Abstract
Fibroblast growth factor 21 (FGF21) is a hormone produced by fat and the liver that plays an important role in lipid metabolism. FGF21 expression is induced by peroxisome proliferator-actived receptor α in response to physiological conditions requiring increased fatty acid oxidation. Retinoic acid receptor-related receptor α (RORα) is another nuclear receptor that plays a critical role in lipid metabolism as well as in regulation of the circadian rhythm. In this study we demonstrate that RORα directly regulates the expression and secretion of FGF21. A canonical ROR response element was identified in the proximal promoter of the FGF21 gene and shown to exhibit functional activity. Overexpression of RORα in HepG2 cells resulted in increased expression and secretion of FGF21. Suppression of RORα expression caused a decrease in FGF21 expression and secretion, suggesting that RORα contributes to the basal expression of FGF21. These data suggest that one mechanism by which RORα regulates lipid metabolism may be by modulation of FGF21 secretion. Furthermore, this study identifies a clear link between RORα, a key regulator of the mammalian clock, and FGF21, an important hormone regulating glucose and lipid homeostasis.
Keywords: Diabetes, Dyslipidemia, Lipid, Nuclear Receptors, Steroid Hormone Receptor
Introduction
The regulation of lipid metabolism is tightly controlled and several nuclear hormone receptors (NHRs)2 play an essential role in maintaining metabolic homeostasis. The retinoic acid receptor-related orphan receptors RORα (NR1F1) and RORγ (NR1F3) have been implicated in metabolic pathways, energy homeostasis, and thymopoiesis. In addition, RORs regulate the expression of several components of the circadian clock and the expression of several downstream genes involved in metabolism.
Like all NHRs, the RORs display a typical nuclear receptor domain structure consisting of four major functional domains: an amino-terminal (A/B) domain followed by a highly conserved DNA-binding domain, a hinge domain, and a carboxyl-terminal ligand-binding domain. RORs regulate gene expression by binding as monomers to specific “half-site” DNA response elements (ROREs) consisting of an AGGTCA sequence with a 5′ AT-rich extension. RORs recruit coactivators resulting in constitutive activation of target gene transcription (1–3).
RORα has been shown to regulate several genes involved in lipid metabolism. Detailed examination of staggerer mice (RORαsg/sg) in which there is a frameshift and a premature stop codon, rendering RORα inactive, have revealed significant alterations in lipid metabolism including low levels of total plasma cholesterol, triglycerides, and apolipoprotein CIII (4). RORα directly regulates the expression of genes for both apoA1 and apoCIII (5, 6). Consistent with this phenotype, the staggerer mice are less susceptible to high fat diet-induced obesity and hepatic stetosis (7).
RORγ-deficient mice exhibit normal levels of plasma cholesterol and triglycerides (8). However, when crossed with the staggerer mouse, the RORα/γ-deficient mice present with hypoglycemia, suggesting a role for these receptors in the maintenance of glucose metabolism (8). This study also demonstrated that RORα and RORγ display significant redundancy in function, which is consistent with plasma glucose levels remaining unaffected unless both receptors were lost. Recently, a role for RORα in glucose metabolism was established when Chopra et al. (9) found that loss of the NHR coactivator SRC-2 resulted in a murine phenotype similar to von Gierke disease which is associated with severe hypoglycemia and abnormal accumulation of glucose in the liver.
Fibroblast growth factor 21 (FGF21), a member of the FGF family, is a hepatic hormone that regulates peripheral glucose tolerance, torpor, and hepatic lipid metabolism (10–12). In vivo administration of FGF21 in rodent models of diabetes lowers both plasma glucose and triglyceride levels and improves insulin sensitivity and glucose clearance (12). Transgenic mice overexpressing FGF21 in the liver display resistance to diet-induced obesity and have improved glycemic control (14). FGF21 administration in diabetic monkeys results in a similar phenotype, including improvements in lipoprotein profiles (13). The ability of FGF21 to improve and protect against metabolic disorders like diet-induced obesity makes it an attractive drug candidate for the treatment of obesity and other metabolic diseases (15–17). Here, we demonstrate that RORα regulates both the expression and secretion of FGF21, providing another link between this NHR and lipid metabolism.
EXPERIMENTAL PROCEDURES
Plasmids and Viruses
The FGF21 promoter (−1738 to +71) was amplified from genomic DNA of HepG2 cells and cloned into pTAL-Luc luciferase report vector (Clontech, CA). The FGF21 promoter mutant construct was made by using QuikChange II site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions. The RORE (−1564 to −1554) was muted from TTCTAGGTCA to TTCTAGGGGG. PGL4.73 reporters were from Promega. PTrex-RORα and pTrex-RORγ were from Phenex Pharmaceuticals AG. RORα was tagged with FLAG and subcloned into pAd/CMV/V5-DEST vector through GatewayTM technique (Invitrogen). The adenovirus with FLAG-RORα was produced according to the manufacturer's instructions.
Site-directed Mutagenesis
The FGF21 promoter mutant construct was made using QuikChange II site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions. The RORE (−1564 to −1554) was mutated from TTCTAGGTCA to TTCTAGGGGG. The mutant primers targeting ROR binding site were: CACCTCTTGCCTTCTAGGGGGCTTCTCACAATGTC (forward) and GACATTGTGAGAAGCCCCCTAGAAGGCAAGAGGTG (reverse). The mutant primers were used to amplify mutant plasmid from pTAL-FGF21 reporter using PfuUltra HF DNA polymerase. The PCR product were treated with DpnI to select for mutation-containing synthesized DNA and then transformed into XL1-Blue supercompetent cells. Positive clones were picked and grown overnight in LB media. The plasmid were isolated using QIAprep spin miniprep kit (Qiagen). The mutant construct was verified by sequencing.
Cell Culture and Luciferase Assay
HEK293 cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum at 37 °C under 5% CO2. 24 h prior to transfection, HEK293 cells were plated in 96-well plates at a density of 15 × 103 cells/well. Transfections were performed using LipofectamineTM 2000 (Invitrogen). 8 h post-transfection, the cells were treated with vehicle or ligands. 24 h post-treatment, the luciferase activity was measured using the Dual-GloTM luciferase assay system (Promega).
Overexpression and Knockdown
The HepG2 cells were maintained in minimum essential medium supplemented with 10% fetal bovine serum at 37 °C under 5% CO2. HepG2 cells were plated in 6-well plate 1 day before infection. The cells were infected with adenovirus for 24 h and then switched to regular growth media. 24 h later, the media were collected for ELISA, and the cells were harvested to isolate total RNA. For knockdown assay, the control siRNA, human RORα siRNA (Thermo Scientific) were transfected with LipofectamineTM RNAiMAX (Invitrogen) by using reverse transfection. After 24 h, the cells were harvested to perform quantitative PCR assay or Western blot. The media were subjected to ELISA.
cDNA Synthesis and Quantitative PCR
Total RNA extraction and cDNA synthesis were performed as described before (18). The quantitative PCR was performed using ABI Prism 7900 HT detection system (Applied Biosystems, Foster City, CA). The primers for quantitative PCR are: human RORα, AAACAAGCAGCGGGAGGTGA (forward) and TGGCAAACTCCACCACATAC (reverse); human FGF21, ACCTGGAGATCAGGGAGGAT (forward) and GCACAGGAACCTGGATGTCT (reverse); and human CYPB, GCAAATTCCATCGTGTAATCAAG (forward) and CGTAGATGCTCTTTCCTCCTG (reverse). The expression of target gene was normalized to housekeeping gene CYPB.
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assays were performed according to the manufacturer's instructions (Millipore) and as we have described previously (18–21). HepG2 cells were plated in 100-mm plates 24 h before infection. The cells were infected with adenovirus (AD-lacZ or AD-RORα) for 24 h and then switched to regular growth media. After overnight incubation, the cells were cross-linked for 10 min with 1% formaldehyde and stopped with 125 mm glycine for 5 min at room temperature. The cells were washed twice with ice-cold phosphate-buffered saline and then harvested in 1 ml of phosphate-buffered saline with protease inhibitor. Cell pellets were obtained by centrifugation at 700 × g at 4 °C and resuspended in SDS lysis buffer (1% SDS, 10 mm EDTA, 50 mm Tris, pH 8.1) with protease inhibitor. Cross-linked chromatins were sheered to fragments in length between 200 and 1000 base pairs by sonication. The cell lysates were centrifuged at 12,000 × g at 4 °C for 10 min to remove insoluble material. Equal amounts of chromatin were diluted 10-fold in dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mm EDTA, 16.7 mm Tris-HCl, pH 8.1, 167 mm NaCl.) and precleared with protein G-agarose for 1 h at 4 °C. Immunoprecipitation with the following antibodies was performed at 4 °C overnight: Anti-mouse IgG (Millipore), anti-α-acetyl histone H3 (Millipore), anti-FLAG (Sigma). Antibody-chromatin complexes were collected with protein G-agarose beads followed by sequential washes with low salt, high salt, lithium, and Tris-EDTA buffers. Chromatins were eluted and reverse cross-linked for 4 h at 65 °C in the presence of NaCl. The proteins were digested with proteinase K, and the DNA fragments were column-purified. FGF21 ChIP primers used in PCR were GTAACGGGTGCCTTCCCAGA (forward) and ACCAGGAAACAACCGGTGG (reverse).
ELISA
HepG2 cells were plated in 6-well plate 1 day before infection. The cells were infected with adenovirus for 24 h and then switched to regular growth media. 24 h later, the media were used to perform FGF21 ELISA according to the manufacturer's instructions (Thermo Scientific).
Western Blot
Control siRNA or human RORα siRNA (Thermo Scientific) were transfected into HepG2 cells using Lipofectamine RNAiMAX (Invitrogen). After 24 h, cell medium was switched to minimum essential medium with 10% fetal bovine serum. 48 h post-transfection, HepG2 cells were washed once with phosphate-buffered saline and then incubated for 10 min at 4 °C in 100 μl of TNT lysis buffer (50 mm Tris-Cl, pH 7.5, 150 mm NaCl, and 1% Triton X-100) and a complete miniprotease inhibitor mixture (Roche Applied Science). The samples were then scraped and harvested into 1.5-ml microcentrifuge tubes, vortexed for 30 s, and then centrifuged (425 × g for 10 min). Protein levels in the supernatants were determined using a Coomassie protein assay kit (Bio-Rad), and 20 μg of protein from each sample was separated by SDS-PAGE (Bio-Rad; 10%) and then transferred to a polyvinylidene difluoride membrane (Millipore, Milford, MA) and immunoblotted with primary antibodies: RORα (BioLegend) or α-tubulin (Sigma) and horseradish peroxidase-conjugated secondary antibodies (Jackson Immunoresearch). Detection of the bound antibody by enhanced chemiluminescence was performed according to the manufacturer's instructions (Santa Cruz).
Statistical Analysis
All of the individual cotransfection experiments were performed in quadruplicate wells, and each experiment was performed at least three times. Results from the cotransfection experiments were normalized and combined for presentation, and the mean ± S.E. is indicated. The FGF21 secretion experiments were performed three times, and statistical analysis was performed on the combined data. Statistical analysis was performed using GraphPad Prism and Student's t test. The differences were considered statistically significant if p < 0.05. Representative blots/gels are shown for the ChIP experiment and Western blot experiments.
RESULTS
Identification of a Putative RORE in the FGF21 Promoter
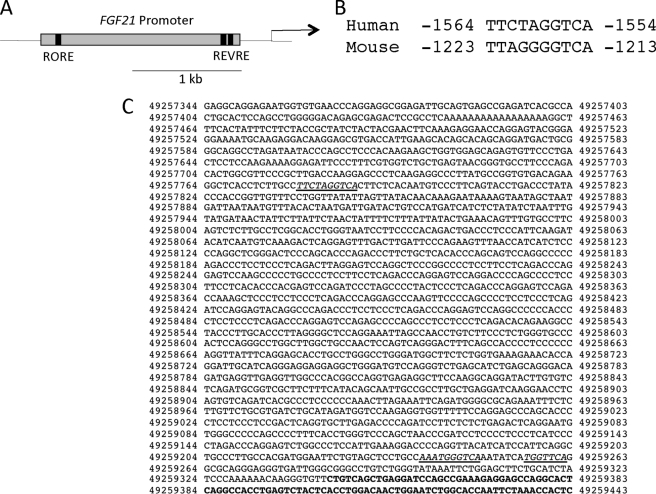
We analyzed the promoters of several genes involved in lipid metabolism for the presence of putative ROREs that are conserved between humans and mice using MAPPER (22, 23). A site within the FGF21 promoter was identified (Fig. 1A) that displayed characteristics of a classical RORE with an AGGTCA “half-site” and an AT-rich 5′ sequence (Fig. 1B). The position of the site was from −1564 to −1554 within the promoter 1359-bp upstream of the putative REV-ERB response element recently suggested to mediate REV-ERB responsiveness to the FGF21 gene (Fig. 1C) (24).
FIGURE 1.
The FGF21 promoter contains an evolutionarily conserved RORE. A, schematic of the FGF21 promoter. The FGF21 promoter contains a RORE in its proximal promoter region and the two recently identified REV-ERB response elements (REVRE) in its distal region. B, the RORE is evolutionarily conserved between humans and mice. C, sequence of the human FGF21 promoter region. The first underlined sequence depicts the RORE that we have identified. The second and third underlined sequences are the two REV-ERB response elements that were recently identified by Estall et al. (24).
Characterization of a Functional RORE within the FGF21 Promoter
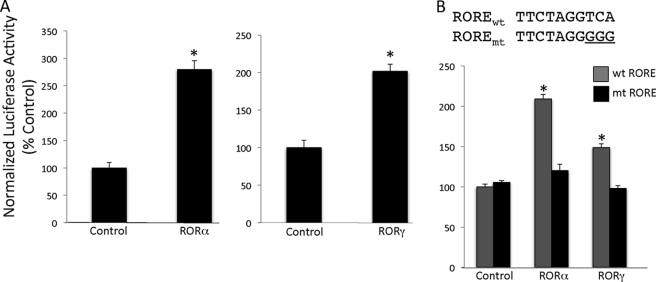
We cloned the FGF21 promoter (−1738 to +71) into the pTAL-Luc luciferase reporter vector and assessed the ability of RORs to modulate FGF21 activity in a cotransfection assay. HEK293 cells were transfected with RORα along with the FGF21 reporter construct. Fig. 2A demonstrates that RORα can induce the transcription of a FGF21 promoter-driven reporter. Inclusion of RORα resulted in an approximate 3-fold increase in FGF21 promoter-driven transcription. RORγ is structurally similar to RORα and displays an overlapping pattern of expression with RORα, and these two receptors cooperate to regulate diverse physiological functions including glucose metabolism and Th17 cell development. We hypothesized that RORγ would also induce the expression of FGF21 similar to RORα. HEK293 cells were transfected with a FGF21 reporter construct and RORγ. Fig. 2A demonstrates that, like RORα, RORγ can induce the expression of FGF21. To confirm that this event is ROR-specific, we mutated the RORE in the FGF21 promoter (Fig. 2B). This mutation rendered the FGF21 promoter unresponsive to both RORα and RORγ, demonstrating the critical role for this sequence in mediating ROR responsiveness (Fig. 2B).
FIGURE 2.
RORα and RORγ increase FGF21 promoter-driven transcription. A, RORα and RORγ increase the transcriptional activity of a FGF21 promoter-driven luciferase construct. HEK293 cells were transfected with RORα or RORγ along with the FGF21 reporter construct. Luciferase values were normalized using Renilla luciferase. *, p < 0.05 versus control vector. B, FGF21 transcriptional activity is ROR-specific. HEK293 cells were transfected with RORα or RORγ along with the FGF21 reporter construct containing the mutated RORE. The data are presented as the means ± S.E. *, p < 0.05 versus the mutant construct. wt, wild type; mt, mutant.
RORα Overexpression Increases FGF21 Expression
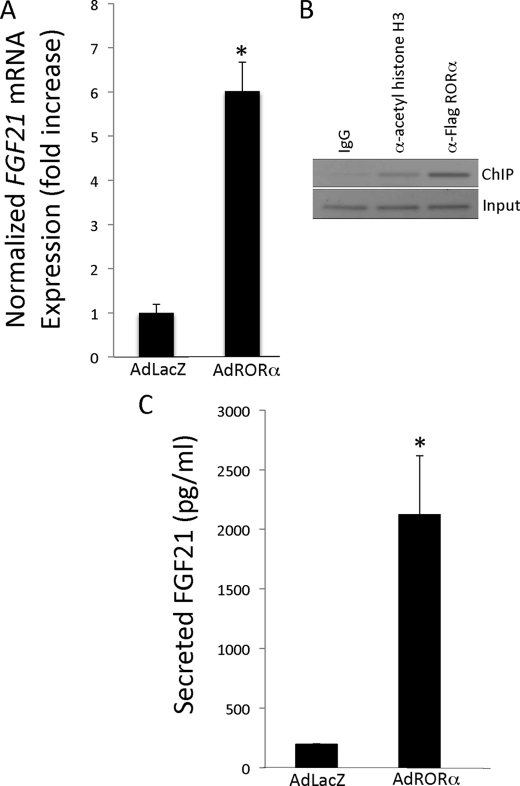
To investigate the potential role of RORα in FGF21 regulation, we overexpressed adenoviral RORα in HepG2 cells and measured the expression of FGF21 by reverse transcription-PCR. As seen in Fig. 3A, the addition of RORα dramatically increased the expression of FGF21 compared with control adenovirus (∼5.5-fold). We examined whether RORα was directly modulating FGF21 expression via binding to the RORE in its promoter sequence by ChIP in these HepG2 cells. As illustrated in Fig. 3B, we detect significant FGF21 promoter occupancy by RORα.
FIGURE 3.
Overexpression of RORα stimulates FGF21 expression. A, adenoviral overexpression of RORα in HepG2 cells increases FGF21 expression at the mRNA level as measured by reverse transcription-PCR. B, RORα binds the RORE within the promoter region of FGF21. IgG was used as a negative control, and α-acetylated histone H3 was used as a positive control. A representative gel is shown. C, adenoviral overexpression of RORα in HepG2 cells increases the expression of secreted FGF21 protein as measured by ELISA. The data are presented as the means ± S.E. *, p < 0.05 versus Ad-LacZ control.
RORα Overexpression Stimulates FGF21 Secretion
HepG2 cells have been used as a model to examine the effects of fatty acids on FGF21 secretion (25); thus we used this model to determine whether overexpression of RORα would result in an alteration of FGF21 secretion. RORα was overexpressed in these cells using the RORα adenovirus or control virus, and after 24 h the amount of FGF21 in the medium was measured using an ELISA. As illustrated in Fig. 3C, overexpression of RORα led to a 10-fold increase in secreted FGF-21.
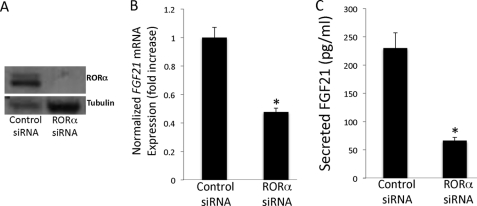
Knockdown of RORα Results in Decreased FGF21 Expression
To further characterize the physiological role of RORα on FGF21 expression, HepG2 cells were treated with either control siRNA or RORα siRNA. As depicted in Fig. 4A, Western blot analysis confirmed that the RORα siRNA significantly decreased RORα protein expression. Furthermore, Fig. 4B demonstrates that a loss of RORα expression leads to a significant 60% decrease in FGF21 gene expression as measured by reverse transcription-PCR. Thus, RORα contributes to the basal expression of FGF21.
FIGURE 4.
Knock down of RORα results in decreased FGF21 expression and secretion. A, HepG2 cells were treated with either control siRNA or RORα siRNA. Western blot analysis confirms the decrease in RORα protein expression. The blot was then reprobed with α-tubulin to confirm loading. A representative blot is shown. B, decreased expression of RORα in HepG2 cells leads to decreased expression of FGF21 as measured by reverse transcription-PCR. C, decreased expression of RORα in HepG2 cells leads to decreased secretion of FGF21. The data are presented as the means ± S.E. *, indicates p < 0.05 versus control siRNA.
A RORα/γ Inverse Agonist Represses FGF21 Promoter-driven Transcription
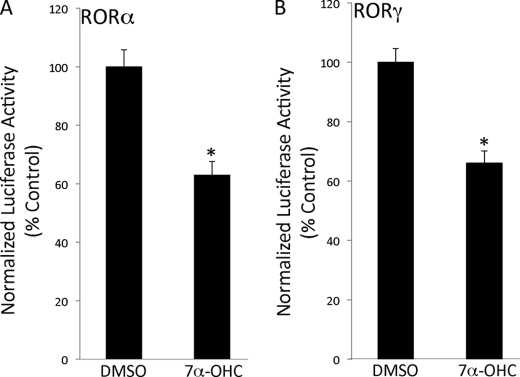
We recently published that the transcriptional activity of RORα and RORγ is modulated by 7-oxygenated sterols. Specifically, we demonstrated that 7α-hydroxycholestrol (7α-OHC) acts as a RORα/γ inverse agonist. Furthermore, 7α-OHC-modulated expression of ROR target genes and suppressed glucose output from primary hepatocytes (21). Due to our recent findings, we hypothesized that 7α-OHC-mediated inhibition of RORα and RORγ transcriptional activity would in turn suppress FGF21 expression. HEK293 cells were transfected with the FGF21 promoter luciferase reporter construct and expression vectors for either RORα or RORγ. 24 h post-transfection, the cells were treated for 24 h with 7α-OHC or vehicle control. Fig. 5 illustrates the ability of 7α-OHC to suppress transcription driven by the FGF21 promoter presumably via repression of RORα and RORγ activity.
FIGURE 5.
7α-Hydroxycholesterol represses FGF21 promoter-driven transcription. The ability of RORα (A) and RORγ (B) to activate FGF21 promoter-driven transcription is repressed by 7α-OHC in HEK293 cells. 7α-OHC was evaluated at a concentration of 10 μm. The data are presented as the means ± S.E. *, p < 0.05 versus dimethyl sulfoxide (DMSO).
DISCUSSION
FGF21 and RORα have been demonstrated to play an important role in lipid metabolism. Both are highly expressed in the liver, and mice with null mutations in either of these two genes have aberrant lipid metabolism (14, 26). Here, we show that the FGF21 promoter contains a functional RORE and that RORα contributes to the basal expression of FGF21. Furthermore, overexpression of RORα leads to a significant increase in the secretion of FGF21.
Another nuclear receptor subclass, the REV-ERBs (REV-ERBα (NR1D1) and REV-ERBβ (NR1D2)) also recognize RORE sites and are often coexpressed with the RORs (27). As opposed to the RORs, the REV-ERBs are repressors of transcription. Until recently, the REV-ERBs were thought to be constitutive repressors of transcription. However, work from our lab as well as that from the Lazar lab has demonstrated that the porphyrin heme functions as a ligand for both REV-ERBs and is required for repression of transcription (18, 28). RORs and REV-ERBs share a number of target genes, indicating that they coordinately regulate physiological processes. Interestingly, a very recent study demonstrated that PGC1α regulates hepatic FGF21 expression by modulation of the heme/REV-ERBα axis (24). PGC-1α modulates the levels of the REV-ERBα ligand, heme, by activation of transcription of the rate-limiting enzyme in heme synthesis, aminolevulinic acid synthase. Elevated levels of heme lead to increased REV-ERBα repressor activity directly at the FGF21 promoter. The putative REV-ERB response element (REVRE) identified by this group is a site distinct from the site we identified in this study. Our mutation studies indicate that the RORα activity is mediated entirely by the upstream site. Although RORs and REV-ERBs typically bind to similar sites, it is conceivable that they recognize different sites on the FGF21 promoter. Ultimately, these data still indicate that ROR and REV-ERBs coordinately regulate the expression of FGF21. Furthermore, these data suggest that the heme (REV-ERB ligand) and oxysterol (RORα ligand) synthetic pathways may regulate lipid and glucose metabolism via modulation of FGF21 secretion.
These two NHRs play an essential role in regulation of the mammalian clock that in turn coordinates various physiological processes (29–34). Studies have suggested that FGF21 expression is regulated in a circadian manner (35), leading to the possibility that RORα and REV-ERBα may be contributing to its circadian pattern of expression. The circadian rhythm is tightly coupled to metabolic regulation, and among several stimuli that can entrain the clock is food (36). This food-entrainable oscillator controls food-anticipatory behavior as well as other physiological alterations in anticipation of a meal. FGF21 levels are induced by fasting and suppressed by refeeding (10, 11, 37), and although another NHR, the peroxisome proliferator-activated receptor α, has been shown to play an essential role in regulation of FGF21 expression, especially as it relates to the fasting-induced expression of this hormone (10–11, 35, 37–39), it is possible that there may also be a circadian regulatory component to FGF21 secretion mediated by RORα.
An aberrant circadian rhythm can also lead to development of metabolic syndrome. Clock mutant mice display hepatic steatosis, hyperlipidemia, high glucose levels, and low insulin levels (40). In humans, epidemiological data indicates that shift work is associated with increased risk for development of diabetes, obesity, and cardiovascular disease (41, 42). A recent study in humans demonstrated that controlled misalignment of the circadian rhythm led to metabolic alterations, with some of the healthy subjects exhibiting postprandial glucose responses expected of individuals in a prediabetic state (43). Recent studies have shown administration of FGF21 to be beneficial in treating hyperglycemia and insulin resistance (16, 44). Our recent work demonstrating that RORα can be regulated by a synthetic ligand (20) suggests that one may be able to modulate FGF21 levels by targeting this NHR.
This work was supported, in whole or in part, by National Institutes of Health Grant DK080201 (to T. P. B.).
- NHR
- nuclear hormone receptor
- FGF
- fibroblast growth factor
- ROR
- retinoic acid receptor-related receptor
- RORE
- ROR response element
- ELISA
- enzyme-linked immunosorbent assay
- siRNA
- small interfering RNA
- ChIP
- chromatin immunoprecipitation
- 7α-OHC
- 7α-hydroxycholestrol.
REFERENCES
- 1.Carlberg C., Hooft van Huijsduijnen R., Staple J. K., DeLamarter J. F., Becker-André M. (1994) Mol. Endocrinol. 8, 757–770 [DOI] [PubMed] [Google Scholar]
- 2.Giguère V., Tini M., Flock G., Ong E., Evans R. M., Otulakowski G. (1994) Genes Dev. 8, 538–553 [DOI] [PubMed] [Google Scholar]
- 3.Hirose T., Smith R. J., Jetten A. M. (1994) Biochem. Biophys. Res. Commun. 205, 1976–1983 [DOI] [PubMed] [Google Scholar]
- 4.Hamilton B. A., Frankel W. N., Kerrebrock A. W., Hawkins T. L., FitzHugh W., Kusumi K., Russell L. B., Mueller K. L., van Berkel V., Birren B. W., Kruglyak L., Lander E. S. (1996) Nature 379, 736–739 [DOI] [PubMed] [Google Scholar]
- 5.Vu-Dac N., Gervois P., Grötzinger T., De Vos P., Schoonjans K., Fruchart J. C., Auwerx J., Mariani J., Tedgui A., Staels B. (1997) J. Biol. Chem. 272, 22401–22404 [DOI] [PubMed] [Google Scholar]
- 6.Raspé E., Duez H., Gervois P., Fiévet C., Fruchart J. C., Besnard S., Mariani J., Tedgui A., Staels B. (2001) J. Biol. Chem. 276, 2865–2871 [DOI] [PubMed] [Google Scholar]
- 7.Lau P., Fitzsimmons R. L., Raichur S., Wang S. C., Lechtken A., Muscat G. E. (2008) J. Biol. Chem. 283, 18411–18421 [DOI] [PubMed] [Google Scholar]
- 8.Kang H. S., Angers M., Beak J. Y., Wu X., Gimble J. M., Wada T., Xie W., Collins J. B., Grissom S. F., Jetten A. M. (2007) Physiol. Genomics 31, 281–294 [DOI] [PubMed] [Google Scholar]
- 9.Chopra A. R., Louet J. F., Saha P., An J., Demayo F., Xu J., York B., Karpen S., Finegold M., Moore D., Chan L., Newgard C. B., O'Malley B. W. (2008) Science 322, 1395–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Badman M. K., Pissios P., Kennedy A. R., Koukos G., Flier J. S., Maratos-Flier E. (2007) Cell Metab. 5, 426–437 [DOI] [PubMed] [Google Scholar]
- 11.Inagaki T., Dutchak P., Zhao G., Ding X., Gautron L., Parameswara V., Li Y., Goetz R., Mohammadi M., Esser V., Elmquist J. K., Gerard R. D., Burgess S. C., Hammer R. E., Mangelsdorf D. J., Kliewer S. A. (2007) Cell Metab. 5, 415–425 [DOI] [PubMed] [Google Scholar]
- 12.Kharitonenkov A., Shiyanova T. L., Koester A., Ford A. M., Micanovic R., Galbreath E. J., Sandusky G. E., Hammond L. J., Moyers J. S., Owens R. A., Gromada J., Brozinick J. T., Hawkins E. D., Wroblewski V. J., Li D. S., Mehrbod F., Jaskunas S. R., Shanafelt A. B. (2005) J. Clin. Invest. 115, 1627–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kharitonenkov A., Wroblewski V. J., Koester A., Chen Y. F., Clutinger C. K., Tigno X. T., Hansen B. C., Shanafelt A. B., Etgen G. J. (2007) Endocrinology 148, 774–781 [DOI] [PubMed] [Google Scholar]
- 14.Badman M. K., Koester A., Flier J. S., Kharitonenkov A., Maratos-Flier E. (2009) Endocrinology 150, 4931–4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kharitonenkov A., Shanafelt A. B. (2009) Curr. Opin. Investig. Drugs 10, 359–364 [PubMed] [Google Scholar]
- 16.Xu J., Lloyd D. J., Hale C., Stanislaus S., Chen M., Sivits G., Vonderfecht S., Hecht R., Li Y. S., Lindberg R. A., Chen J. L., Jung D. Y., Zhang Z., Ko H. J., Kim J. K., Véniant M. M. (2009) Diabetes 58, 250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chavez A. O., Molina-Carrion M., Abdul-Ghani M. A., Folli F., Defronzo R. A., Tripathy D. (2009) Diabetes Care 32, 1542–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raghuram S., Stayrook K. R., Huang P., Rogers P. M., Nosie A. K., McClure D. B., Burris L. L., Khorasanizadeh S., Burris T. P., Rastinejad F. (2007) Nat. Struct. Mol. Biol. 14, 1207–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y., Rogers P. M., Su C., Varga G., Stayrook K. R., Burris T. P. (2008) J. Biol. Chem. 283, 26332–26339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar N., Solt L. A., Conkright J. J., Wang Y., Istrate M. A., Busby S. A., Garcia-Ordonez R. D., Burris T. P., Griffin P. R. (2010) Mol. Pharmacol. 77, 228–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Kumar N., Solt L. A., Richardson T. I., Helvering L. M., Crumbley C., Garcia-Ordonez R. D., Stayrook K. R., Zhang X., Novick S., Chalmers M. J., Griffin P. R., Burris T. P. (2010) J. Biol. Chem. 285, 5013–5025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marinescu V. D., Kohane I. S., Riva A. (2005) BMC Bioinformatics 6, 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marinescu V. D., Kohane I. S., Riva A. (2005) Nucleic Acids Res. 33, D91–D97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Estall J. L., Ruas J. L., Choi C. S., Laznik D., Badman M., Maratos-Flier E., Shulman G. I., Spiegelman B. M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 22510–22515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mai K., Andres J., Biedasek K., Weicht J., Bobbert T., Sabath M., Meinus S., Reinecke F., Möhlig M., Weickert M. O., Clemenz M., Pfeiffer A. F., Kintscher U., Spuler S., Spranger J. (2009) Diabetes 58, 1532–1538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mamontova A., Séguret-Macé S., Esposito B., Chaniale C., Bouly M., Delhaye-Bouchaud N., Luc G., Staels B., Duverger N., Mariani J., Tedgui A. (1998) Circulation 98, 2738–2743 [DOI] [PubMed] [Google Scholar]
- 27.Burris T. P. (2008) Mol. Endocrinol. 22, 1509–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin L., Wu N., Curtin J. C., Qatanani M., Szwergold N. R., Reid R. A., Waitt G. M., Parks D. J., Pearce K. H., Wisely G. B., Lazar M. A. (2007) Science 318, 1786–1789 [DOI] [PubMed] [Google Scholar]
- 29.Akashi M., Takumi T. (2005) Nat. Struct. Mol. Biol. 12, 441–448 [DOI] [PubMed] [Google Scholar]
- 30.Duez H., Staels B. (2008) FEBS Lett. 582, 19–25 [DOI] [PubMed] [Google Scholar]
- 31.Guillaumond F., Dardente H., Giguère V., Cermakian N. (2005) J. Biol. Rhythms 20, 391–403 [DOI] [PubMed] [Google Scholar]
- 32.Jetten A. M. (2009) Nucl. Recept. Signal 7, e003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato T. K., Panda S., Miraglia L. J., Reyes T. M., Rudic R. D., McNamara P., Naik K. A., FitzGerald G. A., Kay S. A., Hogenesch J. B. (2004) Neuron 43, 527–537 [DOI] [PubMed] [Google Scholar]
- 34.Preitner N., Damiola F., Lopez-Molina L., Zakany J., Duboule D., Albrecht U., Schibler U. (2002) Cell 110, 251–260 [DOI] [PubMed] [Google Scholar]
- 35.Oishi K., Uchida D., Ishida N. (2008) FEBS Lett. 582, 3639–3642 [DOI] [PubMed] [Google Scholar]
- 36.Green C. B., Takahashi J. S., Bass J. (2008) Cell 134, 728–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundåsen T., Hunt M. C., Nilsson L. M., Sanyal S., Angelin B., Alexson S. E., Rudling M. (2007) Biochem. Biophys. Res. Commun. 360, 437–440 [DOI] [PubMed] [Google Scholar]
- 38.Gälman C., Lundåsen T., Kharitonenkov A., Bina H. A., Eriksson M., Hafström I., Dahlin M., Amark P., Angelin B., Rudling M. (2008) Cell Metab. 8, 169–174 [DOI] [PubMed] [Google Scholar]
- 39.Christodoulides C., Dyson P., Sprecher D., Tsintzas K., Karpe F. (2009) J. Clin. Endocrinol. Metab. 94, 3594–3601 [DOI] [PubMed] [Google Scholar]
- 40.Turek F. W., Joshu C., Kohsaka A., Lin E., Ivanova G., McDearmon E., Laposky A., Losee-Olson S., Easton A., Jensen D. R., Eckel R. H., Takahashi J. S., Bass J. (2005) Science 308, 1043–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knutsson A., Akerstedt T., Jonsson B. G., Orthgomer K. (1986) Lancet 2, 89–92 [DOI] [PubMed] [Google Scholar]
- 42.Knutsson A., Akerstedt T., Jonsson B. G. (1988) Scand. J. Work Environ. Health 14, 317–321 [DOI] [PubMed] [Google Scholar]
- 43.Scheer F. A., Hilton M. F., Mantzoros C. S., Shea S. A. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 4453–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kharitonenkov A., Shanafelt A. B. (2008) BioDrugs 22, 37–44 [DOI] [PubMed] [Google Scholar]