Abstract
Age determination of unknown human bodies is important in the setting of a crime investigation or a mass disaster because the age at death, birth date, and year of death as well as gender can guide investigators to the correct identity among a large number of possible matches. Traditional morphological methods used by anthropologists to determine age are often imprecise, whereas chemical analysis of tooth dentin, such as aspartic acid racemization, has shown reproducible and more precise results. In this study, we analyzed teeth from Swedish individuals using both aspartic acid racemization and radiocarbon methodologies. The rationale behind using radiocarbon analysis is that aboveground testing of nuclear weapons during the cold war (1955–1963) caused an extreme increase in global levels of carbon-14 (14C), which has been carefully recorded over time. Forty-four teeth from 41 individuals were analyzed using aspartic acid racemization analysis of tooth crown dentin or radiocarbon analysis of enamel, and 10 of these were split and subjected to both radiocarbon and racemization analysis. Combined analysis showed that the two methods correlated well (R2 = 0.66, p < 0.05). Radiocarbon analysis showed an excellent precision with an overall absolute error of 1.0 ± 0.6 years. Aspartic acid racemization also showed a good precision with an overall absolute error of 5.4 ± 4.2 years. Whereas radiocarbon analysis gives an estimated year of birth, racemization analysis indicates the chronological age of the individual at the time of death. We show how these methods in combination can also assist in the estimation of date of death of an unidentified victim. This strategy can be of significant assistance in forensic casework involving dead victim identification.
The identification of human bodies, where there are no clues as to the identity from circumstantial data, poses a difficult problem to the investigator. The determination of age and sex of the body can be crucial to the investigator to limit the search for individuals that could possibly match missing person lists and therefore minimize efforts involving very unlikely alternatives. Whereas gender today can be determined with DNA methods, age determination is not as straightforward. Age estimation in children and adolescents often depends on morphological methods, such as radiological examination of skeletal and dental development. In adults, however, age estimation based on these methods is much less accurate. Current methods of age estimation include simple, yet less precise morphological methods (such as evaluation of dental or skeletal morphology) or more complex, yet more accurate laboratory methods (such as racemization of aspartic acid in dentin or tooth enamel or radiocarbon dating of tooth enamel). Both of the latter methods result in high precision age estimation. Analysis based on morphological methods can yield age estimation error margins of greater than ±10 years (for reviews, see Refs. 1 and 2), whereas precise methods such as aspartic acid racemization and radiocarbon analysis report accuracies of ±3 and ±1–2 years, respectively (3–7). As such, aspartic acid racemization and radiocarbon dating currently represent the methods of choice for precise age estimation.
Assessment of age using aspartic acid racemization methodology was first described in 1975 by Helfman and Bada (5) and has since been widely used in forensic age estimation. During the course of aging, l-forms of amino acids are transformed by racemization to the d-forms. At a temperature of +25 °C, it would take ∼100,000 years for all l-forms of amino acids present in living tissues to undergo complete racemization to the d-amino acid form (5). Thus, the extent of racemization of amino acids may be used to estimate the age of various tissues. Of all stable amino acids, aspartic acid has one of the fastest racemization rates and is therefore the amino acid most commonly used for age estimation. Rates of change of l-form amino acids to d-forms are influenced by various factors, such as temperature, humidity, pH, etc. Because of the continuous formation and removal or degradation of amino acids, tissues with low metabolic rates provide better age estimates than those with high metabolic rates. With this in mind, teeth are the tissue of choice for age estimation analysis. If the post-mortem interval is long, bones and teeth may be all the forensic expert can work with.
Aspartic acid racemization analysis for the purpose of age estimation has been performed on both tooth enamel and crown dentin with analysis of crown dentin shown to yield more accurate age estimation than dental enamel (5, 6). These results have been repeated by several investigators, and typically a high correlation between aspartic acid racemization ratio and age has been reported (for a review, see Ref. 7). Despite these overall positive results, not all groups report the same level of accuracy, and inconsistencies do exist (for a review, see Ref. 2).
In comparison, radiocarbon analysis of tooth enamel is a relatively new technique (first described in 2005 (4)) and yet to be significantly tested by the forensic community. Unlike amino acid racemization analysis, radiocarbon dating of enamel provides information about the date of birth of an individual. When the deceased date of death is also known, chronological age can be determined. Radiocarbon dating of dental enamel has recently been used with very high precision to determine the date of birth of identified and unidentified individuals (3, 4). The rationale of the method is to determine the year of tooth formation based on levels of radiocarbon present in tooth enamel. Radiocarbon, or carbon-14 (14C), is produced naturally in the atmosphere by cosmic ray interactions with nitrogen-14. Single carbon atoms in the atmosphere are chemically active and are quickly oxidized to form CO2. CO2 is then incorporated into plants via the process of photosynthesis. Until recently, 14C levels in the atmosphere had been relatively stable for several thousands of years (with respect to all carbon). However, atmospheric (aboveground) detonations of nuclear weapons during the period of the cold war (1955–1963) doubled the concentration of 14C/12C in the atmosphere (8, 9). Although nuclear weapon testing was conducted at only a few locations, excess levels of 14C in the atmosphere rapidly dispersed and equalized around the globe. Since 1963, as a result of a worldwide test ban treaty, 14C levels in the atmosphere have been decreasing exponentially with a mean half-life of 16 years. 14C levels have not decreased because of radioactive decay (14C has a half-life of 5,730 years), but rather 14C has moved out of the atmosphere due to mixing with large marine and terrestrial carbon reservoirs. Because radiocarbon is incorporated into all living things, this pulse (known as the “bomb curve”) forms an isotopic chronometer of the past 60 years.
Atmospheric radiocarbon concentrations are reflected in the isotopic carbon content of new plant growth. New leaves are produced in weeks, whereas larger fruit and vegetables form over a period of a few months. Herbivores lag the atmosphere slightly because their primary carbon source is removed from the atmosphere in weeks to months. Omnivores and carnivores lag the atmosphere further because their carbon sources are another step removed. The date of formation of a tissue can be estimated from the bomb curve by considering these lags in incorporation and relating the 14C concentration with the date. Enamel formation can occur over several years in humans (10, 11). For radiocarbon analysis of tooth age, the upper limit of enamel formation has been used (i.e. the time approaching enamel laydown completion); this balances out lag periods of 14C incorporation from the atmosphere to the body (12).
Teeth from Swedish individuals with a known date of birth and chronological age were analyzed using both aspartic acid racemization of dentin and radiocarbon analysis of enamel. Radiocarbon analysis of enamel yielded precise date of birth information, and because the date of extraction of all teeth was recorded, the chronological age could also be estimated. A comparison showed that chronological age was more precisely estimated using radiocarbon dating of dental enamel than with analysis of aspartic acid racemization of dentin.
In an ongoing Swedish homicide case where the victim's identity remains unknown, we illustrate how both methodologies can be combined to provide important information as to the year of birth of the deceased, the estimated age of the person at the time of death, and hence the estimated date of death. The precision of radiocarbon dating of tooth enamel to determine the year of birth of an individual is a particularly valuable forensic tool for police authorities to use to define the group of possible matches in the early phase of the investigation. When the date of death cannot be well established, radiocarbon analysis can be used in conjunction with aspartic acid racemization analysis of tooth dentin to provide year of death and chronological age of the victim.
EXPERIMENTAL PROCEDURES
Forty-four teeth were collected from dental clinics in Sweden with the informed consent of all patients. Tooth number, date of extraction, date of birth (year and month), and gender were recorded. This information and analytical data for each tooth used in the study are shown in supplemental Table 1. Four teeth belonging to an unsolved homicide case were received by the Swedish police authorities; in this case, date of birth and date of death were not known. In cases where aspartic acid racemization analysis of dentin and radiocarbon analysis of enamel were performed on the same tooth, half of the whole tooth and the complete root (where possible) were used for aspartic acid racemization, and the remaining half of the crown was retained for radiocarbon analysis.
Procedures for Racemization Analysis
The aspartic acid racemization analysis was performed according to a previously described protocol (7). Briefly, about 1-mm-thick median longitudinal sections were made by cutting the teeth with a low speed cutter (Isomet, 11-1180, Buehler, Chicago, IL). Other areas except dentin were carefully removed from the sections with the cutter, and the dentin was rinsed with ultrasonic waves sequentially in 0.2 m HCl, distilled water (three times), ethanol, and ethyl ether for 5 min, respectively. Then the dentin sections were pulverized in an agate mortar, and 10 mg of the powder was used as the specimen for determination of the racemization ratio. d-Asp and l-Asp were measured by gas chromatography using a glass capillary (GC-17A, Shimadzu, Kyoto, Japan) after hydrolysis and derivatization. The column of the glass capillary was 30 m in length, 0.3 mm in internal diameter, and coated with Chirasil-Val (GL Science, Tokyo, Japan). The concentration of d-Asp was related to the content of l-Asp, and the racemization ratio was expressed as ln[(1 + d/l)/(1 − d/l)]. Plotting the chronological age on the x axis and the racemization ratio on the y axis, we derived the following linear regression equation by the least square method,
where ln[(1 + d/l)/(1 − d/l)] represents the log-transformed racemization ratio, t is the chronological age, and k is the racemization rate constant. To estimate the chronological age, we plotted the age on the y axis and the racemization ratio on the x axis and derived the following linear regression equation by the least square method.
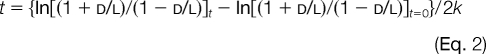 |
The estimated age was obtained by substituting the d/l ratio in this linear regression equation with that of the specimen to be estimated. For a detailed flowchart illustrating the method of racemization analysis, see Ref. 13.
Accelerator MS Sample Preparation
Enamel Preparation
The tooth crown was cut away from the root at the level of the cervical line and incubated in 10 n NaOH at room temperature in a water bath sonicator (Branson 150). Every 24 h NaOH was replaced, and the non-enamel structures were removed mechanically using an odontologic electric drill. Purified enamel was then washed three times with ddH2O,1 resubmersed in 10 n NaOH, and placed again into the sonicator water bath. This procedure was repeated every day for 3–5 days (until all dentin and soft structures were stripped from the enamel). The enamel was then rinsed several times in ddH2O and dried at room temperature overnight. Ten teeth were processed separately using a modified, simplified protocol. For these teeth, the crown was also separated from the root of the tooth, although this time the entire crown was washed in ddH2O and crushed using a liquid nitrogen-filled cryogenic impact grinder (SPEX 6850 Freezer Mill). A fine powder was obtained using a standard program with a run time of 15 min. One whole tooth crown was also washed with ddH2O and sent directly as is for AMS analysis. The enamel, crushed teeth, and the whole crown were weighed and kept sealed in a glass tube until pretreated for AMS analysis.
AMS Pretreatment
Aliquots of the enamel samples were placed in culture tubes for pretreatment to remove the surface carbon that may have coated the enamel between formation and analysis. Because the carbon content of enamel is 0.4–0.6%, 80–150-mg aliquots are typically used to get full sized samples containing 0.4–0.9 mg of carbon for 14C analysis. Enamel samples were immersed in 1.0 n HCl at room temperature for 1.5 h, rinsed three times with ddH2O, and placed on a heating block at 95 °C under a loose aluminum foil tent to dry overnight. Powdered samples react vigorously in 1.0 n HCl and were immersed for only a minute or two, rinsed five times with ddH2O, and placed on a heating block at 95 °C under a loose aluminum foil tent to dry overnight. The acid pretreatment was designed to remove the outer surface of the enamel that was exposed to the harsh alkali environment earlier without dissolving too much of the enamel. Base always contains some carbonate that can potentially exchange with the enamel during the preparation step. Furthermore, alkali solutions remove CO2 from the atmosphere and produce carbonate and bicarbonate in solution that can precipitate. Each dried enamel sample was broken into 5–10 pieces, placed in an individual single use reactor, and again weighed to the nearest 0.1 mg. The acid etching method dissolves a couple milligrams of exterior enamel surface in a 100-mg enamel sample. The enamel samples placed in individual reaction chambers were evacuated, heated, and acidified with concentrated orthophosphoric acid at 90 °C. The evolved CO2 was purified, trapped, and reduced to graphite in the presence of iron catalyst in individual reactors (14, 15). With the enamel aliquots used, nearly all CO2 samples were >500 μg of carbon. The CO2 was split, and δ13C was measured by stable isotope ratio mass spectrometry (supplemental Table 1). Background values were controlled by consistently following procedures, frequently baking sample tubes, periodically cleaning rigs, and maintaining a clean laboratory (16).
AMS Sample Measurement and Analysis
Graphite targets were measured using the 10-MV High Voltage Engineering Europa FN-class tandem electrostatic AMS system at the Center for Accelerator Mass Spectrometry at the Lawrence Livermore National Laboratory (LLNL). The operation is similar to that described by Guilderson et al. (17) when performing high precision measurements of 18,000-year-old turbidites used as secondary standards. Details on the design of the LLNL AMS system and its operation can be found in the literature (17–20). The system uses an LLNL-designed high output negative ion solid graphite cesium sputter source (18), which emits 250–350 μΑ of 12C− from a full-size sample, corresponding to approximately 900 14C counts/s from a contemporary sample. The FN AMS system routinely achieves 15% total system efficiency for carbon (19). Enamel samples are usually full-size and contemporary, so analysis times are relatively rapid, generally less than 5 min. The enamel samples are measured for 30,000 14C counts/cycle for 4–7 cycle repetitions and achieve standard deviations of 0.3–0.8%.
Corrections for background contamination introduced during AMS sample preparation are made by establishing the contributions from contemporary and fossil carbon following published procedures (21, 22). All data are normalized with six identically prepared NIST SRM 4990B (Oxalic Acid I) primary standards. NIST SRM 4990C (Oxalic Acid II), IAEA-C6 (23), and Third International Radiocarbon Intercomparison wood (24) are used as secondary standards and quality controls to monitor spectrometer performance. The ratio of NIST SRM 4990C to NIST SRM 4990B (Oxalic Acid II/Oxalic Acid I) measured between February 2005 and July 2009 on 22 different sample wheels containing enamel samples had an average value of 1.291 ± 0.002 (1 S.D.) in agreement with the certified value of 1.293 ± 0.001. 14C-free calcite serves as background material for processing the enamel samples. The enamel samples are organized in groups of 10–14 unknowns bracketed by primary standards with one primary standard in the middle of the group. The secondary standards, primary standards, and group of unknowns are measured consecutively as a cycle. Upon completion of a cycle, the set of primary standards, secondary standards, and unknown samples is measured again until desired precision is achieved. A typical group of 14 enamel samples is measured completely in 2–3 h. The measurement error is determined for each sample and generally ranges between ±0.2 and 0.8% (1 S.D.). All 14C data are reported using the F14C fraction modern nomenclature developed for postbomb data (25). F14C is a concentration unit (14C/C) denoting enrichment or depletion of 14C relative to oxalic acid standard normalized for isotope fractionation. Data are also reported as decay-corrected Δ14C following published nomenclature (22). Δ14C was calculated using the equation,
where λ = 1/8267 year−1 and y is the year of measurement after 1950 A.D.
Determining Year of Birth from 14C Data
The average age at which enamel formation is completed for each specific tooth has been determined previously and is dependent on the tooth number and gender of the person (3, 4, 10, 11). If the sex of a person is unknown, the average time for enamel completion for males and females is calculated. The 14C concentration measured in the tooth enamel is plotted onto a curve of atmospheric 14C against time to determine the year of enamel synthesis and date of birth of the individual. The time (in years) taken for the enamel to form is subtracted from the year obtained to give an estimated date of birth (Fig. 2 and Table I). Calibrated ages were obtained by using the CALIbomb Levin data set where the smoothing in years was set at 1.0 and 2 σ error was used). For 14C tooth enamel values that fell between 1955 and 1960, the CALIbomb program was not used. In CALIbomb, these values are predicted using a straight line from prebomb values to 1960. For these age ranges, values from the Hua and Barbetti (26) were used.
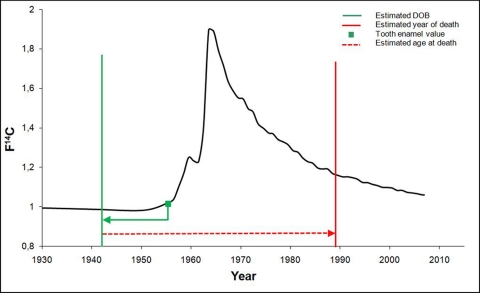
Fig. 2.
Application of combined analysis of radiocarbon and aspartic acid racemization on four teeth collected from homicide case. Three teeth with relatively short enamel laydown times showed no bomb spike-derived 14C in the enamel. However, the fourth tooth, an upper jaw third molar with a much longer enamel formation period, did and matched atmospheric 14C levels equating to 1955.0 (green filled square). This indicates a birth date of 1942.0. By adding the average age (red dashed line) estimated from the racemization analysis, the year of death was calculated (red vertical line) to be 1988.8. DOB, date of birth.
Table I. Raw data for radiocarbon- and aspartic acid racemization-analyzed teeth.
DOB, date of birth (in years); M, male; F, female.
| Person no. | Case no. | Sex | DOB | Known age | Enamel formation time | Radiocarbon analysis |
Aspartic acid racemization analysis |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DOB estimation | Error (2 σ) | Error from true DOB ± | Average absolute error/person | Estimated age | DOB estimation | Error from true DOB ± | Average absolute error/person | ||||||
| yr | yr | yr | yr | yr | yr | yr | |||||||
| 1 | FM01 | M | 1991.3 | 15.5 | 5.6 | 1989.4 | 2.1 | −1.9 | 1.9 | 15.7 | 1991.1 | 0.2 | 0.2 |
| 2 | FM02 | M | 1991.8 | 15.1 | 5.2 | 1990.6 | 2.0 | 1.2 | 1.2 | −1.2 (set at 0) | 2006.9 | −15.1 | 15.1 |
| 3 | FM03 | M | 1993.2 | 13.6 | 4.2 | 1993.5 | 2.1 | 0.3 | 0.3 | 5.5 | 2001.3 | 8.1 | 8.1 |
| 4 | FM05 | F | 1988.8 | 18.1 | 4.2 | 1988.0 | 1.5 | −0.8 | 0.8 | 9.8 | 1997.1 | 8.3 | 8.3 |
| 5 | FM06 | F | 1988.8 | 19.1 | 4.9 | 1985.7 | 1.0 | −3.1 | 3.1 | 20.9 | 1986.0 | 2.8 | 2.8 |
| 6 | FM10 | M | 1982.0 | 24.8 | 3.2 | 1981.2 | 0.7 | −0.8 | 0.8 | 35.9 | 1970.9 | 11.1 | 11.1 |
| 7 | FM25 | F | 1993.6 | 13.4 | 4.4 | 1994.0 | 2.2 | 0.4 | 0.4 | 11.0 | 1996.0 | −2.4 | 2.4 |
| 8 | FM30 | F | 1989.1 | 17.8 | 4.9 | 1987.3 | 1.6 | −1.8 | 1.8 | 16.9 | 1990.0 | −0.9 | 0.9 |
| 9 | FM89 | M | 1947.3 | 60.7 | 5.6 | Prebomb | Prebomb | Prebomb | Prebomb | 54.0 | 1953.9 | −6.7 | 7.8 |
| FM89 | M | 60.7 | 6.6 | Prebomb | Prebomb | Prebomb | Prebomb | 51.8 | 1956.1 | −8.9 | |||
| Average | 1.3 | Average | 6.3 | ||||||||||
| S.D. | 0.9 | S.D. | 5.0 | ||||||||||
| 10 | OC79 | F | 1937.0 | 67.9 | 5.6 | Prebomb | Prebomb | Prebomb | Prebomb | ||||
| 11 | OC66 | M | 1949.4 | 55.6 | 12.6 | 1949.8 | 0.2 | 0.4 | 0.4 | ||||
| 12 | OC65 | M | 1964.9 | 40.1 | 13.0 | 1964.0 | 1.1 | −0.9 | 0.9 | ||||
| 13 | OC73 | F | 1988.6 | 16.3 | 5.6 | 1988.3 | 1.9 | −0.3 | 0.3 | ||||
| 14 | FM12 | F | 1991.3 | 15.5 | 4.9 | 1992.2 | 2.3 | 0.9 | 0.9 | ||||
| 15 | FM42 | M | 1993.2 | 13.8 | 5.6 | 1989.7 | 2.0 | −1.1 | 1.1 | ||||
| 16 | FM27 | M | 1990.8 | 16.1 | 5.6 | 1992.6 | 2.3 | −0.6 | 0.6 | ||||
| 17 | OC45 | M | 1958.2 | 46.7 | 2.5 | 1957.9 | 0.7 | −0.3 | 0.3 | ||||
| 18 | OC54 | F | 1949.0 | 55.9 | 4.4 | Prebomb | Prebomb | Prebomb | Prebomb | ||||
| 19 | OC60 | F | 1955.8 | 49.1 | 5.8 | 1954.3 | 1.4 | −1.5 | 1.5 | ||||
| 20 | OC62 | M | 1956.7 | 48.2 | 12.6 | 1955.8 | 0.5 | −0.9 | 0.9 | ||||
| 21 | FM50 | F | 1988.3 | 18.7 | 4.9 | 17.5 | 1989.4 | −1.2 | 1.2 | ||||
| 22 | FM53 | M | 1990.9 | 16.3 | 5.6 | 11.2 | 1996.0 | −5.1 | 5.1 | ||||
| 23 | FM67 | F | 1936.5 | 70.6 | 3.8 | 69.0 | 1938.1 | −1.6 | 1.6 | ||||
| 24 | FM69 | F | 1947.6 | 59.4 | 2.5 | 55.3 | 1951.6 | −4.1 | 4.1 | ||||
| 25 | FM85 | F | 1963.7 | 44.1 | 5.6 | 48.7 | 1959.2 | 4.6 | 3.2 | ||||
| FM85 | F | 44.1 | 5.8 | 42.3 | 1965.6 | −1.8 | |||||||
| 26 | FM43 | M | 1993.2 | 13.8 | 5.1 | 10.5 | 1996.5 | −3.3 | 3.3 | ||||
| 27 | FM45 | M | 1990.9 | 16.1 | 5.6 | 20.6 | 1986.4 | 4.5 | 4.5 | ||||
| 28 | FM109 | F | 1957.4 | 51.0 | 3.8 | 60.1 | 1948.3 | 9.1 | 9.1 | ||||
| 29 | FM111 | F | 1960.8 | 46.6 | 5.8 | 56.9 | 1950.5 | 10.3 | 10.3 | ||||
| 30 | FM116 | F | 1939.9 | 68.2 | 2.5 | 73.0 | 1935.1 | 4.8 | 4.8 | ||||
| 31 | FM117 | M | 1943.6 | 64.6 | 6.5 | 69.4 | 1938.8 | 4.8 | 4.8 | ||||
| Average | 0.8 | Average | 4.7 | ||||||||||
| S.D. | 0.4 | S.D. | 2.8 | ||||||||||
| Total average | 1.0 | Total average | 5.4 | ||||||||||
| Total S.D. | 0.6 | Total S.D. | 4.2 | ||||||||||
If it is not obvious whether an individual is born before or after the peak of the bomb tests, then two teeth with different enamel laydown times need to be analyzed; this will distinguish whether the 14C measurement relates to the rising or falling part of the curve (4). Additional studies by Cook et al. (3) also show that radiocarbon analysis on the collagen component of the tooth root (combined dentin and cementum) also allows one to determine whether an individual is born on the rising or falling part of the bomb curve. Because determining which side of the bomb peak a person falls has previously been demonstrated, we do not include the analysis of two teeth for this purpose here but have rather chosen the age range appropriate to the known date of birth of the individual.
Case Report: Unidentified Homicide Victim
In the summer of 2006, hunters found a human skeleton covered by a tarpaulin in a Swedish forest. Forensic examination revealed that the person had been shot in the back of his skull. Although the skeleton was almost complete, the consultant forensic anthropologist could not determine the deceased age with any precision but stated that it was a male and suggested he had been about 30–40 years old at the time of death. The anthropologist further estimated that the body had been dead for less than 15 years. Four teeth were extracted and subjected to both 14C analysis and aspartic acid racemization as described previously.
RESULTS
Forty-seven teeth from a total of 41 Swedish individuals were analyzed for aspartic acid racemization and/or radiocarbon analysis. Teeth from individuals with dates of birth ranging from 1936 through 1994 (aged 13–70 years) were analyzed (see supplemental Table 1). All subject information and raw data are listed in supplemental Table 1 and Table III.
Table III. Radiocarbon and aspartic acid racemization analyses of an unsolved homicide case.
DOB, date of birth; N/A, not applicable.
| Case no. | Tooth no. | Enamel formation time | Radiocarbon analysis |
Aspartic acid analysis |
Estimated year of death | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Δ14C | ± | F14C | ± | Estimated DOB | d/l ratio | Estimated Age | ||||
| yr | yr | |||||||||
| FMH1 | 41 | 2.5 | −31.1 | 4.1 | 0.9756 | 0.0041 | 1952.4 or earlier | 0.0934 | 45.0 | 1987.0 |
| FMH2 | 48 | 13.0 | 100.7 | 4.2 | 1.1083 | 0.0042 | 1942.0 | 0.0970 | 47.8 | 1989.8 |
| FMH3 | 11 | 3.2 | −28.0 | 3.9 | 0.9788 | 0.0039 | 1951.3 or earlier | 0.0944 | 45.8 | 1987.8 |
| FMH4 | 17 | 6.5 | −29.2 | 3.8 | 0.9775 | 0.0038 | 1948.4 or earlier | 0.0980 | 48.6 | 1990.7 |
| Estimated DOB | 1942.0 | Average | 46.8 | 1988.8 | ||||||
| S.D. | N/A | S.D. | 1.7 | 2.1 | ||||||
Radiocarbon Dating of Tooth Enamel Precisely Determines Date of Birth
Regression analysis of date of birth estimations using radiocarbon dating of tooth enamel and aspartic acid racemization analysis of crown dentin reveal a strong correlation between the two methods for teeth formed during and after the period of bomb testing (Fig. 1). Although both methods are in good agreement with each other, radiocarbon dating offered more precise age estimations than aspartic acid racemization analysis. Radiocarbon analysis of tooth enamel from nine Swedish individuals (10 teeth), where the date of birth was known, yielded a 14C-based date of birth estimation accuracy of 1.3 years (S.D. = 0.9 years). Two teeth that were formed prior to the period of nuclear weapon testing showed prebomb radiocarbon values. Aspartic acid racemization analysis of crown dentin using the same teeth yielded an average error of age estimation of 6.3 years (S.D. = 5.0 years). Teeth from an additional 22 individuals were also analyzed: 11 individuals were analyzed using radiocarbon dating of enamel to estimate date of birth and were compared against teeth from 11 age-matched individuals analyzed using aspartic acid racemization analysis (Table I). As in the first round, radiocarbon dating gave a more precise estimate of chronological age than aspartic acid racemization analysis (radiocarbon dating age estimate precision was 0.8 years (S.D. = 0.4 years) compared with the aspartic acid racemization age estimation average of 4.7 years (S.D. = 2.8 years). An analysis of all cases demonstrated the average age estimate precision of radiocarbon dating to be 1.0 years (S.D. = 0.6 years; n = 17) and of aspartic acid racemization to be 5.4 years (S.D. = 2.8 years; n = 20) (Table I). Teeth from the same number of individuals were studied for each method; however, three individuals analyzed for radiocarbon dating showed prebomb levels of radiocarbon in their enamel. As such, one can only conclude that the said individual is born at least x number of years before the beginning of the bomb pulse (1955) where x indicates the enamel laydown time. In all cases where the analyzed tooth was formed before the period of nuclear bomb testing, AMS readings indicated prebomb radiocarbon values.
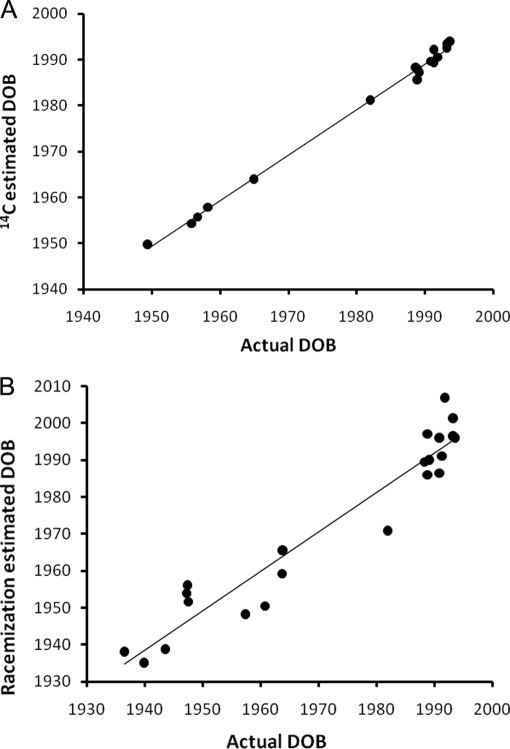
Fig. 1.
Linear regression analysis. a, the estimated date of birth (DOB) based on 14C analysis (y axis) and the actual date of birth (x axis); R2 = 0.996, p < 0.001. b, the estimated date of birth based on aspartic acid racemization analysis (y axis) and the actual date of birth (x axis); R2 = 0.923, p < 0.001. For details regarding each case, see Table I and supplemental Table 1. The two 14C cases giving prebomb values are omitted.
Analysis of Whole Crown Yields Good Date of Birth Estimation
Analysis of the whole crushed crown (as opposed to purified enamel) also results in precise date of birth estimation (Table II). Crushing the complete tooth crown significantly simplifies the processing needed for radiocarbon analysis and cuts the preprocessing time down to 15 min. Very good date of birth estimation was also obtained for one whole tooth crown subjected to AMS analysis without any pretreatment (see “Experimental Procedures” and Table II, case FM101).
Table II. Radiocarbon analysis of whole tooth crown.
DOB, date of birth; M, male; F, female.
| Person no. | Case no. | Sex | Tooth no. | Enamel formation time | Radiocarbon analysis |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Δ14C | ± | F14C | ± | Estimated DOB | Error (2σ) | Year of birth | Error ± | Absolute error | |||||
| yr | |||||||||||||
| 32 | FM26 | M | 24 | 5.6 | 94.2 | 4.6 | 1.1019 | 0.0046 | 1992.4 | 2.4 | 1993.1 | −0.7 | 0.7 |
| 33 | FM28 | M | 34 | 5.1 | 99.3 | 4.6 | 1.1071 | 0.0046 | 1992.2 | 2.3 | 1990.0 | 2.2 | 2.2 |
| 34 | FM42 | M | 24 | 5.6 | 93.8 | 4.3 | 1.1013 | 0.0043 | 1992.7 | 2.3 | 1993.2 | −0.5 | 0.5 |
| 35 | FM81 | F | 21 | 3.2 | 381.4 | 5.8 | 1.3911 | 0.0058 | 1971.6 | 0.6 | 1970.4 | 1.2 | 1.2 |
| 36 | FM100 | M | 41 | 2.5 | 19.5 | 5.2 | 1.0267 | 0.0052 | 1949.5 | 0.4 | 1936.4 | 13.1 | 13.1 |
| 37 | OC03 | M | 28 | 12.6 | 311.5 | 4.8 | 1.3207 | 0.0048 | 1965.8 | 0.8 | 1967.3 | −1.5 | 1.5 |
| 38 | OC04 | M | 27 | 6.5 | 97.1 | 3.6 | 1.1048 | 0.0036 | 1951.7 | 0.5 | 1952.3 | −0.6 | 0.6 |
| 39 | OC11 | M | 42 | 3.0 | 377.1 | 6.7 | 1.3868 | 0.0067 | 1959.5 | 0.4 | 1960.6 | −1.1 | 1.1 |
| 40 | OC17 | F | 18 | 11.2 | 29.7 | 4.4 | 1.0369 | 0.0044 | 1946.3 | 0.5 | 1946.6 | −0.3 | 0.3 |
| 41 | OC49 | F | 47 | 5.6 | 223.4 | 5.0 | 1.2320 | 0.0050 | 1977.6 | 0.8 | 1977.8 | −0.2 | 0.2 |
| 42 | FM101a | F | 28 | 11.2 | 286.6 | 3.9 | 1.2957 | 0.0039 | 1968.4 | 0.6 | 1968.3 | 0.1 | 0.1 |
| Average | 2.0 | ||||||||||||
| S.D. | 3.7 | ||||||||||||
a Whole tooth crown.
Radiocarbon Enamel Dating and Aspartic Acid Racemization Together Yield Date of Birth, Victim Age, and Date of Death Information
Four teeth from an ongoing homicide case in Sweden were analyzed for enamel radiocarbon content as well as aspartic acid racemization of crown dentin. Radiocarbon analysis showed that three of the teeth, with enamel laydown times ranging from 2.5 to 6.5 years, were devoid of any bomb spike-derived radiocarbon, indicating that the victim was born in 1948.4 or earlier (see Table III). One tooth, a third molar from the upper jaw (tooth number 48), did contain bomb carbon in the enamel. Given that the enamel laydown time for this tooth is 13 years, the victim's date of birth was estimated at 1942.0. Because no exact date of death is known for this case, it is not possible using radiocarbon analysis alone to determine the chronological age of the victim. Aspartic acid racemization analysis of crown dentin from the same four teeth indicated that the victim was 46.8 years at the time of death. Combining the age of the victim with the date of birth, it is possible to estimate the date of death as being 1988.8 ± 2.1 years (Table III and Fig. 2).
DISCUSSION
The resistance of teeth to environmental influence makes them particularly valuable in the forensic setting. Teeth can remain intact for an appreciable time and allow for an odontologic identification of even very old skeletons provided ante-mortem data are available. Furthermore, no exchange of carbon will take place in the enamel of the mature tooth during life and generally not after death, making mature permanent teeth particularly suitable for radiocarbon analysis.
We show that both aspartic acid racemization and AMS radiocarbon analyses can be performed on a single tooth and that the combined analysis can provide information about the year of birth and year of death of an individual. Both methods correlate well. The application of radiocarbon analysis of enamel for year of birth determination has previously been reported to show a high precision with an average estimation error of ±1.6 years (4). In this study, we analyzed almost double the number of individuals reported in the first study and made minor adjustments to the methods. We now report an average absolute error of date of birth estimation of 1.0 years. Aspartic acid racemization analysis of dentin also allowed for a fair prediction of the age of the person with a precision comparable to that reported previously (for a review, see Ref. 2).
Both methods have strengths and limitations. The radiocarbon birth dating method can tell the birth date of the person regardless of the time of death. However, the time window for this analysis is limited to subjects born after the early 1940s because the calculations are based on the measurement of bomb pulse-derived 14C. For older subjects, analysis of third molars (if available) may be necessary to detect bomb carbon because of the delay in their enamel laydown time. This is well illustrated by the described homicide case. Four teeth were analyzed for enamel radiocarbon content with enamel laydown time ranging from 2.5 to 13 years. Although no radiocarbon could be detected in the three teeth with the shortest enamel laydown period (2.5–6.5 years), the analysis of a third molar tooth (enamel laydown time of 13 years) showed bomb spike-derived radiocarbon in the tooth enamel. From this, we could deduce that the victim was born in 1942.0. Aspartic acid racemization analysis of the four teeth estimated the victim to be 46.8 years at the time of death, placing the date of death at 1988.8 (±2.1 years). Despite Interpol contacts, this person was never identified. The police do have an idea of who this person might be, a foreigner believed to be in his forties who was suspected for having set fire to a restaurant in 1988 but then disappeared. The results of the radiocarbon and aspartic acid racemization analyses provide additional support for this theory and illustrate how combining both methodologies can provide precise and valuable information to assist police authorities.
Aspartic acid racemization analysis of dentin provides information about the chronological age of an individual at death because the chemical conversion from the l-enantiomer to the d-enantiomer will typically stop completely after death. Thus, analysis is independent of the calendar years in which the person was born and died. To determine these calendar years, other methods must be used, and usually the only alternative is to estimate the post-mortem interval. The wide window for this interval given by the experienced anthropologist in the homicide case serves as an example of the difficulties this often poses.
One advantage of aspartic acid racemization analysis is that it is independent of the bomb spike and hence can be used for age determination of subjects born long before the beginning of aboveground nuclear weapon testing. Several factors, however, will affect the precision of this method. Because the racemization process is basically a function of temperature and time, teeth are exposed to different ambient temperatures depending on their location. Ohtani et al. (27) demonstrate that racemization rates differ between the same tooth in middle-aged versus elderly individuals. They suggest that in elderly individuals racemization in teeth that have been situated deep in the oral cavity for a long time (and thus are exposed to higher ambient temperatures) are more influenced by the environment than by the period of tooth formation. Selection of which tooth to analyze may therefore yield different results depending on the age of the individual. The types of teeth best suited for racemization analysis are single rooted teeth such as mandibular incisors or mandibular premolars (13, 27). In these teeth, all the dentin can be easily collected, and test results have shown that analysis using whole dentin yields a more accurate age estimate than analysis using only part of the dentin (28). The age estimation precision reported here for aspartic acid racemization of dental enamel was conducted on an assortment of teeth and would most likely have been significantly higher if mandibular single rooted teeth had been available and used for analysis. In addition, some of the teeth were collected from teenagers where complete root formation may not have been completed. In such cases, it is impossible to collect whole dentin, resulting in poorer age estimation (as described above). In the ideal situation, the same type of teeth to be used for age estimation analysis should be used as standards to obtain the best precision (13).
For the radiocarbon analysis, we expedited the standard extraction procedure reported previously (4) by using an electric odontological drill to remove dentin. Hence, enamel could typically be isolated within 4 days. The freezing mill used to crush teeth may turn out to be an even more attractive alternative to more rapidly obtain a birth dating. It should be pointed out that age determination is particularly important to limit the search for possible missing person matches and that this information may be badly needed in the early phase of an investigation whether it concerns a crime or a mass disaster. Because radiocarbon analysis also gave good precision with the crushed teeth where the whole crown was ground down, we conclude that the exchange of carbon between the dentin within the crown and the environment typically is negligible. This has also been shown by aspartic acid racemization of crown dentin (28). One of these teeth, however, unexpectedly showed a postbomb value (although a low value). We do not have a good explanation for this other than that contamination might have occurred because of an insufficiently cleaned tube in the freezing mill or some undetected dental procedure (because the whole crown was crushed). Age estimation in all other teeth was excellent (average absolute error, 1.0 years).
The power of the radiocarbon method described here is likely to only improve in the coming years. Three reasons for this are as follows. (i) The status of the teeth in the population has improved substantially over time, particularly because of more effective control of caries and interventions to treat paraodontological problems. (ii) The population exposed to bomb spike radiocarbon rapidly increases for every year. This means that a growing number of unknown dead bodies are expected to have remaining teeth displaying bomb spike-derived 14C. (iii) The precision of AMS analysis improving, implying that a lesser amount of intact enamel will be necessary for analysis.
We have shown that radiocarbon dating of tooth enamel provides an extremely precise estimation of an individual's date of birth. AMS determines the date of tooth formation, aspartic acid racemization determines age of death. The combination of these methodologies offers considerable power to the forensic pathologist and police authorities to help determine the identity and time of death of unidentified individuals. We expect this strategy to become particularly valuable in homicide investigations and in the identification work associated with mass disasters.
Supplementary Material
Acknowledgments
Special thanks are given to the dentists and individuals in Stockholm who facilitated this work.
Footnotes
* This work was supported, in whole or in part, by National Institutes of Health Grant RR13461 from the National Center for Research Resources. This work was also supported by the Human Frontiers Science Program. This work was performed in part under the auspices of the United States Department of Energy by Lawrence Livermore National Laboratory under Contract DE-AC52-07NA27344.
 This article contains supplemental Table 1.
This article contains supplemental Table 1.
1 The abbreviations used are:
- ddH2O
- double distilled H2O
- AMS
- accelerator MS
- NIST
- National Institute of Standards and Technology
- LLNL
- Lawrence Livermore National Laboratory
- SRM
- Standard Reference Materials.
REFERENCES
- 1.Ritz-Timme S., Cattaneo C., Collins M. J., Waite E. R., Schütz H. W., Kaatsch H. J., Borrman H. I. (2000) Age estimation: the state of the art in relation to the specific demands of forensic practise. Int. J. Legal Med 113, 129–136 [DOI] [PubMed] [Google Scholar]
- 2.Waite E. R., Collins M. J., Ritz-Timme S., Schutz H. W., Cattaneo C., Borrman H. I. (1999) A review of the methodological aspects of aspartic acid racemization analysis for use in forensic science. Forensic Sci. Int 103, 113–124 [DOI] [PubMed] [Google Scholar]
- 3.Cook G. T., Dunbar E., Black S. M., Xu S. (2006) A preliminary assessment of age at death determination using the nuclear weapons testing 14C activity of dentine and enamel. Radiocarbon 48, 305–310 [Google Scholar]
- 4.Spalding K. L., Buchholz B. A., Bergman L. E., Druid H., Frisén J. (2005) Forensics: age written in teeth by nuclear tests. Nature 437, 333–334 [DOI] [PubMed] [Google Scholar]
- 5.Helfman P. M., Bada J. L. (1975) Aspartic acid racemization in tooth enamel from living humans. Proc. Natl. Acad. Sci. U.S.A 72, 2891–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helfman P. M., Bada J. L. (1976) Aspartic acid racemisation in dentine as a measure of ageing. Nature 262, 279–281 [DOI] [PubMed] [Google Scholar]
- 7.Ohtani S., Yamamoto T. (2005) Strategy for the estimation of chronological age using the aspartic acid racemization method with special reference to coefficient of correlation between D/L ratios and ages. J. Forensic Sci 50, 1020–1027 [PubMed] [Google Scholar]
- 8.De Vries H. (1958) Atomic bomb effect: variation of radiocarbon in plants, shells, and snails in the past 4 years. Science 128, 250–251 [DOI] [PubMed] [Google Scholar]
- 9.Nydal R., Lövseth K. (1965) Distribution of radiocarbon from nuclear tests. Nature 206, 1029–1031 [DOI] [PubMed] [Google Scholar]
- 10.Bolanos M. V., Manrique M. C., Bolanos M. J., Briones M. T. (2000) Approaches to chronological age assessment based on dental calcification. Forensic Sci. Int 110, 97–106 [DOI] [PubMed] [Google Scholar]
- 11.Nolla C. M. (1960) The development of the permanent teeth. J. Dent. Child 27, 254–263 [Google Scholar]
- 12.Buchholz B. A., Spalding K. L. (2010) Year of birth determination using radiocarbon dating of dental enamel. Surf. Interface Anal, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohtani S., Yamamoto T. (2010) Age estimation by amino acid racemization in human teeth: five case studies. J. Forensic Sci, in press [DOI] [PubMed] [Google Scholar]
- 14.Santos G. M., Southon J. R., Druffel-Rodrigez K., Griffin S., Mazon M. (2004) Magnesium perchlorate as an alternative water trap in AMS graphite sample preparation: a report on sample preparation at KCCAMS at the University of California, Irvine. Radiocarbon 46, 165–173 [Google Scholar]
- 15.Vogel J. S., Southon J. R., Nelson D. E. (1987) Catalyst and binder effects in the use of filamentous graphite for AMS. Nucl. Instrum. Methods Phys. Res. B 29, 50–56 [Google Scholar]
- 16.Zermeño P., Kurdyla D. K., Buchholz B. A., Heller S. J., Kashgarian M., Frantz B. R. (2004) Prevention and removal of elevated radiocarbon contamination in the LLNL/CAMS natural radiocarbon sample preparation laboratory. Nucl. Instrum. Methods Phys. Res. B 223, 293–297 [Google Scholar]
- 17.Guilderson T. P., Southon J. R., Brown T. A. (2003) High-precision AMS C-14 results on TIRI/FIRI turbidite. Radiocarbon 45, 75–81 [Google Scholar]
- 18.Davis J. C., Proctor I. D., Southon J. R., Caffee M. W., Heikkinen D. W., Roberts M. L., Moore T. L., Turteltaub K. W., Nelson D. E., Lloyd D. H., Vogel J. S. (1990) LLNL/UC AMS Facility and Research Program. Nucl. Instrum. Methods Phys. Res. B 52, 269–274 [Google Scholar]
- 19.Fallon S. J., Guilderson T. P., Brown T. A. (2007) CAMS/LLNL ion source efficiency revisited. Nucl. Instrum. Methods Phys. Res. B 259, 106–110 [Google Scholar]
- 20.Southon J. R., Roberts M. L. (2000) Ten years of sourcery at CAMS/LLNL—evolution of a Cs ion source. Nucl. Instrum. Methods Phys. Res. B 172, 257–261 [Google Scholar]
- 21.Brown T. A., Southon J. R. (1997) Corrections for contamination background in AMS 14C measurements. Nucl. Instrum. Methods Phys. Res. B 123, 208–213 [Google Scholar]
- 22.Stuiver M., Pollach H. A. (1977) Discussion. Reporting of 14C data. Radiocarbon 19, 355–363 [Google Scholar]
- 23.Rozanski K., Stichler W., Gonfiantini R., Scott E. M., Beukens R. P., Kromer B., van der Plicht J. (1992) The IAEA C-14 intercomparison exercise 1990. Radiocarbon 34, 506–519 [Google Scholar]
- 24.Scott E. M. (2003) The Third International Radiocarbon Intercomparison (TIRI) and the Fourth International Radiocarbon (FIR)—1999–2002—results, analysis and conclusions. Radiocarbon 45, 293–408 [Google Scholar]
- 25.Reimer P. J., Brown T. A., Reimer R. W. (2004) Discussion. Reporting and calibration of post-bomb C-14 data. Radiocarbon 46, 1299–1304 [Google Scholar]
- 26.Hua Q., Barbetti M. (2004) Review of Tropospheric bomb 14C data for carbon cycle modeling and age calibration purposes. Radiocarbon 46, 1273–1298 [Google Scholar]
- 27.Ohtani S., Ito R., Yamamoto T. (2003) Differences in the D/L aspartic acid ratios in dentin among different types of teeth from the same individual and estimated age. Int. J. Legal Med 117, 149–152 [DOI] [PubMed] [Google Scholar]
- 28.Ohtani S. (1997) Different racemization ratios in dentin from different locations within a tooth. Growth Dev. Aging 61, 93–99 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.