Abstract
T cell receptor-stimulated NF-κB activation requires CARMA1 and is negatively regulated by the deubiquitinase CYLD. Recent studies suggest that CARMA1 regulates regulatory T cell (Treg) development, although the role of NF-κB in this event is incompletely understood. We show that CYLD deficiency causes constitutive NF-κB activation in thymocytes, which is associated with enhanced frequency of Treg cells. The NF-κB activation in CYLD-deficient thymocytes is independent of CARMA1, because the NF-κB activation was also detected in CYLD/CARMA1 double knock-out thymocytes. Interestingly, although loss of CYLD causes NF-κB activation in the CARMA1-deficient thymocytes, the CYLD deficiency fails to rescue the defect of CARMA1 knock-out mice in Treg development. Furthermore, inhibition of canonical NF-κB by an IκBα transgene only partially inhibits Treg development. We demonstrate that CARMA1 regulates IL-2 receptor signaling and controls the IL-2-stimulated maturation of Treg precursors to mature Tregs. These results suggest that the role of CARMA1 in Treg regulation involves both NF-κB activation and IL-2 receptor signaling.
Keywords: Immunology, NF-κB, Signal Transduction, T-cell Receptor, Thymocyte, CARMA1, CYLD, IkB, Treg, IL-2 Signaling
Introduction
Regulatory T cells (Tregs)4 play critical roles in maintaining self-tolerance and preventing autoimmunity (1, 2). There are two major types of Tregs: thymic-driven naturally occurring Tregs (nTregs) and Tregs that are inducibly developed from naïve CD4 T cells in the periphery (iTregs) (2). Tregs express cell surface markers, particularly CD4 and CD25, along with the lineage-specific transcription factor, Foxp3. Expression of functional Foxp3 is required for the development and function of Tregs, as well as the maintenance of self-tolerance. Indeed, both mice and humans with Foxp3 mutations develop autoimmune phenotype with marked hyperactivation of T cells and tissue inflammation (3–5).
Despite significant research efforts directed at understanding the process of Treg development, the underlying mechanism is still incompletely understood. T cell receptor (TCR) signal is one of the key requirements for Treg development (1, 2). Studies using TCR transgenic mice suggest the requirement of cognate antigens in nTreg production (6–8). Additionally, common-gamma chain (γc) cytokines, particularly IL-2, provide the second critical signal for nTreg development (9). The deficiency of IL-2 or its receptor components, CD25 and IL-2Rβ, leads to drastic reduction in nTregs, although two other γc cytokines, IL-7 and IL-15, also contribute to the nTreg development (10–12). Consistently, STAT5, a signaling molecule downstream of γc-receptors, also has a non-redundant role in Treg development, because its disruption leads to complete depletion of Tregs (13). Recent studies suggest a two-step model in nTreg development (14, 15). The first step is generation of CD25+Foxp3− Treg precursors, which is instructed by the TCR signal. In the second step, the Treg precursors further develop into mature CD25+Foxp3+ Tregs in a TCR-independent and IL-2-dependent manner.
The signaling molecules that regulate the developmental steps of Tregs are still poorly defined. Recently, a scaffold protein, CARMA1, has been shown to play a critical role in nTreg development (16–18), although it is dispensable for iTreg production (16). CARMA1 is known as a signaling molecule that mediates TCR-stimulated activation of the transcription factor NF-κB (19, 20). TCR engagement leads to activation of protein kinase C theta, which then phosphorylates CARMA1 and triggers recruitment of Bcl10 and MALT1 to form the CARMA1-Bcl10-MALT1 (CBM) signaling complex (21, 22). The CBM complex activates the IκB kinase (IKK), leading to phosphorylation and degradation of the NF-κB inhibitor, IκBα, and nuclear translocation of NF-κB (23). This signaling pathway involves lysine 63 (K63) ubiquitination of different signaling components and is negatively regulated by a K63-specific deubiquitinase, CYLD (24). CYLD deficiency causes constitutive activation of NF-κB in thymocytes and peripheral T cells (25).
The requirement of CARMA1 in nTreg development suggests a role for NF-κB activation in this biological process. However, because CARMA1 is dispensable for iTreg development (16), precisely how CARMA1 and NF-κB regulate the different steps in Treg development is unclear. In this study, we examined the role of CARMA1 and NF-κB in Treg development using different mouse models. We show that loss of CYLD causes constitutive NF-κB activation in a CARMA1-independent manner, which is associated with enhanced nTreg frequencies in the thymus and spleen. Interesting, however, the NF-κB activation is insufficient for rescuing the defect of the CARMA1−/− thymocytes in nTreg development, because the CYLD−/−CARMA1−/− double knock-out mice show a similar level of nTreg deficiency to that observed in the CARMA1−/− mice. Furthermore, blockade of canonical NF-κB activation by an IκBα transgene in thymocytes only partially inhibited nTreg development. We provide evidence that CARMA1 has an important role in IL-2-stimulated maturation of Treg precursors and that CARMA1 is required for IL-2R signaling. Our data thus suggest that the role of CARMA1 in Treg regulation involves both NF-κB activation and IL-2R signaling.
EXPERIMENTAL PROCEDURES
Mice
The CARMA1 knock-out mice (in C57BL6/129 genetic background) were provided by Dr. Josef Penninger (26), and the CYLD knock-out mice (in C57BL6/DBA genetic background) were described previously (27). Heterozygous breeding was performed to generate control (+/+) and knock-out (−/−) mice. To generate CARMA1/CYLD dKO mice, the CARMA1 and CYLD KO mice were first bred to produce CARMA1+/−CYLD+/− mice, which were then further intercrossed to produce CARMA1−/−CYLD−/− double knock-out (dKO) and CARMA1+/+CYLD+/+ wild-type (WT) mice. Age- and sex-matched control, KO, and dKO mice were used in the experiments. CARMA1−/− mice were also mated to Foxp3-GFP mice (Jackson Laboratory) to generate CARMA1+/−Foxp3-GFP mice, which were then intercrossed to generate CARMA1+/+Foxp3-GFP and CARMA1−/−Foxp3-GFP littermates. IκBαΔNtg mice (in C57BL6 genetic background) were obtained from Jackson Laboratory {C57BL/6-Tg (LCK-NFKBIA)5Dwb/J}. These are transgenic mice expressing a degradation-resistant form of IκBα (IκBαΔN) under the control of the T-cell specific Lck proximal promoter and a CD2 locus control region (28). The IκBαΔNtg mice were bred with C57BL/6 mice to generate IκBαΔNtg and littermate control non-transgenic mice. All mice were maintained in the specific pathogen-free facility of The University of Texas MD Anderson Cancer Center, and all animal experiments were done in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center.
Antibodies and Reagents
Fluorescence-labeled anti-mCD4 (L3T4; eFluor® 450), anti-mCD8 (53–6.7), anti-mCD25 (PC61.5), anti-mCD45.1 (A20), and anti-mFoxp3 (FJK-16s) were purchased from eBioscience. Fluorescence-labeled anti-phospho-STAT5(pY694) (clone 47) was from BD Biosciences, and non-conjugated anti-phospho-STAT5(pY694), anti-STAT5, and anti-phospho-JAK1 (Tyr-1022/1023) were from Cell Signaling Technology. Antibodies for CARMA1 (CARD11, sc-20458), JAK1, and tubulin (TU-02) were from Santa Cruz Biotechnology. Human IL-2 was provided by NCI Biological Resources Branch.
Flow Cytometry and Cell Sorting
Cells from thymus, spleen, and lymph nodes were prepared via gentle homogenization of organs over nylon mesh (Small Parts, Inc.). Mononuclear cells were isolated after RBC lysis (Sigma) and debris filtration over nylon mesh. Flow cytometry data were acquired on LSRII (BD Bioscience) and analyzed using FlowJo (TreeStar). To purify splenic T cells, anti-CD90 magnetic beads (Miltenyi) were used. For isolating purified nTreg precursor populations, CD4 single positive thymocytes were first enriched by depleting CD8+ cells using CD8 MicroBeads (Miltenyi). Tregs (CD4+CD8−CD25+GFP+) and nTreg precursors (CD4+CD8−CD25+GFP− and CD4+CD8−CD25−GFP−) were isolated by flow cytometry sorting using FACSAria (BD Bioscience). Purity of isolated population was verified to be >98%.
Cytokine Stimulation
For Foxp3 induction, purified nTreg precursors were incubated for 5 days in the presence of hIL-2 (50 units/ml). Foxp3 staining was performed according to manufacturer's recommendations (eBioscience). To examine cytokine signaling, cells were stimulated with indicated amounts of hIL-2 for 20–30 min at 37 °C. For immunoblotting (IB), cells were washed in cold phosphate-buffered saline (PBS) and rapidly lysed to obtain whole cell lysates. For flow cytometry, cells were washed in cold PBS and fixed in 4% paraformaldehyde for 10 min at 37 °C. Cells were spun down and permeabilized in ice-cold 90% methanol for 30 min on ice. Following two washes with incubation buffer (PBS + 0.5% bovine serum albumin), cells were stained with p-STAT5 antibody and other appropriate cell surface antibodies for 1 h at room temperature. Cells were washed twice with incubation buffer and data were acquired by flow cytometry.
Real-time Quantitative RT-PCR
Total RNA was isolated using TRI reagent (Molecular Research Center, Inc.) and subjected to cDNA synthesis using RNase H-reverse transcriptase (Invitrogen) and oligo (dT) primers. Real-time quantitative PCR was performed in triplicates, using iCycler Sequence Detection System (Bio-Rad) and iQTM SYBR Green Supermix (Bio-Rad). The expression of individual genes was calculated by a standard curve method and normalized to the expression of GAPDH. The murine IL-2 primers were 5′-CCTGAGCAGGATGGAGAATTACA-3′ (forward) and 5′-TCCAGAACATGCCGCAGAG-3′ (reverse).
RESULTS
Loss of CYLD Causes Constitutive Activation of NF-κB in a CARMA1-independent Manner
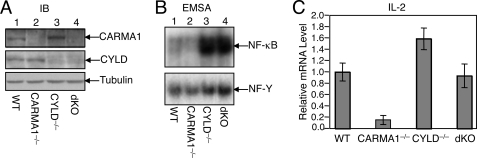
We have previously shown that CYLD-deficient thymocytes and peripheral T cells display constitutive NF-κB activity (25). Loss of CYLD causes ubiquitination and activation of Tak1, as well as its downstream kinase, IKK. These findings suggest that deregulated ubiquitination of the Tak1/IKK signaling complex may trigger constitutive activation of NF-κB, although the involvement of homeostatic TCR signaling cannot be excluded. To examine the latter possibility, we crossed the CYLD knock-out mice with CARMA1 knock-out mice to generate age-matched WT, CYLD−/−, CARMA1−/−, and CYLD−/−CARMA1−/− dKO mice (Fig. 1A). Because CARMA1 is essential for TCR-stimulated NF-κB activation, the NF-κB activity should be at least partially reduced in the dKO thymocytes if the TCR signaling is indeed involved. As we previously reported (25), the CYLD−/− thymocytes had high levels of constitutive NF-κB DNA binding activity in the nucleus (Fig. 1B, lane 3), whereas the CARMA1−/− thymocytes displayed reduced nuclear NF-κB activity (Fig. 1B, lane 2). Interestingly, potent constitutive NF-κB activity was also detected in the CYLD/CARMA1 dKO thymocytes (Fig. 1B, lane 4). These findings suggest that loss of CYLD leads to NF-κB activation independently of CARMA1 and probably the TCR signal.
FIGURE 1.
Loss of CYLD causes CARMA1-independent NF-κB activation and IL-2 gene expression in thymocytes. A, cell lysates were prepared from thymocytes of WT, CARMA1−/−, CYLD−/−, or CARMA1−/−CYLD−/−dKO mice. IB was performed to detect CARMA1, CYLD, and the loading control tubulin. B, nuclear extracts were prepared from the indicated mouse strains and subjected to EMSA using 32P-radiolabeled probes for NF-κB or the control nuclear protein, NF-Y. C, total RNA was isolated from the thymocytes of the indicated mouse strains and subjected to IL-2 real-time PCR. Data are presented as mean ± S.D. of fold of induction compared with the WT sample.
The CARMA1-deficient thymocytes had reduced expression of the IL-2 gene, as determined by real-time PCR analyses (Fig. 1C). Consistent with their hyperactivation of NF-κB, the CYLD-deficient thymocytes had increased levels of IL-2 gene expression. Furthermore, the level of IL-2 gene expression in the CYLD/CARMA1 dKO thymocytes was comparable to that of the WT cells (Fig. 1C). Thus, loss of CYLD not only caused activation of NF-κB but also rescued the expression of IL-2 in the CARMA1-deficient thymocytes.
Activation of NF-κB in CARMA1−/− Thymocytes Can Not Rescue Their Defect in Treg Development
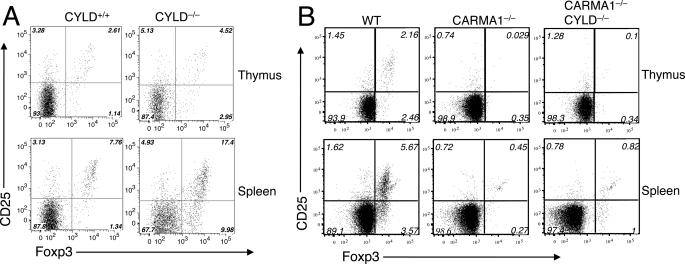
Recent studies suggest a critical role of CARMA1 in regulating the development of nTregs, although not the inducible production of iTregs (16–18). Because an important role of CARMA1 is to mediate NF-κB activation by the TCR, we reasoned that the NF-κB signaling defect in these mutant cells might contribute to the blockade of nTreg development. The CYLD/CARMA1 dKO mice provided a powerful tool to examine this possibility. We performed flow cytometry assays to determine the frequency of CD4+CD25+Foxp3+ Treg cells in both the thymus and spleen of the control and knock-out mice. Consistent with the involvement of NF-κB in Treg development (29–33), CYLD−/− mice displayed increased frequency of Treg cells in both the thymus and the spleen (Fig. 2A). It is important to note, however, the increased Treg frequency in CYLD−/− mice could be due to the reduction in the conventional T cells, because the absolute number of Tregs was even lower than that of control mice (data not shown). Nevertheless, these data suggest that in contrast to its requirement in conventional thymocyte development (27), CYLD is dispensable for Treg cell production.
FIGURE 2.
CYLD deficiency increases the frequency of Treg cells but does not rescue the Treg defect of CARMA1−/− mice. A, thymocytes (upper panels) and splenocytes (lower panels) of CYLD homozygous knock-out (CYLD−/−) and age-matched control (CYLD+/+) mice were subjected to flow cytometry for analyzing the frequency of Treg cells based on expression of CD25 and Foxp3. The numbers indicate percentages of the specific subsets within CD4+CD8−-gated population. Data are representative of three experiments with 3 or more mice per group. B, thymocytes and splenocytes of age-matched wild-type controls (WT), CARMA1−/−, and CARMA1−/−CYLD−/− double knock-out mice were subjected to Treg analysis as in A.
We next examined whether the loss of CYLD could rescue the Treg defect in CARMA1−/− mice. As previously reported, the CARMA1−/− mice had a severe reduction in the frequency of Treg cells in both the thymus and the spleen (Fig. 2B). To our surprise, the CYLD/CARMA1 dKO mice still showed a drastic reduction in the frequency of Tregs, despite the competent NF-κB activation and IL-2 gene expression in their thymocytes (Fig. 1, B and C). These results suggest that NF-κB activation is insufficient for rescuing the Treg development defect of the CARMA1−/− mice.
Inhibition of Canonical NF-κB by IκBα Partially Diminishes Treg Development
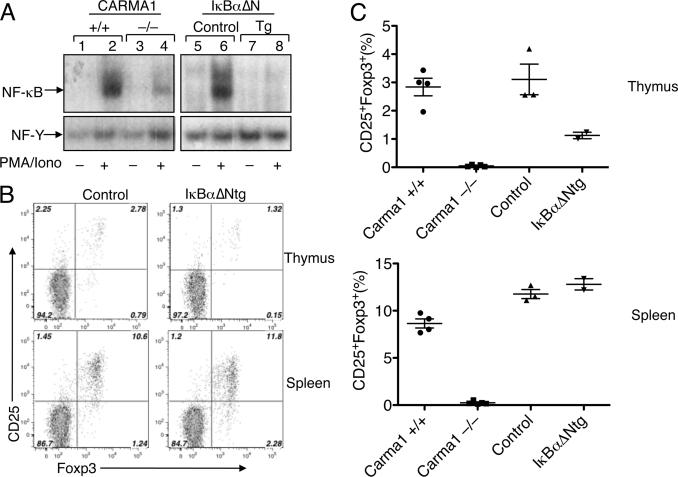
To further examine the role of NF-κB in Treg development, we employed transgenic mice expressing a degradation-resistant form of IκBα (IκBαΔNtg) (28). We compared the NF-κB activation and Treg development between the IκBαΔNtg and CARMA1−/− mice. As expected, mitogen-stimulated NF-κB activation was attenuated in the CARMA1−/− thymocytes, and a more severe defect in NF-κB activation was detected in the IκBαΔNtg thymocytes (Fig. 3A). Interestingly, despite their severe defect in NF-κB activation, the IκBαΔNtg mice had only a partial reduction in the thymic Treg cells and no reduction in the splenic Treg cells (Fig. 3, B and C). As seen with the CARMA1−/− mice (Fig. 2B), the IκBαΔNtg mice had a lower frequency of CD25+Foxp3− cells in the thymus (Fig. 3B), which are known as thymic Treg precursor cells (14, 15). However, the IκBΔNtg mice displayed a much milder reduction in the frequency of CD25+Foxp3+ mature Treg cells than that observed in CARMA1−/− mice (compare Figs. 3, B and C and 2B). These results, together with those obtained with the CYLD/CARMA1 dKO mice (Fig. 2B), suggest that the role of CARMA1 in Treg development involves mechanisms other than NF-κB activation.
FIGURE 3.
Inhibition of NF-κB by IκBα transgene does not block Treg development. A, total thymocytes from CARMA1+/+, CARMA1−/−, control (no IκBαΔN transgene), and IκBαΔNtg mice were subjected to nuclear extract preparation following PMA/ionomycin stimulation. EMSAs were performed using 32P-radiolabeled probes for NF-κB (upper panels) and a constitutive transcription factor, NF-Y (lower panels). B, thymocytes (upper panels) or splenocytes (lower panels) from control and IκBαΔNtg mice were subjected to flow cytometry to analyze the Treg frequency as described in Fig. 2. C, thymocytes or splenocytes from the indicated mice were analyzed for frequency of CD25+Foxp3+ Tregs within CD4+CD8− populations. Data are presented as mean ± S.D. of multiple mice.
CARMA1 Is Required for IL-2-stimulated Maturation of Treg Precursors
Recently, a two-step model of nTreg development has been proposed (14, 15). In the first step, TCR signaling induces the generation of CD4+CD25+Foxp3− Treg precursors that become sensitive to IL-2 stimulation. In the second step, which is TCR-independent, these precursor cells respond to IL-2 stimulation to acquire the ability to express Foxp3 and become the mature CD4+ CD25+Foxp3+ Tregs. As a critical regulator of TCR-induced NFκB activation, CARMA1 played a role in regulating the generation of Treg precursors (Fig. 2B and Ref. 18). However, the moderate reduction in Treg precursor frequency could not account for the severe mature Treg deficiency in CARMA1−/− mice, because the IκBαΔNtg mice also had reduced frequency of Treg precursors despite having normal frequency of mature Tregs (Fig. 3B, upper panel).
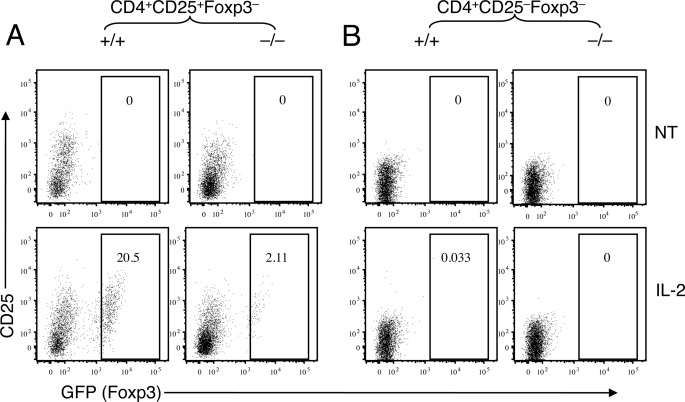
We next examined whether CARMA1 plays a role in the maturation of Treg precursor cells. To facilitate the purification of nTreg precursor populations, we crossed the CARMA1−/− mice to Foxp3-GFP reporter mice. We purified the CD4+CD8−CD25+GFP− Treg precursor cells and the CD4+CD8−CD25−GFP− control cells by flow cytometric sorting from the thymocytes of CARMA1+/+Foxp3-GFP and CARMA1−/−Foxp3-GFP mice. Purified Treg precursors were incubated in vitro in the presence of IL-2 without further TCR stimulation. Consistent with prior studies (14, 15), IL-2 induced Foxp3 expression in the CD4+CD8−CD25+GFP− Treg precursor cells (Fig. 4A, left panels) but not in the CD4+CD8−CD25−GFP− thymocytes (Fig. 4B, left panels). Interestingly, the IL-2-stimulated Foxp3 expression was significantly diminished in the CARMA1−/− CD4+CD8−CD25+GFP− Treg precursor cells (Fig. 4A, right panels). These results thus reveal a critical role for CARMA1 in regulating IL-2-stimulated maturation of the Treg precursor cells.
FIGURE 4.
CARMA1 is required for IL-2-stimulated maturation of Treg precursors. Thymocytes were prepared from CARMA1+/+Foxp3-EGFP or CARMA1−/−Foxp3-EGFP mice and subjected to flow cytometric sorting to purify Foxp3− (GFP-negative) cell populations. The sorted CD4+CD25+Foxp3− (A) and CD4+CD25−Foxp3− (B) cells were cultured in vitro in the presence of IL-2. After 5 days, the percentages of Foxp3+ (GFP-positive) Tregs were determined in each culture conditions by flow cytometry. The percentage of Treg cells was indicated.
CARMA1 KO Mice Have IL-2 Signaling Defect
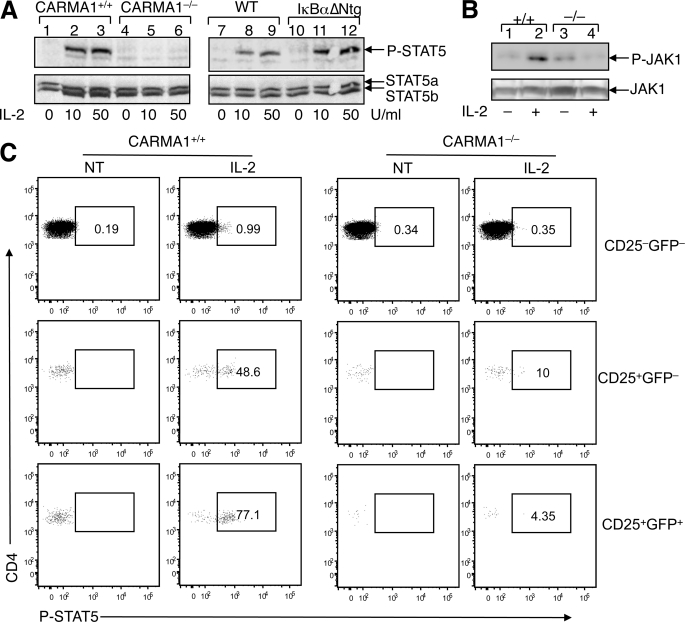
Because CARMA1−/− Treg precursors showed diminished response to IL-2, we examined the potential involvement of CARMA1 in IL-2R signaling. Total thymocytes were isolated from CARMA1+/+ and CARMA1−/− mice and stimulated with IL-2, followed by examining STAT5 phosphorylation by IB (Fig. 5A). In WT thymocytes, IL-2 stimulated strong tyrosine phosphorylation of STAT5 (Fig. 5A, lanes 1–3). Remarkably, this signaling event was severely attenuated in the CARMA1−/− thymocytes (Fig. 5A, lanes 4–6). This signaling defect was unlikely due to the blockade of typical NF-κB activation, because competent IL-2R signaling was detected in IκBαΔNtg thymocytes (Fig. 5A, lanes 10–12) despite their lack of NF-κB activation (Fig. 3A). To understand the molecular mechanism by which Carma1 regulates IL-2 signaling, we examined IL-2-stimulated phosphorylation of an upstream kinase, JAK1. As seen with STAT5 phosphorylation, the IL-2-stimulated tyrosine phosphorylation of JAK1 was defective in CARMA1−/− cells (Fig. 5B). We next analyzed the IL-2 signaling pathway in different nTreg precursors. For these studies, thymocytes from CARMA1+/+Foxp3-GFP and CARMA1−/−Foxp3-GFP mice were stimulated with IL-2 and subjected to phospho-flow cytometry to measure the phosphorylation of STAT5. In CARMA1+/+ CD4 single positive thymocytes, both the CD25+GFP− Treg precursor cells and the CD25+GFP+ mature Tregs rapidly phosphorylated STAT5 upon IL-2 stimulation, although the CD25−GFP− cells barely responded to IL-2 (Fig. 5C). Moreover, in CARMA1−/− thymocytes, IL-2 signaling was significantly attenuated in both the Treg precursor and mature Treg populations (Fig. 5C). Together, these results suggest that CARMA1 plays an important role in regulating IL-2 signaling in nTreg precursors and mature Treg cells.
FIGURE 5.
CARMA1−/− nTreg precursors have IL-2 signaling defect. A, total thymocytes were isolated from CARMA1+/+ and CARMA1−/− mice or IκBαΔNtg and its control non-transgenic mice. The cells were stimulated with the indicated doses of IL-2 for 20 min and lysed for IB analysis of tyrosine-phosphorylated STAT5 (P-STAT5) (upper panel). The IB membrane was re-probed with regular anti-STAT5 to show the expression level of STAT5a and STAT5b (lower panel). B, total thymocytes from CARMA1+/+ (+/+) and CARMA1−/− (−/−) mice were incubated for 20 min either in the absence (−) or presence (+) of IL-2 (50 units/ml). JAK1 was isolated by IP (using anti-JAK1) followed by IB to detect phosphorylated JAK1 (P-JAK1) using anti-phospho-JAK1 (Tyr-1022/1023) (upper panel). Total JAK1 level in cell lysates was detected by IB using anti-JAK1 (lower panel). C, CARMA1+/+Foxp3-EGFP and CARMA1−/−Foxp3-EGFP thymocytes were stimulated with IL-2 (50 units/ml) for 30 min and immediately subjected to phospho-flow cytometry to detect tyrosine-phosphorylated STAT5 (P-STAT5) within the subsets of CD4+ single positive thymocyte populations. GFP was used as a marker for Foxp3 expression. Cells were gated on the CD25−GFP− cells, the CD25+GFP− Treg precursors, and the CD25+GFP+ mature Tregs. The percentage of P-STAT5-positive cells within each of the subsets is indicated.
DISCUSSION
nTreg development requires a set of signaling events, most notably engagement of the TCR and stimulation by IL-2 (1). The TCR and IL-2 signals regulate the generation of Treg precursors and their subsequent maturation, respectively (14, 15). In this report, we provide evidence that CARMA1, a signaling molecule known to mediate TCR-stimulated NF-κB activation, regulates IL-2 receptor signaling and the maturation of Treg precursor cells. We found that the CARMA1−/− mice have a moderate reduction in the frequency of nTreg precursor cells; however, the CARMA1-deficient Treg precursors are attenuated in IL-2-stimulated maturation, leading to an almost complete loss of mature Tregs in the CARMA1−/− thymocytes and splenocytes.
NF-κB was initially implicated in the regulation of Treg development based on the finding that genetic deficiency in upstream signaling factors, such as protein kinase C theta and CBM components, reduces Treg frequency (2). While our work was under review, several recent reports provided further evidence for the involvement of NF-κB in nTreg development (29–33). However, how NF-κB exerts its function in Treg development is still incompletely understood. A previous study suggests that the IκBαΔNtg mice do not show reduced frequency of CD4+CD25+ T cells in the periphery (34). Consistently, our current study reveals that the IκBαΔNtg mice have normal frequency of peripheral Tregs and moderately reduced frequency of thymic Tregs. The similar phenotype was also detected in a related transgenic mouse that expresses the IκB super-repressor (IκB-SR Tg) (31). These results are in sharp contrast to the near complete loss of thymic and splenic Treg cells in the CARMA1−/− mice. Of note, the IκBαΔNtg thymocytes have a similar or higher level of NF-κB defect than the CARMA1−/− thymocytes. It is thus logical to propose that the role of CARMA1 in Treg regulation may involve both NF-κB activation and additional mechanisms.
We have now obtained genetic and biochemical evidence for the involvement of CARMA1 in IL-2R signaling. Our data suggest that CARMA1 regulates the maturation of Treg precursors, a step of Treg development that is independent of TCR function but is dependent on IL-2R signaling (14, 15). Indeed, the CARMA1−/− thymocytes are severely attenuated in IL-2R signaling. Although how CARMA1 regulates IL-2R signaling remains to be further examined, it seems that canonical NF-κB is not involved because inhibition of NF-κB activation by the IκBαΔN transgene has no effect on IL-2R signaling. On the other hand, our data cannot exclude the involvement of an atypical NF-κB signaling pathway in CARMA1-mediated IL-2R signaling and Treg maturation. In this regard, T cells with knock-in of a degradation-resistant mutant of the IκB-like molecule p105 have severe defect in IL-2R signaling (35), although it is unclear whether the p105 pathway contributes to CARMA1-mediated IL-2R signaling and Treg maturation. It is also unknown whether p105 directly regulates IL-2R signaling or controls the expression of an IL-2R signaling component. We have found that the CARMA1−/− thymocytes have reduced frequency of cells that express CD25, the α-subunit of the high-affinity IL-2R. However, the reduced frequency of CD25+ cells cannot be a major reason for their IL-2R signaling defect, because the similar defect was detected in purified CD4+CD25+ CARMA1−/− thymocytes. Moreover, the IκBαΔNtg thymocytes also have reduced frequency of CD25+ cells, but they are fully competent in IL-2R signaling. Obviously, additional studies are needed to better understand how CARMA1 regulates IL-2R signaling.
A major IL-2R downstream signaling event is activation of STAT5, a molecular event that is also critical for Treg development. The finding that CARMA1 regulates IL-2-stimulated STAT5 phosphorylation is consistent with the defect of the CARMA1−/− CD25+Foxp3− Treg precursors in IL-2-stimulated maturation. However, regulation of STAT5 activation may not be the only function of CARMA1 in Treg development, since overexpression of a constitutively active form of STAT5 could not rescue the defect of the CARMA1−/− mice in Treg production (18). Based on the previous findings and our current data, it is conceivable that CARMA1 may regulate both IL-2R signaling and NF-κB activation, thereby achieving a critical role in the Treg developmental program.
The CYLD/CARMA1 dKO mice provide a useful model for studying the mechanism of Treg development as well as the mechanism of NF-κB regulation by deubiquitination. We found that loss of CYLD causes constitutive activation of NF-κB even in CARMA1−/− thymocytes. Because CARMA1 is essential for NF-κB activation by the TCR, our finding suggests a TCR-independent mechanism of NF-κB activation in CYLD-deficient cells. We propose that CYLD negatively regulates the homeostatic ubiquitination and, thus, the activation of IKK signaling components. This hypothesis is also supported by our previous finding that CYLD knockdown or knock-out in T cells causes spontaneous ubiquitination of TRAF2 and TAK1 (25, 36). The increased Treg frequency in CYLD−/− mice supports a role for NF-κB in promoting Treg development. However, it is important to note that the Treg phenotype of CYLD−/− mice differs significantly from that of mice overexpressing a constitutively active IKKβ (IKKEE-Tg) (31). The IKKEE-Tg mice have abnormally high levels of Foxp3+ thymocytes, but these cells do not express CD25 or display Treg functions. In contrast, the CYLD−/− Treg cells have typical surface markers and suppress the proliferation of naive CD4 T cells in vitro (data not shown). Moreover, while the CYLD deficiency fails to rescue the Treg defect in CARMA1−/− mice, the IKKEE transgenic expression induces Foxp3 expression even in the absence of CARMA1 (31). It is possible that different NF-κB signaling pathways are activated in CYLD−/− and IKKEE-Tg thymocytes, thus contributing to their phenotypic differences. However, it is also likely that the overexpression of IKKEE may drive the expression of Foxp3 independently of the IL-2 signal. These possibilities remain to be examined in future studies. Notwithstanding, our current work clearly demonstrates the involvement of CARMA1 in both the TCR-dependent and the IL-2-dependent steps of Treg development.
Acknowledgments
We thank Dr. J. M. Penninger for providing the CARMA1 mice and the NCI Biological Resources Branch for providing human IL-2. We also thank the flow cytometry core facilities of MD Anderson Cancer Center for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants AI064639, GM084459, and GM084459S1.
- Treg
- regulatory T cell
- TCR
- T-cell receptor
- CBM
- CARMA1-Bcl10-MALT1
- IKK
- IκB kinase
- K63
- lysine 63
- dKO
- double knock-out
- WT
- wild type
- IB
- immunoblotting
- GFP
- green fluorescent protein
- IL
- interleukin.
REFERENCES
- 1.Sakaguchi S., Yamaguchi T., Nomura T., Ono M. (2008) Cell 133, 775–787 [DOI] [PubMed] [Google Scholar]
- 2.Josefowicz S. Z., Rudensky A. (2009) Immunity 30, 616–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chatila T. A., Blaeser F., Ho N., Lederman H. M., Voulgaropoulos C., Helms C., Bowcock A. M. (2000) J. Clin. Invest. 106, R75–R81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett C. L., Christie J., Ramsdell F., Brunkow M. E., Ferguson P. J., Whitesell L., Kelly T. E., Saulsbury F. T., Chance P. F., Ochs H. D. (2001) Nat. Genet. 27, 20–21 [DOI] [PubMed] [Google Scholar]
- 5.Wildin R. S., Ramsdell F., Peake J., Faravelli F., Casanova J. L., Buist N., Levy-Lahad E., Mazzella M., Goulet O., Perroni L., Bricarelli F. D., Byrne G., McEuen M., Proll S., Appleby M., Brunkow M. E. (2001) Nat. Genet. 27, 18–20 [DOI] [PubMed] [Google Scholar]
- 6.Apostolou I., Sarukhan A., Klein L., von Boehmer H. (2002) Nat. Immunol. 3, 756–763 [DOI] [PubMed] [Google Scholar]
- 7.Jordan M. S., Boesteanu A., Reed A. J., Petrone A. L., Holenbeck A. E., Lerman M. A., Naji A., Caton A. J. (2001) Nat. Immunol. 2, 301–306 [DOI] [PubMed] [Google Scholar]
- 8.Kawahata K., Misaki Y., Yamauchi M., Tsunekawa S., Setoguchi K., Miyazaki J., Yamamoto K. (2002) J. Immunol. 168, 4399–4405 [DOI] [PubMed] [Google Scholar]
- 9.Turka L. A., Walsh P. T. (2008) Front. Biosci. 13, 1440–1446 [DOI] [PubMed] [Google Scholar]
- 10.Fontenot J. D., Rasmussen J. P., Gavin M. A., Rudensky A. Y. (2005) Nat. Immunol. 6, 1142–1151 [DOI] [PubMed] [Google Scholar]
- 11.Burchill M. A., Yang J., Vogtenhuber C., Blazar B. R., Farrar M. A. (2007) J. Immunol. 178, 280–290 [DOI] [PubMed] [Google Scholar]
- 12.Vang K. B., Yang J., Mahmud S. A., Burchill M. A., Vegoe A. L., Farrar M. A. (2008) J. Immunol. 181, 3285–3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao Z., Kanno Y., Kerenyi M., Stephens G., Durant L., Watford W. T., Laurence A., Robinson G. W., Shevach E. M., Moriggl R., Hennighausen L., Wu C., O'Shea J. J. (2007) Blood 109, 4368–4375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burchill M. A., Yang J., Vang K. B., Moon J. J., Chu H. H., Lio C. W., Vegoe A. L., Hsieh C. S., Jenkins M. K., Farrar M. A. (2008) Immunity 28, 112–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lio C. W., Hsieh C. S. (2008) Immunity 28, 100–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes M. J., Krebs P., Harris N., Eidenschenk C., Gonzalez-Quintial R., Arnold C. N., Crozat K., Sovath S., Moresco E. M., Theofilopoulos A. N., Beutler B., Hoebe K. (2009) PLoS Biol. 7, e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medoff B. D., Sandall B. P., Landry A., Nagahama K., Mizoguchi A., Luster A. D., Xavier R. J. (2009) Eur. J. Immunol. 39, 78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molinero L. L., Yang J., Gajewski T., Abraham C., Farrar M. A., Alegre M. L. (2009) J. Immunol. 182, 6736–6743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin X., Wang D. (2004) Semin. Immunol. 16, 429–435 [DOI] [PubMed] [Google Scholar]
- 20.Thome M. (2004) Nat. Rev. Immunol. 4, 348–359 [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto R., Wang D., Blonska M., Li H., Kobayashi M., Pappu B., Chen Y., Wang D., Lin X. (2005) Immunity 23, 575–585 [DOI] [PubMed] [Google Scholar]
- 22.Sommer K., Guo B., Pomerantz J. L., Bandaranayake A. D., Moreno-García M. E., Ovechkina Y. L., Rawlings D. J. (2005) Immunity 23, 561–574 [DOI] [PubMed] [Google Scholar]
- 23.Blonska M., Lin X. (2009) Immunol. Rev. 228, 199–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun S. C., Ley S. C. (2008) Trends Immunol. 29, 469–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiley W. W., Jin W., Lee A. J., Wright A., Wu X., Tewalt E. F., Leonard T. O., Norbury C. C., Fitzpatrick L., Zhang M., Sun S. C. (2007) J. Exp. Med. 204, 1475–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hara H., Wada T., Bakal C., Kozieradzki I., Suzuki S., Suzuki N., Nghiem M., Griffiths E. K., Krawczyk C., Bauer B., D'Acquisto F., Ghosh S., Yeh W. C., Baier G., Rottapel R., Penninger J. M. (2003) Immunity 18, 763–775 [DOI] [PubMed] [Google Scholar]
- 27.Reiley W. W., Zhang M., Jin W., Losiewicz M., Donohue K. B., Norbury C. C., Sun S. C. (2006) Nat. Immunol. 7, 411–417 [DOI] [PubMed] [Google Scholar]
- 28.Boothby M. R., Mora A. L., Scherer D. C., Brockman J. A., Ballard D. W. (1997) J. Exp. Med. 185, 1897–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isomura I., Palmer S., Grumont R. J., Bunting K., Hoyne G., Wilkinson N., Banerjee A., Proietto A., Gugasyan R., Li W., McNally A., Steptoe R. J., Thomas R., Shannon M. F., Gerondakis S. (2009) J. Exp. Med. 206, 3001–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jana S., Jailwala P., Haribhai D., Waukau J., Glisic S., Grossman W., Mishra M., Wen R., Wang D., Williams C. B., Ghosh S. (2009) Eur. J. Immunol. 39, 2571–2583 [DOI] [PubMed] [Google Scholar]
- 31.Long M., Park S. G., Strickland I., Hayden M. S., Ghosh S. (2009) Immunity 31, 921–931 [DOI] [PubMed] [Google Scholar]
- 32.Ruan Q., Kameswaran V., Tone Y., Li L., Liou H. C., Greene M. I., Tone M., Chen Y. H. (2009) Immunity 31, 932–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Visekruna A., Huber M., Hellhund A., Bothur E., Reinhard K., Bollig N., Schmidt N., Joeris T., Lohoff M., Steinhoff U. (2010) Eur. J. Immunol. 40, 671–676 [DOI] [PubMed] [Google Scholar]
- 34.Zhou P., Balin S. J., Mashayekhi M., Hwang K. W., Palucki D. A., Alegre M. L. (2005) J. Immunol. 174, 3447–3453 [DOI] [PubMed] [Google Scholar]
- 35.Sriskantharajah S., Belich M. P., Papoutsopoulou S., Janzen J., Tybulewicz V., Seddon B., Ley S. C. (2009) Nat. Immunol. 10, 38–47 [DOI] [PubMed] [Google Scholar]
- 36.Reiley W., Zhang M., Wu X., Graner E., Sun S. C. (2005) Mol. Cell. Biol. 25, 3886–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]