Abstract
Little is known about the rate at which protein turnover occurs in living tendon and whether the rate differs between tendons with different physiological roles. In this study, we have quantified the racemization of aspartic acid to calculate the age of the collagenous and non-collagenous components of the high strain injury-prone superficial digital flexor tendon (SDFT) and low strain rarely injured common digital extensor tendon (CDET) in a group of horses with a wide age range. In addition, the turnover of collagen was assessed indirectly by measuring the levels of collagen degradation markers (collagenase-generated neoepitope and cross-linked telopeptide of type I collagen). The fractional increase in d-Asp was similar (p = 0.7) in the SDFT (5.87 × 10−4/year) and CDET (5.82 × 10−4/year) tissue, and d/l-Asp ratios showed a good correlation with pentosidine levels. We calculated a mean (±S.E.) collagen half-life of 197.53 (±18.23) years for the SDFT, which increased significantly with horse age (p = 0.03) and was significantly (p < 0.001) higher than that for the CDET (34.03 (±3.39) years). Using similar calculations, the half-life of non-collagenous protein was 2.18 (±0.41) years in the SDFT and was significantly (p = 0.04) lower than the value of 3.51 (±0.51) years for the CDET. Collagen degradation markers were higher in the CDET and suggested an accumulation of partially degraded collagen within the matrix with aging in the SDFT. We propose that increased susceptibility to injury in older individuals results from an inability to remove partially degraded collagen from the matrix leading to reduced mechanical competence.
Keywords: Aging, Extracellular Matrix/Collagen, Methods/HPLC, Protein/Connective Tissues, Protein/Turnover, Collagen, Protein Degradation, Aspartic Acid Racemization, Equine, Tendon
Introduction
Tendons play a key role in locomotion by providing the mechanical link between muscle and bone. High mechanical strength is an important prerequisite as stresses of up to 50 MPa (1) can be imposed on the tendon, and this is provided largely by the highly organized collagen component. More specialized tendons, such as the Achilles tendon in humans and the superficial digital flexor tendon (SDFT)2 in horses, in addition to positioning the limb play a vital role in energy storage and release thereby increasing the efficiency of locomotion by up to 36% (2). An appropriate compliance is required by the energy-storing tendons to allow stretching and recoil to occur at a rate in keeping with the gait cycle. These energy-storing tendons have a higher non-collagenous protein content, predominately proteoglycan (3), which is thought to allow sliding movement between collagen fibrils (4).
Maintenance of both tendon material and structural properties is essential for function, and this is achieved by a balance between matrix synthesis and degradation. Recent studies have demonstrated that, contrary to previous thinking, collagen turnover in patellar tendons occurs at a rate comparable to that of collagen in metabolically active tissues such as muscle (5). However, degenerative changes are a common finding, and these changes are more frequent in older aged individuals and in specific tendons (6).
Energy-storing tendons are subjected to much higher stresses and strains than tendons that are designed predominantly for limb placement (positional tendons) and therefore might be expected to experience higher levels of micro-damage thus requiring a greater capacity for matrix turnover. Unexpected findings from our previous work (3), however, suggest that the matrix of the high strain equine SDFT is turned over more slowly than in the low strain positional common digital extensor tendon (CDET). This conclusion was based upon a simple measurement of tissue-associated fluorescence; long-lived proteins such as collagen are subjected to age-related glycation and subsequent spontaneous formation of advanced glycation end-products (AGEs), some of which fluoresce naturally. However, enzymatically derived cross-links, such as hydroxylysyl pyridinoline (HP) and lysyl pyridinoline (LP), which do not accumulate with age in mature equine tendon (7, 8), also fluoresce, and so a difference in cross-link profile, as has been previously shown for the SDFT and CDET (9), could also account for this difference in fluorescence.
A more widely accepted and robust method of assessing molecular age is to measure the rate of amino acid racemization. Amino acids are incorporated into proteins in the l-form, but as a function of time they undergo spontaneous racemization and convert to the d-form. The most rapidly racemizing amino acid is aspartic acid (10), making it most suitable for aging biological tissues. Thus measurement of the ratio of d-aspartate (d-Asp) to l-aspartate can be used to calculate protein half-life (11–14). Another marker widely accepted to give a good indication of protein age is the AGE pentosidine, which accumulates linearly with age in tissues with a low rate of matrix turnover, and also shows strong correlation with d-Asp measurements (15). Matrix age can also be assessed indirectly by measuring markers of collagen degradation. The cross-linked telopeptide of type I collagen (ICTP) (16) and collagenase-generated neoepitope (17) have been used previously as markers of collagen turnover in a variety of tissues.
In this study we assessed the turnover of collagen and non-collagenous protein in healthy equine tendon using the racemization of aspartic acid as a probe and the levels of collagenase-generated neoepitopes and ICTP as markers of degradation. We explored the hypothesis that the positional CDET has a higher rate of matrix turnover than the energy-storing SDFT and that matrix turnover rate decreases with increasing horse age in both tendon types.
EXPERIMENTAL PROCEDURES
Tissue Sampling and Preparation
The SDFT and CDET were harvested from the right forelimb of skeletally mature horses (n = 32) aged between 4 and 30 years euthanized at a commercial equine abattoir for reasons other than tendon injury. Tendons showing macroscopic evidence of degeneration were excluded from the study. A 2-cm section was excised from the mid-metacarpal region of each tendon, snap frozen in hexane, and stored at −80 °C. Prior to processing, the tendon was semi-thawed and the epitenon along with peripheral tissue was removed from each sample. The tendon tissue was lyophilized until a constant weight was reached and reduced to a fine powder by the use of a mikro-dismembrator (Sartorius, Germany).
Separation of Matrix Components and Preparation for Aspartic Acid Racemer Determination
The collagenous and non-collagenous matrix components of the tendons were separated by guanidine HCl (GdnHCl) extraction as described by Birch et al. (3). The remaining collagenous protein-containing pellet was washed twice with deionized H2O to remove the GdnHCl, freeze-dried, and papain-digested in 1 ml of digestion buffer as described previously (18). An aliquot of digest (100 μl) was hydrolyzed in 1 ml of 6 m HCl at 110 °C for 24 h, dried, and re-dissolved in 10 ml of 0.1 m HCl. GdnHCl was removed from the supernatant, which contained the non-collagenous proteins, using centrifugal filter units (Microcon YM-3, 3000 molecular weight cut-off, Millipore) according to the manufacturer's instructions. An aliquot (20 μl) of the remaining concentrated sample was hydrolyzed in 6 m HCl as described above, dried, and re-dissolved in 1 ml of 0.1 m HCl. The efficiency of the matrix separation was determined by collagen and glycosaminoglycan (GAG) assay (see supplemental materials). To measure the ratio of d- to l-Asp in the whole matrix, ∼20 mg of powdered tendon was also papain-digested and hydrolyzed as above.
Determination of d/l-Asp Ratio
The hydrolysates from the entire matrix, and collagenous and non-collagenous matrix components were filtered (0.2-μm filter), and an aliquot (100 μl) of each sample was derivatized with 200 μl of o-phthaldialdehyde-N-acetyl-l-cysteine solution (19). The derivatization reaction was quenched with 200 μl of 0.3 m sodium phosphate buffer (pH 7.5).
The d/l-Asp ratio was determined using high performance liquid chromatography (HPLC) according to Yekkala et al. (19). Standard solutions of d- and l-Asp were used to determine the retention times and fluorescent response of the derivatized products (see supplemental materials). Derivatized standard or samples (50 μl) were injected into the HPLC system (Series 200, PerkinElmer Life Sciences) and the d- and l-Asp derivatives were eluted using a Nova-Pak C8 4-μm column (150 mm × 3.9 mm inner diameter, Waters). Mobile phase A was 10% methanol and 90% 30 mm sodium phosphate buffer (pH 5.5), and mobile phase B was 90% methanol and 10% 30 mm sodium phosphate buffer (pH 5.5). A gradient program was run as follows; 0–8 min 0–100% mobile phase B, 8–13 min 100% mobile phase B, 13–15 min 100–0% mobile phase B, and 15–35 min 0% mobile phase B. The flow rate was 0.5 ml/min. Fluorescence was measured at λex 337 nm and λem 442 nm. The HPLC technique used to quantify the d- and l-Asp derivatives resulted in complete separation of the d- and l-forms; the precision of the technique, as calculated by the relative standard deviation, was 0.69%, and the detection limit was 0.56% d-Asp (see supplemental materials). The ratio of d- to l-Asp was calculated for each tendon sample using the fluorescent response of the standard solutions (1:1.29 d-Asp:l-Asp) and subtraction of any d- and l-Asp present in papain and correction for racemization that occurred during the hydrolysis step (see supplemental materials).
Calculation of Protein Turnover Rates and Half-lives
The calculation of protein turnover rate and protein half-life was carried out essentially as described by Maroudas et al. (11). The accumulation of d-Asp in any tissue depends on the intrinsic rate constant of racemization, i.e. the amount of racemization that occurs when no tissue is turned over (ki), and the protein turnover (kT). Assuming the concentration of aspartic acid is constant over time and that the percentage of d-Asp is <10%, which it is in vivo (11), kT can be calculated using Equation 1.
The accumulation rate (d(d/l)dt) in Equation 1 was approximated by fitting a linear curve to the measured data for the ratio d/l in each matrix component of tendon as a function of time (see Fig. 1 and Table 1).
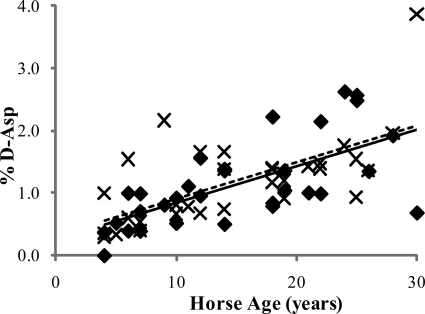
FIGURE 1.
d/l-Asp ratio for the SDFT (♦) and CDET (×) as a function of horse age. A linear equation was found to give the best fit (n = 32, r = 0.69 and n = 32, r = 0.64 for the SDFT (solid line) and CDET (dashed line), respectively). No significant difference was found in the rate of accumulation of d-Asp with age in the SDFT compared with the CDET.
TABLE 1.
Accumulation rates of d-Asp per year in the SDFT and CDET ((d(d/l)dt) in Equation 1)
| Fraction | Accumulation rate of d-Asp (×10−4/year) |
|
|---|---|---|
| SDFT | CDET | |
| Whole tendon | 5.87 | 5.82 |
| Collagenous | 14.06 | 7.27 |
| Non-collagenous | 2.57 | 6.58 |
The rate constant of racemization (ki) is taken as the rate of accumulation of d-Asp in dentin; collagen in dentin is the most stable during the human lifetime, and so negligible collagen turnover occurs in this tissue. The accumulation rate of d-Asp in dentin is 7.87 × 10−4/year (20). However, the rate of racemization is temperature-dependent; if it is assumed that the average temperature of dentin is 33 °C based on tooth surface temperature (21) and the average temperature of tendon is 37 °C (22), then the value of ki can be corrected for the difference in temperature using Equation 2 (10),
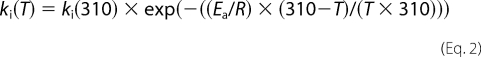 |
where T is temperature in Kelvin, Ea is the activation energy (33.4 × 103 as calculated by Bada et al. (23), and R is the gas constant (1.99). Using this equation, ki at 37 °C was calculated to be 1.604 × 10−3/year. This value for ki was used to calculate kT for the whole matrix and the collagenous component of the matrix. Previous work has found that non-collagenous proteins have a higher intrinsic rate of racemization; the ki value was calculated to be 1.873 × 10−3 in articular cartilage aggrecan (24). Intra-articular temperature is comparable to that of dentin (33 °C) (25), and so when the difference in temperature between articular cartilage and tendon is accounted for using Equation 4 the ki value for non-collagenous protein is 3.73 × 10−3.
The rate of protein turnover was then used to calculate protein half-life (t1/2) using Equation 3.
Quantification of Cross-links
HP, LP, and pentosidine cross-links were quantified using HPLC. Approximately 15 mg of powdered tendon was hydrolyzed in 3 ml of 6 m HCl at 110 °C for 24 h, dried, and re-dissolved in 500 μl of deionized H2O with 1% trifluoroacetic acid. The samples were filtered (0.2-μm filter), and 50 μl of sample was injected into the HPLC system (Series 200, PerkinElmer Life Sciences). The cross-links were eluted using a Hypercarb column (150 × 4.6 mm, 7 μm inner diameter, Thermo Scientific) and a method adapted from Bailey et al. (26). Mobile phase A was deionized H2O with 0.5% trifluoroacetic acid (v/v), and mobile phase B was tetrahydrofuran with 0.5% trifluoroacetic acid (v/v). A gradient program was run as follows: 0–5 min 5% mobile phase B, 5–35 min 5–20% mobile phase B, and flow rate 1 ml/min. From 0 to 20 min fluorescence was measured at λex 295 nm and λem 405 nm to detect HP and LP cross-links, and from 20 to 35 min fluorescence was measured at λex 335 nm and λem 385 nm to detect pentosidine.
Pentosidine concentration was calculated by measuring the peak area of known concentrations of pentosidine (a gift from Nick Avery). HP and LP concentration was calculated using a commercially available standard (Metra Pyd/Dpd HPLC Calibrator, Quidel Inc., San Diego, CA, supplied by Technoclone Ltd., Surrey, UK). Collagen content was calculated by measuring the concentration of the imino acid hydroxyproline (27, 28), assuming it to be present in collagen at 14% (18) in a 100-μl aliquot of the hydrolysate used for cross-link analysis. Cross-link concentration was expressed as millimolar/molar (mm/m) collagen.
Determination of Tissue Fluorescence
Tissue fluorescence was quantified using a fluorometer (LS50B, PerkinElmer Life Sciences) (3). An aliquot (150 μl) of papain-digested tissue was diluted to 3 ml with buffer (10 mm Tris, 1 mm EDTA·Na2, 0.1 mm NaCl, pH 7.4), and fluorescence was measured at λex 348 nm and λem 457 nm. Tissue fluorescence was expressed as arbitrary units/mg of dry weight tissue.
Determination of Glycosaminoglycan Content
Sulfated GAG content was measured in whole tissue following the papain digestion using dimethylmethylene blue dye (29). GAG concentration was calculated by comparing it to a standard curve prepared with bovine trachea chondroitin sulfate, which was diluted in dye to give concentrations from 0 to 10 μg in 3 ml. GAG concentration was expressed as micrograms/mg of dry weight tissue.
ICTP Measurements
ICTP was extracted and quantified as described previously (3). Briefly, protein was precipitated from an aliquot of the GdnHCl extract. A commercially available radioimmunoassay kit (Orion Diagnostica, Finland) for human serum was used to measure levels of ICTP. The assay was validated for use on guanidine hydrochloride-extracted ICTP from tendon samples using a parallel inhibition curve with the reference sample, which showed an excellent correlation with the tendon extract sample (see supplemental materials). ICTP levels were expressed as nanograms/mg of tendon tissue.
Measurement of Collagenase-generated Neoepitope of Type I Collagen
A commercially available competitive enzyme-linked immunosorbent assay kit (IBEX Pharmaceuticals Inc., Montreal, Canada), using an antibody that recognizes an 8-amino acid sequence on the ¾ fragment of type I collagen (C1,2C) was used to measure the collagenase generated neoepitope of type I collagen. This antibody has previously been shown to recognize the neoepitope in equine tissue (17). Lyophilized tendon (30 mg) was suspended in 0.75 ml of TAPSO buffer (100 mm TAPSO, 10 mm CaCl, pH 8.2) and heated at 90 °C for 35 min to denature the collagen. The samples were allowed to cool to 37 °C, and 0.4 ml of trypsin solution was added (10,000 units/ml TAPSO buffer). The samples were digested for 20 h at 37 °C with agitation, and the trypsin was then inhibited with 20 μl of Nα-tosyl-Lys-chloromethyl ketone HCl (18.2 mg/ml TAPSO buffer). A blank sample containing no tissue was included in the digestion and was used to dilute the standards. Standard solutions of neoepitope as supplied with the kit were also heated and digested with trypsin as described above to ensure that the extraction process did not interfere with antibody recognition (see supplemental materials). The enzyme-linked immunosorbent assay kit was used according to the manufacturer's instructions, and the resulting absorbance was read at 450 nm using a plate reader (Bio-Rad, 3550 microplate reader). A non-linear standard curve was constructed using GraphPad Prism curve fitting software (version 5.02). The concentration of the neoepitope was expressed as millimolar/molar collagen.
Statistical Analysis
Statistical tests were performed using SPSS (version 13, Microsoft), SPlus (version 6.1, Insightful), and Minitab (version 15.1). Significance was determined using linear mixed effects, with horse as a random factor and tendon as a fixed factor. The level of significance was p < 0.05. All data are displayed as mean ± S.E.
RESULTS
In Vivo Accumulation of d-Asp in Matrix from the Energy Storing SDFT and Positional CDET
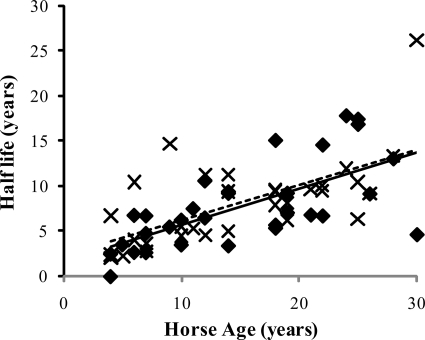
The ratio of d- to l-Asp increased linearly with age in the tissue from both the SDFT and CDET (p < 0.001, Fig. 1 and Table 1). Calculation of matrix half-life (using the accumulation rates given in Table 1) gave a mean (±S.E.) of 7.85 (±0.82) years for the SDFT and 8.02 (±0.84) years for the CDET, which were not significantly different. Matrix half-life increased significantly (p < 0.001) with age in both tendon types (Fig. 2).
FIGURE 2.
Matrix half-life of the SDFT (♦) and CDET (×) as a function of horse age. A linear equation was found to give the best fit (n = 32, r = 0.67 and n = 32, r = 0.65 for the SDFT (solid line) and CDET (dashed line), respectively). No significant difference was found in the half-life of the matrix in the SDFT and CDET.
Separation of the matrix into collagen and non-collagenous protein however revealed significant differences in protein turnover between the tendon types. Collagen half-life was significantly (p < 0.001) higher in the SDFT (197.53 (±18.23) years) than the CDET (34.03 (±3.39) years) and increased significantly with age in the SDFT (p = 0.03). The half-life of the non-collagenous component was considerably lower than that of the collagen component for both tendon types. As with collagen, the non-collagenous proteins showed a significant difference in half-life within the matrix, but in this case the SDFT (2.18 (±0.41) years) showed a lower residence time than the CDET (3.51 (±0.51) years) (p = 0.04). Matrix analysis showed a small but significant difference in total collagen content between the two tendon types and a significantly higher total sulfated glycosaminoglycan content in the SDFT than the CDET (Table 2). The collagen content did not change significantly with increasing horse age (data not shown), whereas the sulfated GAG content showed an aging-related decline in the CDET (p ≤ 0.001) (see supplemental materials).
TABLE 2.
Concentration of markers of matrix age and degradation and matrix components in the SDFT and CDET
Data are displayed as mean ± S.E. Italic numbers represent significant correlation with age. Bold numbers represent significant difference from SDFT.
| Mean± S.E. |
Correlation with age (r) |
|||
|---|---|---|---|---|
| SDFT | CDET | SDFT | CDET | |
| d/l-Asp × 100 (total) | 1.12 ± 0.12 | 1.18 ± 0.12 | 0.694a | 0.637a |
| d/l-Asp × 100 (coll.) | 5.64 ± 0.52 | 4.31 ± 0.42b | 0.376c | 0.244 |
| d/l-Asp × 100 (non-coll.) | 0.88 ± 0.25 | 1.58 ± 0.26b | 0.245 | 0.465c |
| Pentosidine (mm/m) | 13.83 ± 1.69 | 14.80 ± 2.14 | 0.847a | 0.893a |
| HP (mm/m) | 737.84 ± 45.18 | 70.11 ± 4.53a | 0.048 | 0.168 |
| LP (mm/m) | 101.51 ± 19.11 | 84.33 ± 16.33 | 0.390c | 0.481c |
| ICTP (ng/mg) | 86.09 ± 7.31 | 448.21 ± 104.89a | −0.651a | −0.499b |
| C1,2C (mm/m) | 12.65 ± 0.71 | 15.49 ± 0.86c | 0.458c | 0.056 |
| Tissue fluorescence | 58.22 ± 3.43 | 38.33 ± 1.69a | 0.883a | 0.820a |
| Collagen (%) | 75.81 ± 1.45 | 80.38 ± 1.26c | 0.138 | −0.095 |
| GAG (μg/mg) | 10.40 ± 0.86 | 1.83 ± 0.12a | −0.194 | −0.643a |
a p < 0.001.
b p < 0.005.
c p < 0.05.
Cross-link Levels and Tissue Fluorescence
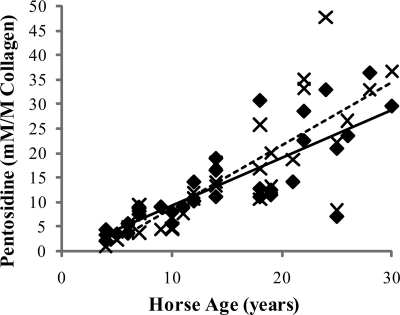
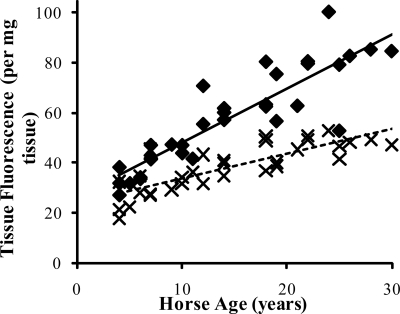
Levels of the AGE pentosidine were 13.83 (±1.69) mm/m collagen (mean ± S.E.) for the SDFT and 14.80 (±2.14) mm/m collagen for the CDET. Levels increased significantly (Fig. 3) with age in both tendon types (p < 0.001) and showed a good correlation with % d-Asp (r = 0.64) but were not different between tendon types. The tissue fluorescence was significantly lower in the CDET than the SDFT (Fig. 4) and increased significantly with horse age for both tendon types. HP levels were significantly lower in the CDET than the SDFT (Table 2) but did not change significantly with increasing horse age. There was no significant difference in LP content between tendons and levels increased significantly with age in both tendons (Table 2).
FIGURE 3.
Accumulation of the AGE pentosidine in the SDFT (♦) and CDET (×) as a function of horse age. A linear equation was found to give the best fit (n = 32, r = 0.85 and n = 32, r = 0.89 for the SDFT (solid line) and CDET (dashed line), respectively). Levels of pentosidine did not differ between tendons.
FIGURE 4.
Tissue fluorescence of the SDFT (♦) and CDET (×) as a function of horse age. A linear equation was found to give the best fit (n = 32, r = 0.88 and n = 32, r = 0.82 for the SDFT (solid line) and CDET (dashed line), respectively). Tissue fluorescence increased at a significantly lower rate in the CDET compared with the SDFT (p < 0.001).
Markers of Collagen Degradation
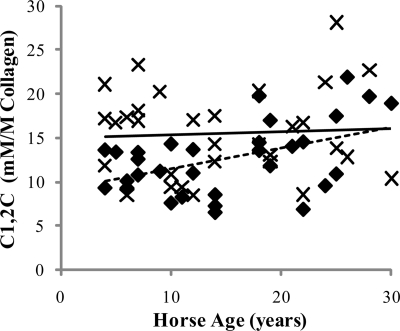
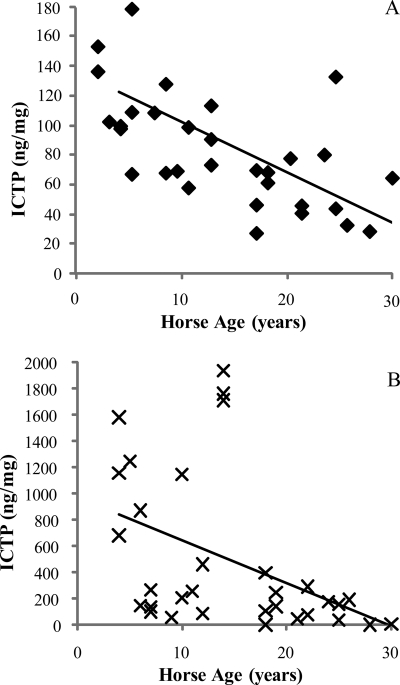
Levels of the collagenase-generated neoepitope (C1,2C) within tendon tissue were significantly (p = 0.007) higher for the CDET than the SDFT (Table 2) and increased significantly with age in the SDFT (p = 0.008) (Fig. 5). The amount of ICTP extractable from the matrix using GdnHCl was significantly (p < 0.001) greater for the CDET than the SDFT (Table 2) and decreased significantly with horse age for both tendon types (p ≤ 0.004) (Fig. 6).
FIGURE 5.
Concentration of the collagenase-generated neoepitope (C1,2C) in the SDFT (♦) and CDET (×) as a function of horse age. A linear equation gave the best fit (n = 32, r = 0.46 for the SDFT (solid line); C1,2C levels were not correlated with age in the CDET (dashed line)). C1,2C levels were significantly higher in the CDET compared with the SDFT (p = 0.007).
FIGURE 6.
A and B, concentration of ICTP extracted from the SDFT (♦) (A) and CDET (×) (B) as a function of horse age. A linear equation was found to give the best fit (n = 32, r = −0.64 and n = 32, r = −0.50 for the SDFT and CDET, respectively). The amount of ICTP extracted from the matrix was significantly greater in the CDET than in the SDFT (p < 0.001).
DISCUSSION
In this study, we aimed to assess molecular matrix age by calculating protein half-life in equine tendons using the racemization of aspartic acid as a probe. It is widely accepted that tendon pathology is a degenerative condition resulting in an accumulation of micro-damage and a diminution of mechanical competence, although the nature of the micro-damage is not clear. Matrix turnover rate gives an indication of the ability of the tenocytes to repair any micro-damage and is therefore of particular relevance to the accumulation of damage with aging in specific tendons. A small amount of d-Asp indicates relatively rapid protein turnover and short residence time, suggesting the cells are able to renew the matrix components. A higher level of d-Asp accumulation indicates that protein turnover is slower, and so it will be more difficult for cells to repair any damage to the matrix, which may then accumulate over time. The results of our study suggest a half-life of 198 and 34 years for the SDFT and CDET collagenous matrix, respectively, and 2.2 and 3.5 years, respectively, for the non-collagenous matrix components. No previous studies have calculated protein half-life in tendon using aspartate racemization, however the d/l ratios have been measured in human supraspinatus and biceps tendon tissue, and the levels recorded (approximately 1%) were similar to those obtained in our study (30) without separation into collagen and non-collagenous components. Collagen half-life has been calculated in other tissues; Sivan et al. (14) reported a half-life of 95–215 years in intervertebral disc collagen depending on donor age group, whereas Verzijl et al. (15) reported 117 years in articular cartilage. Non-collagenous protein half-life has also been calculated in other tissues, with values of 3–25 years reported for aggrecan fractions in articular cartilage (11) and 6–26 years for intervertebral disc aggrecan (12). The half-life values of collagenous and non-collagenous matrix components calculated in these studies are in the same region as the values we report.
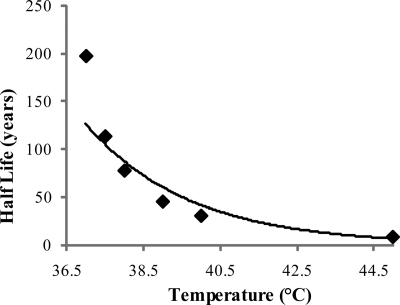
Our half-life value of almost 200 years for the collagenous component of the SDFT suggests that only 0.25% of the collagen is renewed each year. Thus, in biological terms, turnover is insignificant and the collagen is essentially inert. The absolute values however reported in our study and others rely on assumptions made in the calculations, as the accumulation of d-Asp is affected by several factors, including temperature, pH, and protein structure; increasing temperature by 4 °C causes the rate constant of racemization to double. We based our calculations on a tendon temperature of 37 °C and assumed that the pH in dentin and tendon were not significantly different. Tendons in the equine forelimb are likely to be exposed to a wide range of temperatures; during exercise the SDFT has been shown to experience temperatures as high as 45 °C (22), and this temperature variation may alter the rate constant of racemization. The difference in the calculated half-life is significant; for example a 1 °C increase would change the half-life of the collagenous matrix in the SDFT from 198 years to 77 years, whereas an increase to 45 °C would decrease the half-life to 8 years (Fig. 7). However we consider that the half-life of the majority of tendon collagen is likely to be closer to the higher values calculated.
FIGURE 7.
Effect of temperature on predicted average collagen half-life in the SDFT. An exponential equation was found to give the best fit (r = 0.99).
Regardless of absolute values, our studies most interestingly show a clear difference between tendon types, in relation to collagen and non-collagen turnover, which was not appreciated when the whole tissue was analyzed (as shown in Fig. 2). The half-life of the collagenous matrix in the SDFT was approximately five times greater than in the CDET, supporting our hypothesis. The lower tissue fluorescence in the CDET in this study and our previous work also suggests a higher rate of matrix turnover. Based on our results, the lower tissue fluorescence is likely to be due to a combination of the higher rate of collagen turnover in the CDET and the lower levels of the fluorescent pyridinoline cross-links. Pentosidine is also widely accepted as a marker of matrix age and in our study showed a good correlation with d/l aspartate ratios in the whole tissue and an age-related accumulation but did not demonstrate the difference between tendon types. Pentosidine is present at relatively low levels (1 per 70 collagen molecules) and is also not specific for collagen; pentosidine has also been shown to accumulate with age in aggrecan from cartilage (31) and intervertebral discs (12) hence a difference between tendon types is not apparent using pentosidine as a marker of matrix age.
A lower rate of collagen turnover in the high strain SDFT compared with the low strain CDET seems counterintuitive, and there are several possible explanations for this. The difference may reflect a compromise of the synthetic ability of tenocytes within the SDFT due to physiochemical factors such as nutrient availability and oxygen tension. However, other investigators have suggested that collagenous structures, which require very high mechanical strength, are protected from re-modeling that may weaken the structure (32). In a comparable manner, the collagenous matrix in the SDFT may not be turned over in an attempt to preserve its structural integrity and keep it within the narrow range of strength and stiffness required for optimal energy storage. Alternatively, differences in the way that the tendons are loading during high speed locomotion may result in a predisposition to collagen damage over proteoglycan damage in the CDET. This possibility is supported by the lower half-life of the non-collagenous component of the SDFT compared with the CDET and previous work of others showing a high rate of proteoglycan turnover in flexor tendon (33). The viscoelastic behavior of tendon is complex and depends on the rate of deformation. Studies in rat tail tendon have shown that at small strain rates the response is dominated by the elastic component, whereas at high strain rates the response is dominated by viscosity, which is largely provided by the proteoglycan matrix (34). In addition to higher strains, the SDFT is loaded during the stance phase of gallop and is subjected to a faster rate of loading than the CDET.
The levels of collagen degradation markers measured in our study provide further evidence to support the concept of a lower rate of collagen turnover in the SDFT. Interestingly the levels of neoepitope show that ∼1.5% of the collagen molecules have been cleaved by collagenase; a value much higher than the racemization data suggests for turnover. This value of 1.5% is likely to be an underestimate as once the collagen triple helix has been cleaved the exposed neoepitope will be susceptible to other proteases. These data therefore suggest that in the SDFT collagen is enzymatically cleaved but remains in the matrix and continues to undergo age-related racemization and glycation. The significantly higher ICTP levels extracted from the CDET support the half-life data and suggest that collagen turnover occurs more rapidly in this tendon. Age-related changes to the matrix reduce the extractability of cleaved products from the matrix as demonstrated by the reduction in levels of ICTP extracted from the tendon tissue in both tendon types with increasing age.
Although our study separates collagen and non-collagenous components of the matrix, there may, in addition, be pools of collagen that differ in their turnover rate, which we are not able to distinguish between in this study. The extraction technique used in this study does not retain all of the collagen; a small amount of soluble collagen (<13%) is extracted from the matrix, which is likely to represent a more labile pool of collagen. However, the insoluble collagen does also appear to be slowly replaced giving support to other studies that have detected collagen synthesis and degradation in tendon (5, 16, 35) but are not able to distinguish between different pools of collagen. The collagenases (MMP1, -8, and -13) are the only enzymes able to cleave the collagen triple helix; however, collagen fibrils are notably more resistant to collagenolysis than lone collagen monomers (36). The architecture of the fibrils is important as only those collagen molecules on the surface of the fibril are accessible for enzymatic degradation (36). Therefore, areas within the tendon where collagen fibrils are of a smaller diameter, such as inter-fascicular collagen, might be expected to have a much higher turnover rate than intra-fascicular collagen. In the same manner, tendons with smaller collagen fibril diameters, hence a larger surface area, might be expected to show a higher rate of turnover. However this does not appear to be the case as the SDFT has a lower mass average fibril diameter than the CDET (37) but a lower rate of collagen turnover, implying other control mechanisms. Computational and visualization studies suggest that the C-terminal telopeptide of collagen must be proteolytically cleaved before collagenase can gain access to the cleavage site (36). MMP3 can act as a telopeptidase (38), and this action may be the initiating event representing a possible control point.
Age-related modifications to tendon collagen, as identified in our study, are of biological significance if they change the mechanical behavior of the tendon. Although the racemization of amino acids is a useful marker of protein age the effect on mechanical properties is likely to be small. Molecular dynamics calculations suggest that the intermediate formed in the racemization reaction is energetically unfavorable within the constraints of the collagen triple helix, hence racemization is likely to be confined to the telopeptide regions of the molecule (39). In any case, the amounts of the d-form of aspartic acid compared with the l-form are very small. Glycation of collagen is much more likely to have an effect on tendon properties. AGE cross-links can form at numerous lysine and hydroxylysine residues throughout the collagen triple helix. Although the levels of pentosidine are relatively low (1 per 70 collagen molecules in our study) the recently discovered lysine-arginine cross-link derived from glucose, namely glucosepane, reaches levels comparable to those of the lysyl oxidase-derived cross-links (1–5 mol/mol collagen) (40). In vitro studies have shown that glycation of rabbit Achilles tendon increases the stiffness and strength of the tendon (41), and it is well documented that AGEs are strongly associated with diabetic complications (42) such as stiffening of collagenous tissues. However in our previous studies we have not been able to show an age-related increase in equine tendon stiffness, which may now be explained by the accumulation of fragmented collagen, which would have the opposite effect of reducing tendon stiffness.
Interestingly, although our data show age-related associations with matrix modifications, there is considerable individual variation between individual horses. In our previous studies we have also found a wide variation in material properties of tendons from different horses, which are not related to horse size or exercise history (37), indicating that some individuals may be more at risk of tendon damage than others.
In conclusion, our data has revealed new evidence concerning the nature of age-related tendon micro-damage by showing that partially cleaved collagen remains in the matrix and levels increase with age in the SDFT. These data suggest that any mechanically induced micro-damage would not be removed completely and replaced with new collagen. The accumulation of partially degraded collagen has implications for the mechanical integrity and function of tendon, which may explain the increased incidence of tendon injury with increasing age.
Supplementary Material
Acknowledgment
We are grateful to Brendan Jackson for technical assistance with the ICTP assay.
This work was supported by a welfare grant from The Horse Trust, UK (Registered Charity Number 231748).

The on-line version of this article (available at http://www.jbc.org) contains supplemental text, references, and Figs. S1–S5.
- SDFT
- superficial digital flexor tendon
- CDET
- common digital extensor tendon
- AGE
- advanced glycation end-product
- HP
- hydroxylysyl pyridinoline
- LP
- lysyl pyridinoline
- ICTP
- cross-linked C-terminal telopeptide of type I collagen
- GdnHCl
- guanidine hydrochloride
- GAG
- glycosaminoglycan
- HPLC
- high-performance liquid chromatography.
REFERENCES
- 1.Ker R. F. (2002) Comp. Biochem. Phys. A 133, 987–1000 [DOI] [PubMed] [Google Scholar]
- 2.Biewener A. A. (1998) Comp. Biochem. Phys. B 120, 73–87 [DOI] [PubMed] [Google Scholar]
- 3.Birch H. L., Worboys S., Eissa S., Jackson B., Strassburg S., Clegg P. D. (2008) Matrix Biol. 27, 182–189 [DOI] [PubMed] [Google Scholar]
- 4.Scott J. E. (2003) J. Physiol. 553, 335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller B. F., Olesen J. L., Hansen M., Døssing S., Crameri R. M., Welling R. J., Langberg H., Flyvbjerg A., Kjaer M., Babraj J. A., Smith K., Rennie M. J. (2005) J. Physiol. 567, 1021–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rees J. D., Wilson A. M., Wolman R. L. (2006) Rheumatology 45, 508–521 [DOI] [PubMed] [Google Scholar]
- 7.Birch H. L., Bailey J. V., Bailey A. J., Goodship A. E. (1999) Equine Vet. J. 31, 391–396 [DOI] [PubMed] [Google Scholar]
- 8.Lin Y. L., Brama P. A., Kiers G. H., van Weeren P. R., DeGroot J. (2005) J. Vet. Med. A 52, 333–338 [DOI] [PubMed] [Google Scholar]
- 9.Birch H. L., Smith T. J., Draper E. R., Bailey A. J., Avery N. C., Goodship A. E. (2006) Matrix Biol. 25, 74 [Google Scholar]
- 10.Bada J. L. (1984) Methods Enzymol. 106, 98–115 [DOI] [PubMed] [Google Scholar]
- 11.Maroudas A., Bayliss M. T., Uchitel-Kaushansky N., Schneiderman R., Gilav E. (1998) Arch. Biochem. Biophys. 350, 61–71 [DOI] [PubMed] [Google Scholar]
- 12.Sivan S. S., Tsitron E., Wachtel E., Roughley P. J., Sakkee N., van der Ham F., DeGroot J., Roberts S., Maroudas A. (2006) J. Biol. Chem. 281, 13009–13014 [DOI] [PubMed] [Google Scholar]
- 13.Sivan S. S., Tsitron E., Wachtel E., Roughley P., Sakkee N., van der Ham F., DeGroot J., Maroudas A. (2006) Biochem. J. 399, 29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sivan S. S., Wachtel E., Tsitron E., Sakkee N., van der Ham F., DeGroot J., Roberts S., Maroudas A. (2008) J. Biol. Chem. 283, 8796–8801 [DOI] [PubMed] [Google Scholar]
- 15.Verzijl N., DeGroot J., Thorpe S. R., Bank R. A., Shaw J. N., Lyons T. J., Bijlsma J. W., Lafeber F. P., Baynes J. W., TeKoppele J. M. (2000) J. Biol. Chem. 275, 39027–39031 [DOI] [PubMed] [Google Scholar]
- 16.Langberg H., Skovgaard D., Petersen L. J., Bulow J., Kjaer M. (1999) J. Physiol. 521, 299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frisbie D. D., Al-Sobayil F., Billinghurst R. C., Kawcak C. E., McIlwraith C. W. (2008) Osteoarthr. Cartilage 16, 1196–1204 [DOI] [PubMed] [Google Scholar]
- 18.Birch H. L., Bailey A. J., Goodship A. E. (1998) Equine Vet. J. 30, 534–539 [DOI] [PubMed] [Google Scholar]
- 19.Yekkala R., Meers C., Hoogmartens J., Lambrichts I., Willems G., Van Schepdael A. (2007) J. Sep. Sci. 30, 118–121 [DOI] [PubMed] [Google Scholar]
- 20.Helfman P. M., Bada J. L. (1976) Nature 262, 279–281 [DOI] [PubMed] [Google Scholar]
- 21.Fanibunda K. B. (1986) J. Dent. 14, 160–164 [DOI] [PubMed] [Google Scholar]
- 22.Wilson A. M., Goodship A. E. (1994) J. Biomech. 27, 899–905 [DOI] [PubMed] [Google Scholar]
- 23.Bada J. L., Kvenvolden K. A., Peterson E. T. (1973) Nature 245, 308–310 [Google Scholar]
- 24.Maroudas A., Palla G., Gilav E. (1992) Connect. Tissue Res. 28, 161–169 [DOI] [PubMed] [Google Scholar]
- 25.Becher C., Springer J., Feil S., Cerulli G., Paessler H. (2008) BMC Musculoskel. Disord. 9, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey A. J., Sims T. J., Avery N. C., Halligan E. P. (1995) Biochem. J. 305, 385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bergman I., Loxley R. (1963) Analyt. Chem. 35, 1961–1965 [Google Scholar]
- 28.Bannister D. W., Burns A. B. (1970) Analyst 95, 596–600 [DOI] [PubMed] [Google Scholar]
- 29.Farndale R. W., Buttle D. J., Barrett A. J. (1986) Biochim. Biophys. Acta 883, 173–177 [DOI] [PubMed] [Google Scholar]
- 30.Riley G. P., Curry V., DeGroot J., van El B., Verzijl N., Hazleman B. L., Bank R. A. (2002) Matrix Biol. 21, 185–195 [DOI] [PubMed] [Google Scholar]
- 31.Verzijl N., DeGroot J., Bank R. A., Bayliss M. T., Bijlsma J. W., Lafeber F. P., Maroudas A., TeKoppele J. M. (2001) Matrix Biol. 20, 409–417 [DOI] [PubMed] [Google Scholar]
- 32.Laurent G. J. (1987) Am. J. Physiol. 252, 1–9 [DOI] [PubMed] [Google Scholar]
- 33.Rees S. G., Flannery C. R., Little C. B., Hughes C. E., Caterson B., Dent C. M. (2000) Biochem. J. 350, 181–188 [PMC free article] [PubMed] [Google Scholar]
- 34.Puxkandl R., Zizak I., Paris O., Keckes J., Tesch W., Bernstorff S., Purslow P., Fratzl P. (2002) Philos. T. Roy. Soc. B 357, 191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langberg H., Rosendal L., Kjaer M. (2001) J. Physiol. 534, 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perumal S., Antipova O., Orgel J. P. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 2824–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birch H. L. (2007) Int. J. Exp. Pathol. 88, 241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J. J., Lark M. W., Chun L. E., Eyre D. R. (1991) J. Biol. Chem. 266, 5625–5628 [PubMed] [Google Scholar]
- 39.van Duin A. C. T., Collins M. J. (1998) Organic Geochem. 29, 1227–1232 [Google Scholar]
- 40.Sell D. R., Biemel K. M., Reihl O., Lederer M. O., Strauch C. M., Monnier V. M. (2005) J. Biol. Chem. 280, 12310–12315 [DOI] [PubMed] [Google Scholar]
- 41.Reddy G. K., Stehno-Bittel L., Enwemeka C. S. (2002) Arch. Biochem. Biophys. 399, 174–180 [DOI] [PubMed] [Google Scholar]
- 42.Monnier V. M., Sell D. R., Dai Z., Nemet I., Collard F., Zhang J. (2008) Ann. N. Y. Acad. Sci. 1126, 81–88 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.