T, Danielson BG, Wikstrom B. The pharmacokinetics of recombinant human erythropoietin after intravenous and subcutaneous administration to healthy subjects. Br J Clin Pharmacol. 1990;29:709–13. doi: 10.1111/j.1365-2125.1990.tb03692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McMahon FG, Vargas R, Ryan M, et al. Pharmacokinetics and effects of recombinant human erythropoietin after intravenous and subcutaneous injections in healthy volunteers. Blood. 1990;76:1718–22. [PubMed] [Google Scholar]
- 59.Kampf D, Kahl A, Passlick J, et al. Single-dose kinetics of recombinant human erythropoietin after intravenous, subcutaneous and intraperitoneal administration. Preliminary results. Contrib Nephrol. 1989;76:106–10. doi: 10.1159/000417886. discussion 10–1. [DOI] [PubMed] [Google Scholar]
- 60.Nielsen OJ. Pharmacokinetics of recombinant human erythropoietin in chronic haemodialysis patients. Pharmacol Toxicol. 1990;66:83–6. doi: 10.1111/j.1600-0773.1990.tb00710.x. [DOI] [PubMed] [Google Scholar]
- 61.Hughes RT, Cotes PM, Oliver DO, et al. Correction of the anaemia of chronic renal failure with erythropoietin: Pharmacokinetic studies in patients on haemodialysis and CAPD. Contrib Nephrol. 1989;76:122–30. doi: 10.1159/000417888. [DOI] [PubMed] [Google Scholar]
- 62.Krishnan R, Shankaran S, Krishnan M, Kauffman RE, Kumar P, Lucena J. Pharmacokinetics of erythropoietin following single-dose subcutaneous administration in preterm infants. Biol Neonate. 1996;70:135–40. doi: 10.1159/000244358. [DOI] [PubMed] [Google Scholar]