Abstract
Thyroid eye disease (TED) is a debilitating disorder characterized by the accumulation of adipocytes and hyaluronan (HA). Production of HA by fibroblasts leads to remarkable increases in tissue volume and to the anterior displacement of the eyes. Prostaglandin D2 (PGD2), mainly produced by mast cells, promotes orbital fibroblast adipogenesis. The mechanism by which PGD2 influences orbital fibroblasts and their synthesis of HA is poorly understood. We report here that mast cell-derived PGD2 is a key factor that promotes HA biosynthesis by orbital fibroblasts. Primary orbital fibroblasts from TED patients were isolated and used to test the effects of PGD2, prostaglandin J2, as well as prostaglandin D receptor (DP) agonists and antagonists on HA synthesis. The expression of HA synthase (HAS), hyaluronidase, DP1, and DP2 mRNA levels was assessed by PCR. Small interfering RNAs against HAS1 or HAS2 were used to assess the importance of HAS isoforms on HA production. Treatment of human orbital fibroblasts with PGD2 and PGJ2 increased HA synthesis and HAS mRNA. HAS2 was the dominant isoform responsible for HA production by PGD2. The effect of PGD2 on HA production was mimicked by the selective DP1 agonist BW245C. The DP1 antagonist MK-0524 completely blocked PGD2-induced HA synthesis. Human mast cells (HMC-1) produced PGD2. Co-culture of HMC-1 cells with orbital fibroblasts induced HA production and inhibition of mast cell-derived PGD2 prevented HA synthesis. Mast cell-derived PGD2 increased HA production via activation of DP1. Selectively targeting the production of PGD2 and/or activation of DP1 may prevent pathological changes associated with TED.
Keywords: Extracellular Matrix/Hyaluronan, G Protein-coupled Receptors (GPCR), Adenylate Cyclase (Adenylyl Cyclase), Cyclic AMP (cAMP), Fibroblast, Prostaglandins, siRNA, DP1, Thyroid Eye Disease, Hyaluronan Synthase
Introduction
Graves disease is an autoimmune disorder in which 40–60% of patients develop Graves ophthalmopathy, also called thyroid eye disease (TED).3 TED is characterized by expansion of the orbital fat compartment and extraocular muscles (1). Intense inflammation and infiltration of immune cells, including T cells, macrophages, and mast cells, in the retrobulbar space of TED patients are key factors that drive the proliferation and differentiation of orbital fibroblasts to adipocytes (2, 3). In addition to the adipogenic potential of orbital fibroblasts, these cells are also key producers of extracellular matrix glycosaminoglycans. One of the key pathological findings in TED patients is the overproduction and accumulation of the glycosaminoglycan hyaluronan (HA). The extremely hydrophilic nature of HA leads to remarkable increases in tissue volume and to the anterior displacement of the eye, or exophthalmos (4), resulting in the disfigurement and vision impairment (5) characteristic of TED.
HA is synthesized as an acidic, negatively charged, high molecular weight polysaccharide via the actions of hyaluronan synthases (HAS) (6), of which there are three isoforms: HAS1, HAS2, and HAS3. Increased HA synthesis closely correlates with the expression levels of HAS (7), which are themselves induced by growth factors, cytokines (7–9), and prostaglandins (PGs) (10, 11). One PG that may have an important implication in TED is PGD2. PGD2 is a metabolite of arachidonic acid that is formed by the actions of cyclooxygenases and PGD2 synthases (PGDS) (12, 13). Many of the biological actions of PGD2 are mediated through two G protein-coupled receptors: DP receptor 1 (DP1) and DP2 (also called chemoattractant receptor-homologous molecule (CRTH2)) (14–16). These receptors elicit divergent effects by the coupling to either Gs (DP1) or Gi (DP2) to elevate cyclic AMP (cAMP) or intracellular calcium (Ca2+), respectively. PGD2 can also spontaneously undergo a series of dehydration reactions to form the PGJ family of prostaglandins, including 15d-PGJ2, an endogenous ligand for the peroxisome proliferator-activated receptor (PPARγ) (17, 18).
Human orbital fibroblasts express PPARγ (3), and PPARγ is crucial for the differentiation of fibroblasts to adipocytes (19). We recently published that activated human T lymphocytes isolated from patients with TED produce much more PGD2-derived PGs compared with T cells from healthy individuals (3). Mast cells are also a key cellular source of PGs, with PGD2 being the major prostanoid released (3, 20, 21). Mast cells are a central immune cell in the pathogenesis of TED. Not only is there intense mast cell infiltration and degranulation (22) associated with adipocytes (23) in TED patients, but co-culture of mast cells with orbital fibroblasts up-regulates HA synthesis (24). Whether this increase in HA production by orbital fibroblasts is the result of PGD2 acting via the direct modulation of DP receptors is not known. Herein, we report for the first time that PGD2 increases HA synthesis in orbital fibroblasts via the induction of HAS2. Pharmacological inhibition of DP1, but not DP2, prevented the PGD2-induced up-regulation of HA. We also show that inhibition of PGD2 synthesis by mast cells prevents HA synthesis by orbital fibroblasts. These results have important implications for future therapies directed against treating those afflicted with TED. Preventing PGD2 synthesis by mast cells and/or DP1 activation on orbital fibroblasts may reduce the severity of the disease.
EXPERIMENTAL PROCEDURES
Reagents
PGD2, PGJ2, MK0524, ramatroban (BAY-u3405), BW 245C, 4-benzhydryloxy-1-[3-(1H-tetrazol-5-yl)-propyl]-piperidine (HQL-79), 13,14-dihydro-15-keto-PGD2, anti-DP1, anti-hematopoietic type PGDS (H-PGDS), anti-Cox-1 (cyclooxygenase-1), and anti-Cox-2 antibodies were purchased from Cayman Chemical (Ann Arbor, MI). Forskolin, A23187, and isobutylmethylxanthine (IBMX) were purchased from Sigma. Anti-DP2 antibody was purchased from R&D Systems (Minneapolis, MN). All of the drugs were dissolved in DMSO.
Tissue Collection and Cell Culture
Orbital Fibroblasts
Primary orbital fibroblasts were isolated from TED patients undergoing orbital decompression surgery. The protocols for orbital biopsy and blood sample (see below) were approved by the internal review board, and informed, written consent was obtained from all patients. The primary fibroblasts were established by standard explant techniques (25, 26) and cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT), 2-mercaptoethanol (Eastman Kodak Co.), l-glutamine (Invitrogen), HEPES (U.S. Biochemical Corp.), nonessential amino acids, sodium pyruvate, and gentamicin (Invitrogen). Fibroblasts were characterized by their adherent morphology; expression of vimentin and types I and III collagen; and absence of CD45, factor VIII, or cytokeratin. Fibroblasts were used at the earliest passage possible (between passages 4 and 10).
Human T Cells
Lymphocytes were isolated from 60 ml of peripheral blood obtained during orbital decompression surgery. Whole blood was separated over a Ficoll-Paque Plus gradient (Amersham Biosciences) to obtain peripheral blood mononuclear cells. T cells were enriched using CD3/CD28 T cell Expander beads (Invitrogen). Specifically, 5 × 106 lymphocytes were incubated with CD3/CD28 beads at a 1:1 ratio in RPMI 1640 with 10% FBS medium at 37 °C for 2 days. After that, 50 units/ml recombinant interleukin-2 was added to the culture and incubated for another 2–3 days. On day 5, cells were diluted to a concentration of 0.5 × 106 cells/ml in medium containing 50 units/ml recombinant interleukin-2 and incubated for an additional 3–7 days. Cellular purity was assessed using an anti-CD3-PE antibody (BD Biosciences, San Jose, CA) and analyzed on a FACSCalibur flow cytometer (BD Biosciences). The T-cell purity was >95% (data not shown).
Co-culture of Mast Cells with Orbital Fibroblasts
HMC-1 mast cells were allowed to proliferate in Iscove's modified Dulbecco's medium enriched with 5% FBS. Co-cultures were initiated by introducing HMC-1 cells to confluent cultures of fibroblasts as described previously (24). Mast cells were washed with RPMI 1640 medium (0.5% FBS) once before the addition to the fibroblasts (1:1 ratio unless otherwise specified). After 4 h, the medium and HMC-1 cells were removed by gently rinsing in phosphate-buffered saline, and fresh RPMI 1640 medium (0.5% FBS) was used to cover the cells. Alternatively, fibroblasts and mast cells were co-cultured in a transwell system (0.4 μm; Greiner Bio-one, New York) with fibroblasts being cultured on the bottom and the mast cells in the top chamber. The ratio of mast cells to fibroblasts was varied from 5:1 to 20:1.
Quantitation of HA
Aliquots of culture medium were removed at the indicated time points and centrifuged (5 min, 8,000 × g at 4 °C), and HA levels were quantified by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's protocol (R&D Systems). Briefly, samples were incubated in HA-binding protein-coated microwells, allowing HA in samples to react with the immobilized HA-binding protein. After the removal of unbound molecules by washing, biotinylated HA-binding protein was added to the microwells. Following a second wash, streptavidin conjugated to horseradish peroxidase was added. After the last wash, the chromogenic substrate was added, and HA levels were determined using a Varioskan Flash plate reader (Thermo Fisher Scientific, Milford, MA).
Agarose Gel Electrophoresis of HA
Fibroblasts were cultured to confluence in 10-cm2 dishes in RPMI containing 10% FBS, followed by RPMI with 2% FBS for 3 days. Fibroblasts were then treated with 5 μm PGD2 or control (DMSO) for 18 h. Cell culture supernatant was centrifuged (5 min at 8,000 × g at 4 °C), and half of the sample was digested with 1 turbidity-reducing unit/ml Streptomyces hyaluronidase (Sigma) at 37 °C for 4 h; the remainder of the sample was mock-digested. Following digestion with proteinase K (120 μg/ml) (Qiagen, Valencia, CA) at 37 °C for 4 h, the samples were concentrated using a Vivaspin 10,000 Da cut-off ultrafiltration spin column (Sartorius Stedim Biotech, Goettingen, Germany), washed with phosphate-buffered saline, and analyzed by 1% agarose electrophoresis. Select-HA HiLadder (Hyalose LLC, Oklahoma City, OK) and blue dextran (Sigma; molecular mass 2 × 106 Da) were also run as standards. The gel was stained with 0.005% Stains-All (Sigma) in 50% ethanol overnight at room temperature before being scanned on an HP scanner.
Western Blotting
Following treatments, cells were lysed in 1× SDS sample buffer, and protein concentrations were determined using the DC protein assay kit (Bio-Rad). Samples were separated on a 9 or 12% SDS-polyacrylamide gel, transferred to polyvinylidene difluoride membranes, and subjected to immunoblotting with antibodies against DP1 (1:1,000), DP2 (1:500), H-PGDS (1:500), Cox-1 (1:500), or Cox-2 (1:500). As a loading control, membranes were reprobed for glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:3,000; Calbiochem).
Measurement of cAMP
cAMP levels were quantified using a time-resolved fluorescence resonance energy transfer immunoassay from PerkinElmer Life Sciences according to the manufacturer's protocol. Briefly, orbital fibroblasts were detached with a non-enzymatic cell dissociation solution (Versene, Invitrogen) and resuspended in Stimulation Buffer (pH 7.4; 1× HBSS buffer containing 5 mmol/liter HEPES buffer and 0.01–0.1% bovine serum albumin) containing 0.5 mm IBMX and labeled with the Alexa Fluor® 647-labeled anti-cAMP antibody. Finally, the cells were incubated with drugs at the indicated times. Following the addition of the detection buffer, the amount of cAMP signal was measured.
Measurement of PGD2
HMC-1 cells (1 × 106/well) were seeded into 6-well plates and cultured in Iscove's modified Dulbecco's medium with 5% FBS in the absence or presence of HQL-79 (H-PGDS inhibitor, 100 μm) at 37 °C for 60 min. The cells were then washed and resuspended in RPMI 1640 medium with 0.5% FBS. After a 2-h incubation, the cells were stimulated with 10 μm calcium ionophore A23187 for 30 min. The culture media were collected, and PGD2 in the supernatants was quantified by PGD2-MOX EIA kits (Cayman Chemical).
Reverse Transcription (RT)-PCR and Quantitative RT (qRT)-PCR
RNA was isolated from orbital fibroblasts or T cells using an RNeasy minikit (Qiagen) and reverse transcribed to cDNA using an iScript cDNA synthesis kit (Bio-Rad) and diluted 5-fold in RNase-free H2O. Forward and reverse primers for each gene are shown in Table 1. The PCR was performed using the Qiagen Fast Cycling PCR kit for 30 cycles in a Bio-Rad thermal cycler. The PCR product was visualized on a 5% acrylamide gel stained with ethidium bromide. Amplifications without reverse transcriptase were carried out as negative controls. qRT-PCR was performed in a Bio-Rad iCycler containing B-R SYBR Green SuperMix for IQ (Quanta Biosciences, Gaithersburg, MD). Efficiency of the amplification was determined to be >90%.
TABLE 1.
Gene primers
| Gene | Primer sequence | Size |
|---|---|---|
| bp | ||
| HAS1 (NM-001523) | ||
| Forward | 5′-TGTGTATCCTGCATCAGCGGT-3′ | 172 |
| Reverse | 5′-CTGGAGGTGTACTTGGTAGCATAACC-3′ | |
| HAS2 (NM-005328) | ||
| Forward | 5′-GCCTCATCTGTGGAGATGGT-3′ | 181 |
| Reverse | 5′-ATGCACTGAACACACCCAAA-3′ | |
| HAS3 (NM-005329) | ||
| Forward | 5′-GGCATTATCAAGGCCACCTA-3′ | 184 |
| Reverse | 5′-AGGCCAATGAAGTTCACCAC-3′ | |
| HYAL1 (NM-007312) | ||
| Forward | 5′-GATGTCAGTGTCTTCGATGTGGTA-3′ | 79 |
| Reverse | 5′-GGGAGCTATAGAAAATTGTCATGTCA-3′ | |
| HYAL2 (NM-003773) | ||
| Forward | 5′-CTAATGAGGGTTTTGTGAACCAGAATAT-3′ | 79 |
| Reverse | 5′-GCAGAATCGAAGCGTGGATAC-3′ | |
| HYAL3 (NM-003549) | ||
| Forward | 5′-CCTCCAGTGCCCTCTTCC-3′ | 196 |
| Reverse | 5′-ACAAGGTCATCCTGGGACAG-3′ | |
| DP1 (NM-000953) | ||
| Forward | 5′-TCTGCGCGCTACCTTTCATG-3′ | 84 |
| Reverse | 5′-TCCTCGTGGACCATCTGGATA-3′ | |
| DP2 (NM-004778) | ||
| Forward | 5′-TTTCTCAACATGTTCGCCAG-3′ | 131 |
| Reverse | 5′-AAGCACCAGGCAGACTTTGT-3′ | |
| 7 S RNA (NR-002715) | ||
| Forward | 5′-ACCACCAGGTTGCCTAAGGA-3′ | 68 |
| Reverse | 5′-CACGGGAGTTTTGACCTGCT-3′ | |
Amplification of 7 S ribosomal RNA was carried out for each cDNA (in triplicate) for normalization. The threshold cycle number (Ct) of amplification in each sample was determined by Bio-Rad software, and the relative mRNA abundance was calculated as the Ct for amplification of a gene-specific cDNA minus average Ct for 7 S, expressed as a power of 2 (i.e. 2ΔCt).
Gene Knockdown Using siRNA
Orbital fibroblasts were cultured to 80–90% confluence and transfected with 80 nm HAS1, HAS2, or scrambled control (SC) siRNA (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) using Lipofectamine 2000 (Invitrogen). Forty-eight hours post-transfection, RPMI containing 0.5% FBS was added. Twenty-four hours later, the cells were treated with or without 5 μm PGD2 for 2 h (for qRT-PCR analysis) or 18 h (for analysis of HA secretion).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). For comparison between groups of three or more, an analysis of variance with a Newman-Keuls multiple comparison test was used to determine differences between treatments. Error bars represent the S.D. of triplicate samples. A p value of <0.05 is considered significant. All experiments were performed at least three times.
RESULTS
Prostaglandins of the D and J Series Induce HA Synthesis by Human Orbital Fibroblasts
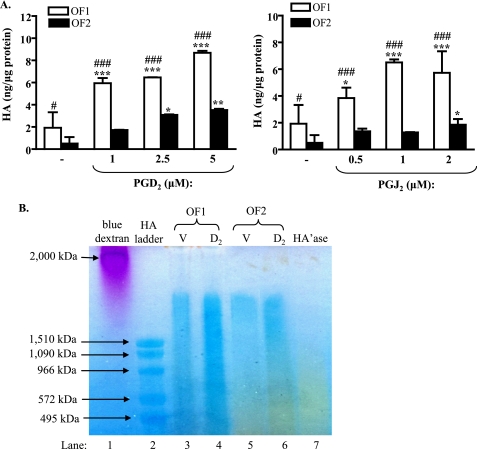
We previously reported that PGD2 and PGJ2 induce adipogenesis in human orbital fibroblasts (3). Here, we examined whether PGD2 or PGJ2 would also induce HA production. Two strains of human orbital fibroblasts (OF1 and OF2) from two different TED patients were treated with increasing concentrations of PGD2 or PGJ2 for 18 h, and HA levels were detected in the cell culture supernatant by a commercial HA ELISA. Fig. 1A shows that basal levels of HA differed significantly between the two orbital fibroblast strains (OF1 (1.9 ± 1.4 ng/μg protein) versus OF2 (0.5 ± 0.6 ng/μg protein); #, p < 0.05). Treatment of the fibroblast strain designated OF1 with either PGJ2 or PGD2 resulted in significantly more HA production compared with OF2. PGD2 and PGJ2 also significantly increased HA synthesis by both fibroblast strains in a dose-dependent manner, with 5 μm PGD2 and 2 μm PGJ2 yielding the largest induction (Fig. 1A). Higher concentrations of PGD2 or PGJ2 did not further increase HA synthesis in either of the two fibroblast strains (data not shown). Therefore, most of the remaining experiments were conducted using 5 μm PGD2 or 2 μm PGJ2.
FIGURE 1.
PGD2 and PGJ2 induce HA synthesis in human orbital fibroblasts. A, confluent strains of human orbital fibroblasts (OF1 and OF2) were cultured in RPMI 1640 with 0.5% FBS for 3 days prior to treatment with PGJ2, PGD2, or vehicle (DMSO) for 18 h. The cell culture medium was assayed for HA by an HA ELISA as described under ”Experimental Procedures.“ There was a significant increase in HA production by two strains of orbital fibroblasts following treatment with PGD2 (1–5 μm) and PGJ2 (2 μm). The experiment was performed in triplicate. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared with vehicle control. #, p < 0.05; ###, p < 0.001, OF1 versus OF2. Results are expressed as the mean ± S.D. (error bars). B, agarose gel HA analysis: Orbital fibroblasts (OF1 and OF2) were cultured in RPMI 1640 with 2% FBS for 3 days and then treated with 5 μm PGD2 (D2) or vehicle (V) for 18 h, and the conditioned medium was analyzed by agarose gel electrophoresis. Both OF1 and OF2 exhibited basal HA (lanes 3 and 5, respectively; blue). When treated with PGD2, there was an increase in color intensity, indicating increased HA production (lanes 4 and 6; compare with lanes 3 and 5). A Streptomyces hyaluronidase-digested sample (HA'ase) (from PGD2-treated OF2) was included as a negative control (lane 7).
We also performed agarose gel electrophoresis to analyze the molecular size(s) of HA produced by the orbital fibroblasts. HA is an extremely high molecular weight (Mr) polysaccharide (∼105 to 4 × 106 Da) and can be separated from other glycosaminoglycans by ultrafiltration combined with agarose gel electrophoresis (27). Fig. 1B confirms the presence of HA in the cell culture media of two fibroblast strains (OF1 and OF2) (lanes 3–6, blue). Treatment with PGD2 (D2) increased HA compared with vehicle (V) (lanes 4 and 6; compare with lanes 3 and 5). OF1 also produces relatively more low Mr HA compared with OF2. We also used Streptomyces hyaluronidase to digest the HA in the samples. Unlike other hyaluronidases, this enzyme is specific for hyaluronic acid and is inactive with chondroitin and chondroitin sulfate (28). The Streptomyces hyaluronidase-digested sample (HA'ase, lane 7) did not show any blue staining, confirming the HA specificity of the assay. Together, these results demonstrate that orbital fibroblasts increase the production of HA when exposed to PGD2.
Differential Expression of PGD2-induced Hyaluronidase (HYAL) in Two Orbital Fibroblast Strains
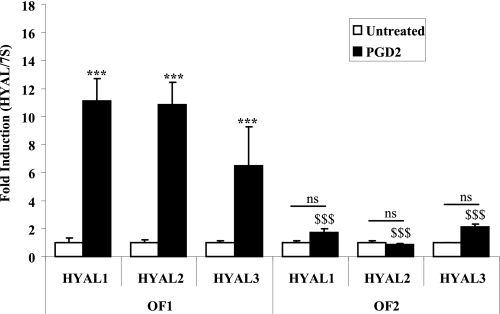
HYALs (HYAL1 to -3) depolymerize HA into low Mr polymers (29). To determine if the apparent increase in low Mr HA in PGD2-treated OF1 fibroblast strain might be the result of increased HYAL expression, we treated OF1 and OF2 with 5 μm PGD2 and assessed mRNA. There was a significant induction in HYAL1, HYAL2, and HYAL3 mRNA following treatment of OF1 with PGD2 (Fig. 2). In contrast, OF2 failed to significantly increase HYAL mRNA expression. The induction in HYAL in OF1 caused by PGD2 was significantly greater than PGD2-treated OF2 HYAL mRNA levels (Fig. 2). These results are the first to demonstrate regulation of HYAL mRNA by PGD2 in human orbital fibroblasts.
FIGURE 2.
Differential induction in HYAL mRNA between two orbital fibroblast strains. cDNA from orbital fibroblast strains OF1 and OF2 treated with 5 μm PGD2 or vehicle (Untreated) for 4 h was assessed by qRT-PCR. There was a significant increase in mRNA expression for all three HYAL isoforms (HYAL1, HYAL2, and HYAL3) when OF1 was treated with 5 μm PGD2 (***, p < 0.0001 compared with untreated). There was no significant increase in HYAL1 to -3 in PGD2-treated OF2 cells (ns, compared with untreated). The induction of HYAL1, HYAL2, and HYAL3 mRNA in PGD2-treated OF1 was significantly higher than PGD2-exposed OF2 HYAL expression ($$$, p < 0.0001 for each respective HYAL). Results are expressed as the mean ± S.D. (error bars) (n = 3).
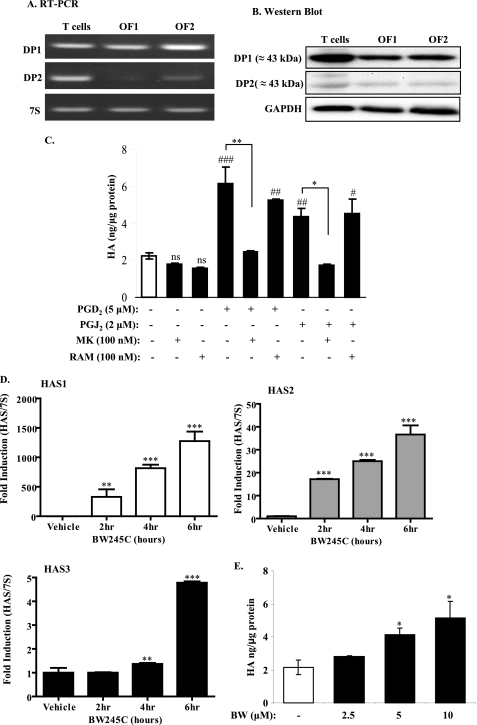
PGD2 Induces HAS mRNA Expression in Human Orbital Fibroblasts
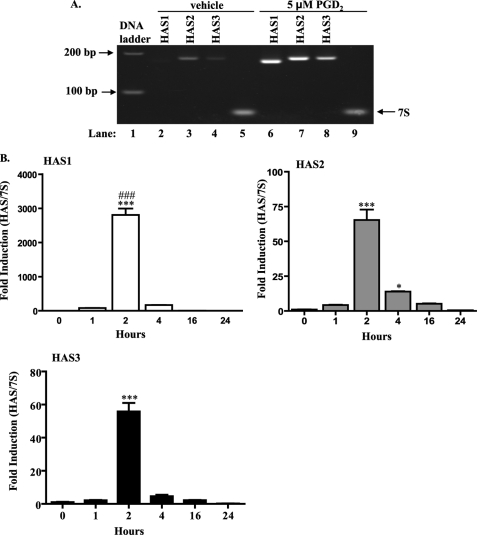
We next evaluated the effect of PGD2 on HAS mRNA expression. Fig. 3A shows that all three HAS genes are expressed in orbital fibroblasts. In untreated cells (vehicle), HAS1 mRNA is very low and scarcely detectable. In contrast, HAS2 and HAS3 mRNA are relatively greater. Following 2 h of PGD2 treatment, the mRNA for all three HAS isoforms increased significantly (Fig. 3, A and B), with HAS1 exhibiting the greatest induction. The increase in HAS1 at 2 h was significantly greater compared with either HAS2 or HAS3 (Fig. 3B; ###, p < 0.001). The mRNA for all three HAS isoforms declined rapidly, where by 16 h, there was no significant difference compared with untreated (0 h). 7 S ribosomal mRNA expression remained the same, regardless of treatment (Fig. 3A, compare lanes 5 and 9) and was used to normalize the qRT-PCR results (Fig. 3B).
FIGURE 3.
PGD2 induces HAS mRNA in human orbital fibroblasts. A, RT-PCR analysis. cDNA from orbital fibroblasts treated with 5 μm PGD2 or vehicle for 2 h was amplified by RT-PCR and separated on 5% acrylamide gels. All three HAS isoforms are expressed in human orbital fibroblasts. Following treatment with PGD2, there was a relative increase in the abundance of HAS1, HAS2, and HAS3 mRNA. 7 S was used as a control. B, qRT-PCR. Total RNA from orbital fibroblasts treated with 5 μm PGD2 for 1–24 h was analyzed by qRT-PCR as described under ”Experimental Procedures.“ There was a significant increase in HAS mRNA levels (HAS1, HAS2, and HAS3) beginning at 2 h (***, p < 0.001 compared with time 0 for each HAS isoform). Of the three, HAS1 yielded the greatest increase (2807 ± 213-fold); this increase in HAS1 was significantly greater than HAS2 or HAS3 (###, p < 0.001). Expression of HAS1 and HAS2 remained significantly elevated through 4 h (*, p < 0.05). By 16 h, mRNA for HAS1, HAS2, and HAS3 was not different from control. Results are expressed as the mean ± S.D. (error bars) of triplicate samples performed on duplicate cultures.
PGD2-induced HA Production by Orbital Fibroblasts Is Dependent on HAS2 Expression
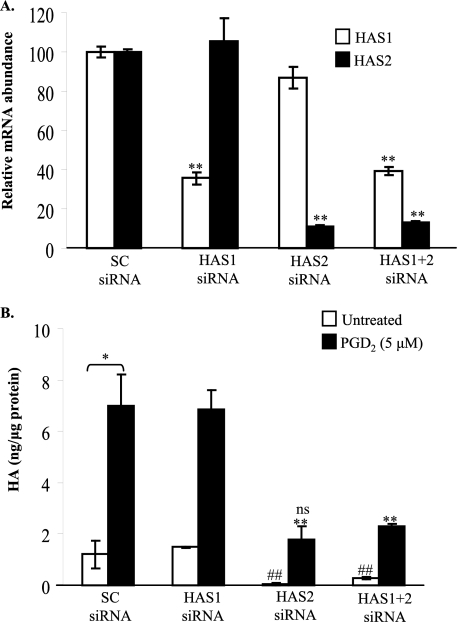
To verify whether or not HA synthesis was directly related to HAS expression, siRNA was used to selectively knock down HAS1 or HAS2 mRNA levels in fibroblasts that were treated with PGD2. Fig. 4A shows that siRNA directed against HAS1 or HAS2 selectively and significantly reduced (by ∼75–80% of mRNA levels) PGD2-induced HAS1 (open bars) and HAS2 (black bars), compared with the SC siRNA.
FIGURE 4.
PGD2-induced HA production by orbital fibroblasts is dependent on HAS2 expression. A, orbital fibroblasts were transfected with siRNA for HAS1, HAS2, or a combination as described under ”Experimental Procedures.“ The cells were cultured for 24 h in RPMI 1640 with 0.5% FBS and treated with 5 μm PGD2 for 2 h, and HAS gene levels were analyzed by qRT-PCR. The mRNA for HAS1 and HAS2 in the PGD2-treated SC siRNA samples was standardized to 100 for comparison of gene expression. Each siRNA reduced its target mRNA expression selectively and significantly (up to 80%). **, p < 0.01. B, orbital fibroblasts transfected with siRNA for HAS1, HAS2, or a combination were cultured in RPMI 1640 with 0.5% FBS for 24 h and then exposed to 5 μm PGD2 for 18 h, and HA was analyzed by HA ELISA. Fibroblasts treated with PGD2 (black bars) increased HA synthesis. Knockdown of HAS2, but not HAS1, significantly reduced the ability of PGD2 to induce HA in human orbital fibroblasts. *, p < 0.05; **, p < 0.01 compared with PGD2-treated SC siRNA-transfected; ##, p < 0.01, untreated SC siRNA versus untreated HAS2 siRNA; ns, no significance, HAS2 siRNA-transfected, PGD2-treated fibroblasts versus untreated SC siRNA-transfected cells.
We then analyzed for HA in orbital fibroblasts that were untreated (open bars) or treated with PGD2 (black bars) after transfection with siRNA against HAS1 and/or HAS2 (Fig. 4B). Those fibroblasts transfected with the SC siRNA exhibited a significant increase in HA synthesis in response to PGD2 (Fig. 4B; compare with Fig. 1A). Despite the significant increase in HAS1 mRNA induced by PGD2 (Fig. 3), attenuation of HAS1 expression did not block PGD2-induced HA production (Fig. 4B). In contrast, reduction in HAS2 mRNA expression significantly reduced both untreated and PGD2-induced HA production when compared with the SC siRNA control (Fig. 4B). This reduction in HA synthesis in the HAS2 siRNA-transfected, PGD2-treated fibroblasts was not significantly different compared with untreated SC siRNA-transfected cells (ns, p = 0.13). These data support that HAS2 is the dominant isoform responsible for the increased HA synthesis by orbital fibroblasts in response to PGD2.
PGD2 and PGJ2 Induce HA Production in Human Orbital Fibroblasts via DP1, but Not DP2, Activation
Both PGD2 and PGJ2 can exert their biological functions via activation of DP1 or DP2 (30). We therefore examined whether or not human orbital fibroblasts express DP receptors. RT-PCR and Western blot analysis revealed that orbital fibroblasts (OF1 and OF2) express both DP1 and DP2 mRNA and protein, respectively (Fig. 5, A and B). Protein expression of DP1 and DP2 in orbital fibroblasts was comparable with that of human T cells, which are well known to express DP receptors, particularly DP2 (15, 31). Both orbital fibroblast strains (OF1 and OF2) expressed considerably more DP1 mRNA and protein compared with DP2 (Fig. 5, A and B).
FIGURE 5.
PGD2 and PGJ2 induce HA production in human orbital fibroblasts via DP1, but not DP2, activation. A, RT-PCR. Human orbital fibroblasts (OF1 and OF2) and T cells express DP1 and DP2 mRNA. Note the variability in the expression of DP2 between the two fibroblast strains. B, Western blot analysis revealed that orbital fibroblasts and T cells express both DP1 and DP2 protein. Membranes were reprobed for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to ensure equal protein loading. C, pharmacological inhibition of DP1, but not DP2, blocks PGD2- and PGJ2-induced HA production. Orbital fibroblasts were left untreated (Vehicle) or were pretreated with 100 nm of the DP1 antagonist MK-0524 (MK) or DP2 antagonist ramatroban (RAM) for 1 h with or without PGD2 or PGJ2 for 18 h, and HA ELISA was performed. Both PGD2 and PGJ2 significantly increased HA production compared with untreated (p < 0.001 (###) and p < 0.01 (##), respectively). Pretreatment of orbital fibroblasts with MK significantly decreased the ability of PGD2 (**, p < 0.01) and PGJ2 (*, p < 0.05) to induce HA levels. RAM was not able to prevent PGD2- and PGJ2-increased HA levels (p < 0.05 (#) and p < 0.01 (##) compared with vehicle control, respectively). ns, not significant compared with untreated (vehicle). D, treatment with the DP1 agonist BW245C significantly increased the expression of HAS mRNA. Orbital fibroblasts were cultured in reduced serum for 3 days and exposed to BW245C (10 μm) for the indicated times. There was a significant increase in HAS1 (329 ± 128-fold increase; **, p < 0.01) and HAS2 (17 ± 0.14-fold increase; ***, p < 0.001) at 2 h compared with vehicle control. HAS3 mRNA increased by 6 h (4.8 ± 0.06; ***, p < 0.01). E, activation of DP1 by the selective agonist BW245C induces HA. There was a significant increase in HA when fibroblasts were treated with 5 and 10 μm BW245C (p < 0.05 (*) and p < 0.01 (**), respectively, compared with vehicle control. Samples were run in duplicate, utilizing three separate human orbital fibroblast strains (representative results are shown).
To determine which DP was involved in the PGD2-mediated induction of HA, we first utilized well described selective pharmacological antagonists directed against either DP1 (MK-0524) (32, 33) or DP2 (ramatroban) (30, 34). Fibroblasts were untreated or were pretreated with either MK-0524 or ramatroban for 1 h with or without PGD2 or PGJ2. Fig. 5C shows that neither MK-0524 (MK) nor ramatroban (RAM) influenced basal HA levels (no significance (ns) compared with untreated; open bar). Fibroblasts treated with either PGD2 (5 μm) or PGJ2 (2 μm) significantly increased HA synthesis (Fig. 5C; compare with Figs. 1A and 4B). Pretreatment with the DP1 antagonist MK-0524 completely blocked the ability of both PGD2 and PGJ2 to induce HA production in orbital fibroblasts. By contrast, pretreatment with the DP2 antagonist ramatroban was unable to prevent the induction of HA by PGD2 or PGJ2. This suggests that DP1 is the dominant receptor subtype involved in the induction of HA by PG of the D and J series.
To confirm the importance of DP1 in the induction of HA in human orbital fibroblasts, we utilized BW245C, a selective DP1 agonist (35), and assessed HAS mRNA expression and HA production. The results presented in Fig. 5D demonstrate that treatment with BW245C (10 μm) significantly induced HAS mRNA expression. Here, there was a significant increase in HAS1 and HAS2 mRNA expression as early as 2 h. HAS3 mRNA increased by 6 h of exposure to BW245C. In addition, BW245C dose-dependently increased HA levels (Fig. 5E). At concentrations of 5 and 10 μm, there was a significant increase in HA production, compared with untreated fibroblasts. In contrast, the high affinity selective DP2 agonist 13,14-dihydro-15-keto-PGD2 (36–38) (in the low micromolar concentration range) did not significantly increase HA levels (data not shown). Taken together, these data strongly suggest that PGD2 and PGJ2 induced HA production in orbital fibroblasts through activation of the DP1.
DP1 Activation Boosts Intracellular cAMP to Increase HA by Orbital Fibroblasts
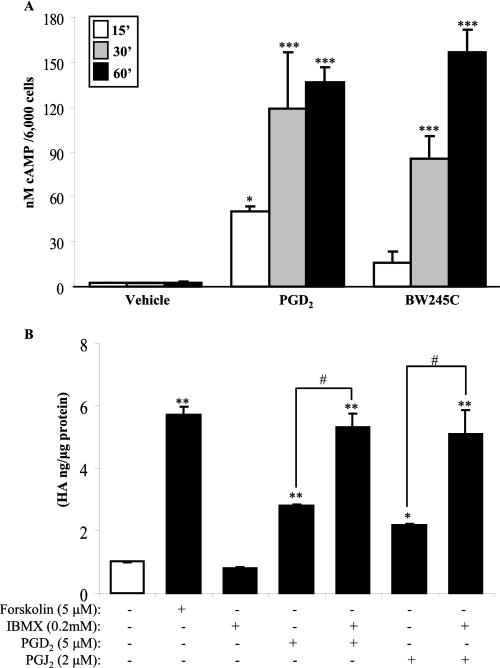
Activation of DP1, a Gs protein-coupled receptor, leads to an increase in intracellular cAMP. We hypothesized that activation of DP1 by PGD2 and subsequent production of HA would require the generation of cAMP. We therefore measured intracellular cAMP following treatment with PGD2 and the DP1 agonist BW245C. Treatment with PGD2 (5 μm) and BW245C (10 μm) significantly increased intracellular cAMP production within 15–30 min (Fig. 6A). cAMP levels remained elevated through 60 min (Fig. 6A, black bars).
FIGURE 6.
PGD2-induced HA synthesis is through the DP1-cAMP signal pathway. A, DP1 activation by PGD2 or BW245C increases intracellular cAMP level. Orbital fibroblasts were treated with PGD2 or BW245C for up to 60 min, and intracellular cAMP was detected as described under ”Experimental Procedures.“ There was a significant increase in cAMP within 15 min of treatment with PGD2 compared with vehicle control (*, p < 0.05). cAMP further increased by 30 and 60 min (***, p < 0.001). B, cultures of confluent orbital fibroblasts were treated with 5 μm forskolin or 200 μm IBMX, with or without 5 μm PGD2 or 2 μm PGJ2, for 18 h, and the cell culture supernatant was collected for HA ELISA. There was a significant increase in HA when cells were treated with forskolin, PGD2, or PGJ2 compared with vehicle-treated (open bar) (*, p < 0.05; **, p < 0.01). Augmenting cAMP (via IBMX) in conjunction with PGD2 or PGJ2 significantly increased HA when compared with PGD2 and PGJ2 alone (#, p < 0.05). Results are expressed as the mean ± S.D. (error bars).
To investigate whether or not the elevation in intracellular levels of cAMP was directly attributable to the increased HA production by orbital fibroblasts, cells were treated with forskolin, which activates adenylyl cyclase to augment intracellular levels of cAMP (39, 40). As we predicted, forskolin alone significantly increased HA production compared with vehicle-treated fibroblasts (Fig. 6B). We then treated the cells with PGD2 or PGJ2 together with IBMX, a nonselective phosphodiesterase inhibitor that boosts cAMP levels by preventing its degradation (41). IBMX, in conjunction with either PGD2 or PGJ2, significantly enhanced the ability of PGD2 or PGJ2 to generate HA (Fig. 6B). Collectively, these data support the idea that PGD2 and PGJ2, via activation of DP1 and generation of cAMP, increase HA production in human orbital fibroblasts.
Mast Cell-derived PGD2 Activates Orbital Fibroblasts to Produce HA via HAS2
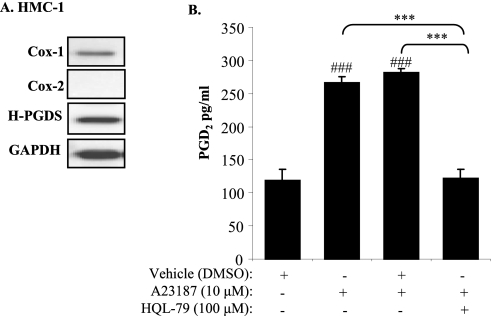
Mast cells are a key immune cell proposed to be involved in the pathogenesis of TED via their ability to activate fibroblasts. Mast cells are also a key cell type that produces PGD2 in vivo. We therefore hypothesized that mast cell-derived PGD2 would increase HA production by orbital fibroblasts. We first sought to determine if human mast cells produce PGD2 in vitro. HMC-1 cells, a commonly used human mast cell line (42, 43), were used to first examine whether mast cells express H-PGDS. Western blot analysis of unstimulated HMC-1 cells revealed that these cells basally express both Cox-1 and H-PGDS (Fig. 7A). We then activated HMC-1 cells with A23187, a calcium ionophore (42), in the presence or absence of HQL-79, a specific inhibitor of H-PGDS (44, 45), and determined PGD2 production. Fig. 7B demonstrates that activated HMC-1 cells significantly increase PGD2 compared with unstimulated cells. Inhibition of H-PGDS with HQL-79 significantly reduced the level of PGD2 in activated HMC-1 cells (Fig. 7B).
FIGURE 7.
PGD2 production by mast cells is dependent on H-PGDS activity. A, Western blot of HMC-1 cells indicates the expression of Cox-1 and H-PGDS. Unactivated HMC-1 cells do not express Cox-2. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a housekeeping protein. B, inhibition of H-PGDS activity ameliorates the production of PGD2 by activated mast cells. HMC-1 cells were treated with HQL-79 for 1 h, followed by activation with A23187, and cell culture supernatant was assessed for PGD2 levels by commercial EIA kit. Activation of HMC-1 cells with A23187 significantly increased PGD2 production (###, p < 0.001 compared with untreated). Inclusion of HQL-79 significantly reduced PGD2 production (***, p < 0.001).
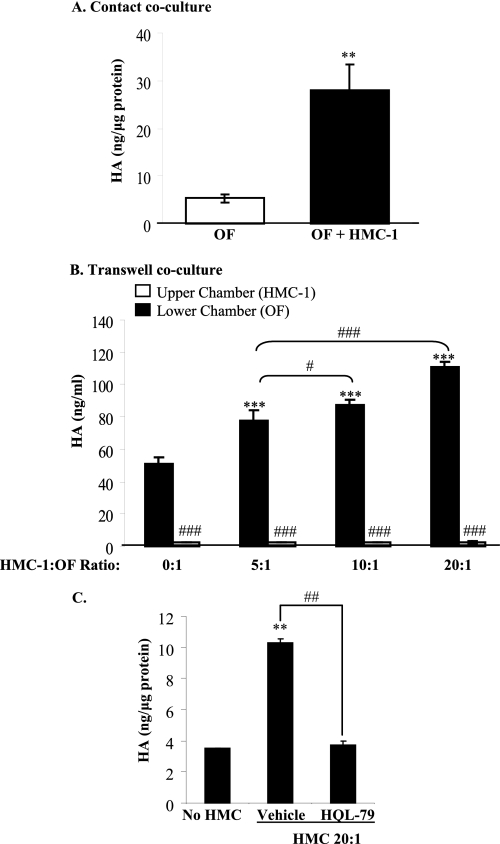
Using co-culture, we further investigated the association among mast cells, orbital fibroblasts, and HA production. First, we cultured orbital fibroblasts with HMC-1 cells (at a ratio of 1:1) for 4 h. Following the removal of the mast cells and the addition of fresh medium, the supernatant was collected for HA analysis. When fibroblasts and mast cells were cultured together, there was a significant increase in HA production compared with fibroblasts alone (Fig. 8A, open versus black bar; **, p < 0.001).
FIGURE 8.
Mast cell-derived PGD2 activates orbital fibroblasts to produce HA. A, contact co-culture. Confluent orbital fibroblasts were seeded with HMC-1 cells at a cell ratio of 1:1 for 4 h. The mast cells were then removed, the fibroblasts were washed, and fresh medium was added for another 18 h. The medium was collected for HA ELISA. Co-culture of orbital fibroblasts with HMC-1 cells significantly increased HA synthesis (**, p < 0.001). B, transwell co-culture. HMC-1 cells and confluent fibroblasts were co-cultured in a transwell system, where the fibroblasts and HMC-1 cells were separated by a 0.4-μm membrane; the HMC-1/orbital fibroblast (OF) ratio was 5:1, 10:1, or 20:1. The conditioned medium from both chambers was collected for HA ELISA. There was a significant difference in HA levels between HMC-1 (upper chamber, open bars) and orbital fibroblasts (lower chamber, black bars) (#, p < 0.05; ###, p < 0.001). Co-culture of orbital fibroblasts with HMC-1 cells significantly increased HA production only by the fibroblasts (lower chamber) (***, p < 0.001 compared with no HMC-1 cells). C, inhibition of PGD2 secretion by HMC-1 cells prevents HA production by orbital fibroblasts. HMC-1 cells were treated with the H-PGDS inhibitor HQL-79 prior to co-culturing (ratio 20:1) with orbital fibroblasts. There was a significant increase in HA synthesis when fibroblasts were cultured with HMC-1 cells (**, p < 0.01 compared with no HMC). This increase in HA was attenuated when PGD2 production in mast cells was prevented by HQL-79 (##, p < 0.001, HQL-79 compared with vehicle).
We then utilized a transwell system, whereby orbital fibroblasts were physically separated from HMC-1 cells. Following 24 h of co-culture, conditioned medium in both chambers was collected for HA ELISA. HA levels were negligible in the chamber (upper) containing only the HMC-1 cells. HA production also did not increase as a consequence of increasing cell number (Fig. 8B, open bars), suggesting that HMC-1 cells do not produce HA in significant quantities. In contrast, HA levels in the lower chamber (orbital fibroblasts) were significantly higher than in the HMC-1 (upper) chamber (Fig. 8B; ###, p < 0.001, upper chamber compared with lower chamber). Co-culture of HMC-1 cells with orbital fibroblasts significantly increased HA levels with increasing ratios of HMC-1/orbital fibroblasts. Thus, even when HMC-1 cells were physically separated from fibroblasts, HA levels increased, suggesting that a factor(s) secreted by the HMC-1 cells drives HA synthesis by fibroblasts.
To determine if PGD2 was the factor secreted by HMC-1 cells that was responsible for driving HA synthesis by orbital fibroblasts, we pretreated HMC-1 cells with HQL-79 (to block PGD2 synthesis) and then added these cells to the upper chamber of the transwell at a ratio of 20:1 (HMC-1/orbital fibroblast). Fig. 8C shows that treatment of HMC-1 cells with HQL-79 significantly attenuated the production of HA by orbital fibroblasts compared with treatment of HMC-1 cells with vehicle alone. These data suggest that PGD2 secreted by mast cells plays a dominant role in HA production by orbital fibroblasts during TED pathogenesis.
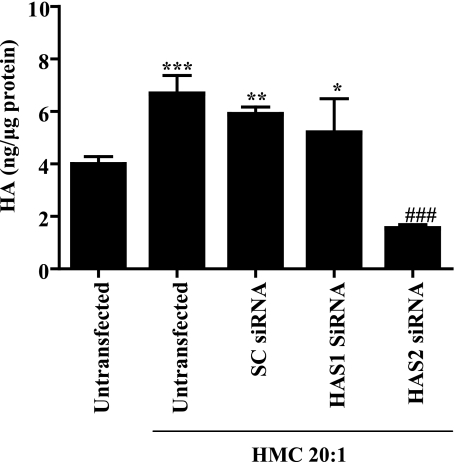
Finally, to determine whether HA production by co-culture of fibroblasts with mast cells was via HAS2, we transfected orbital fibroblasts with control, HAS1, or HAS2 siRNA and co-cultured the transfected fibroblasts with the mast cells. As expected, co-culture with untransfected fibroblasts with HMC-1 cells significantly increased HA production (Fig. 9). Transfection of fibroblasts with either control siRNA or HAS1 siRNA failed to attenuate HA production elicited by co-culture with HMC-1 mast cells. In contrast, transfection of orbital fibroblasts with HAS2 siRNA significantly decreased HA production by orbital fibroblasts. Here, there was a significant decrease in HA basal levels (untransfected, no mast cells) as well as by fibroblasts co-cultured with HMC-1 cells (Fig. 9). Collectively, these data highlight the dominant role of HAS2 in HA production elicited by mast cell-derived PGD2.
FIGURE 9.
siRNA against orbital fibroblast HAS2 prevents mast cell-derived PGD2 induction of HA. Orbital fibroblasts were transfected with HAS1, HAS2, or SC siRNA using Lipofectamine 2000 (Invitrogen). Following this, the fibroblasts were co-cultured for 24 h with HMC-1 cells in a transwell system at a ratio of 20:1 (mast cells/orbital fibroblasts), and the media were collected for HA detection. Untransfected orbital fibroblast co-cultured with HMC-1 cells significantly increased HA production (***, p < 0.0001; compared with untransfected without co-culture). Co-culture of fibroblasts with HMC-1 cells also induced a significant increase in HA in SC siRNA-transfected (**, p < 0.001) and HAS1 siRNA-transfected (*, p < 0.05) orbital fibroblasts. HAS2 siRNA-transfected orbital fibroblasts failed to increase HA synthesis when co-cultured with HMC-1 cells (###, p < 0.0001 compared with untransfected, control siRNA, and HAS1 siRNA). Results are expressed as mean ± S.D. (n = 4–6).
DISCUSSION
TED is a debilitating disorder that causes disfigurement and vision impairment. TED afflicts ∼40% of patients with Graves hyperthyroidism, a thyroid-specific autoimmune disease characterized by the presence of autoantibodies against the thyroid-stimulating hormone receptor. Treatment of moderate to severe TED often involves invasive procedures, including orbital radiotherapy and orbital decompression surgery (46). There are few effective pharmacological treatments for TED due, in part, to a poor understanding the pathogenic mechanisms leading to clinical manifestations of TED. Current evidence, including data from our laboratories (3, 47, 48), suggests that activation of orbital fibroblasts by infiltrating inflammatory cells, particularly T cells and mast cells, plays an important role in TED pathogenesis (49). Orbital fibroblast proliferation and ECM production, particularly HA (50, 51), are key events that contribute to manifestations of TED, such as periorbital edema, exophthalmos, and extraocular motility dysfunction (1, 52). Here, we have provided data indicating that mast cell-derived PGD2 is a key factor that regulates the production of HA by orbital fibroblasts via activation of DP1 (Figs. 1, 5, and 7). Importantly, prevention of DP1 signaling (Fig. 5) or PGD2 production by mast cells (Fig. 8) attenuated HA synthesis by the fibroblasts.
HA is a large negatively charged polysaccharide that is overproduced in the retroocular space of patients with TED (53). HA has remarkable viscosity and ability to retain water, which leads to increases in orbital tissue volume and anterior displacement of the eye, culminating in exophthalmos (4). Fibroblasts are the major source of HA in the orbit. Activation of orbital fibroblasts by immunoglobulins from TED patients (4) and cytokines, such as interleukin-1β (51), interferon-γ (54), and transforming growth factor-β (55), increase HA production. Our study is the first to show that PGD2, a non-cytokine mediator, contributes to HA synthesis by orbital fibroblasts (Fig. 1). The increase in HA production by PGD2 is directly related to increased expression of HAS2 (Figs. 3, 4, and 9). Despite the fact that all three HAS isoforms (HAS1, HAS2, and HAS3) are significantly increased, only the attenuation of HAS2 expression completely abrogated PGD2-induced HA. This is in agreement with observations made in HAS2−/− mouse embryos, which are virtually devoid of HA (56). Of interest, HAS1 mRNA exhibited the most dramatic increase in response to PGD2 yet does not contribute significantly to HA biosynthesis (Fig. 4). This observation may be explained by the fact that HAS1 protein rapidly loses its enzymatic activity (57). Thus, our data suggest that HAS2 is responsible for most, if not all, HA production by orbital fibroblasts in response to PGD2.
The induction of HAS mRNA and subsequent production of HA required the generation of the second messenger cAMP via activation of DP1. We are the first to show that DP receptors are expressed on orbital fibroblasts (Fig. 5). Pharmacological activation of DP1 significantly increased HAS mRNA and HA biosynthesis (Fig. 5). Moreover, the DP1 antagonist MK-0524 completely inhibited PGD2-induced HA production, whereas DP2 antagonist ramatroban had no effect. These findings suggest that PGD2-induced HA production is mediated solely by DP1. MK-0524 (also known as Laropiprant and CORDAPTIVETM) is an orally acting drug that is currently being developed in conjunction with niacin as a cholesterol-lowering drug (58). Our results indicate that selectively targeting the DP1 system (via MK-0524) in the eye may prevent the severity and/or occurrence of proptosis in TED patients by reducing HA production.
PGD2 also regulates the migration of inflammatory cells by facilitating vasodilatation and increasing vascular permeability (59). Within the eye, this would facilitate transendothelial migration of infiltrating mast cells and lymphocytes, thereby contributing to the increase in immune cells characteristic of TED. Association of mast cells with fibroblasts/adipocytes in the orbital tissue is commonly observed in biopsies of TED patients as well as animal models of TED (22, 23, 60–63). Mast cell degranulation and close proximity to adipocytes are suggestive of their participation in the TED process (23). Our data presented here strongly support the hypothesis that mast cell-fibroblast interactions participate in TED. We first demonstrated that fibroblasts robustly respond to PGD2 to increase HA synthesis (Fig. 1). Using both co-culture and transwell systems, we then showed that mast cells increase orbital fibroblast production of HA via PGD2 produced by the mast cell (Fig. 8). This accumulated HA within the orbit can, in turn, facilitate inflammatory cell infiltration (64) to the orbit, thereby perpetuating clinical symptoms associated with TED. These data, in conjunction with our previously published data showing that PGD2 promotes adipogenesis (48), highlights PGD2 as a key regulator of orbital fibroblast function.
It has been well described that fibroblasts are heterogeneous, differing not only between organ systems but also within a given organ (25, 65–68). We previously published that fibroblasts from the lung exhibit significant interindividual variability in their apoptotic response to cigarette smoke (69), a finding that may explain why only a fraction of smokers develop lung diseases, such as chronic obstructive pulmonary disease. Therefore, it is noteworthy that there was significant variability in HA production, HA size, and HYAL expression between orbital fibroblasts derived from two different individuals (Figs. 1 and 2). The increase in low Mr HA by OF1 (Fig. 1B) correlates with increased expression of HYAL1 to -3 (Fig. 2). These enzymes are responsible for the depolymerization of HA, thereby generating HA fragments (29). These low Mr HA fragments are known to promote inflammation (70) by activating immune cells and increasing inflammatory gene expression (71). Thus, increased HA production, coupled with the generation of inflammation-promoting low Mr HA, by orbital fibroblasts from an individual could be a decisive factor in predicting which Graves patients ultimately develop symptomatic TED.
Using a combination of molecular and pharmacological approaches, we have provided data on the role of mast cell-derived PGD2 in promoting HA biosynthesis by orbital fibroblasts, key effector cells in the pathogenesis of TED. We also show that the DP1/cAMP signaling pathway rapidly activates HAS mRNA induction to promote HA production. PGD2, in addition to causing HA accumulation, may also provide an environment conducive to lymphocyte infiltration into orbital tissue via DP1. The lymphocytic accumulation within the orbit may, in turn, lead to additional release of inflammatory mediators, including PGD2. Therefore, selectively targeting the production of lipid mediators (i.e. PGD2) and/or activation of receptor systems (DP1) may reduce or prevent symptoms associated with TED.
This research was supported by EY017123 and EY011708 and a Research to Prevent Blindness Foundation Grant.
- TED
- thyroid eye disease
- cAMP
- cyclic AMP
- DP
- D prostanoid receptor
- ELISA
- enzyme-linked immunosorbent assay
- FBS
- fetal bovine serum
- HA
- hyaluronan
- HAS
- hyaluronan synthase(s)
- PGD and PGJ
- prostaglandin D and J, respectively
- PGDS
- PGD2 synthase
- H-PGDS
- hematopoietic type PGD2 synthase
- HYAL
- hyaluronidase
- RT
- reverse transcription
- qRT
- quantitative RT
- siRNA
- small interfering RNA
- PPARγ
- peroxisome proliferator-activated receptor γ
- PG
- prostaglandin(s)
- IBMX
- isobutylmethylxanthine
- SC
- scrambled control.
REFERENCES
- 1.Prabhakar B. S., Bahn R. S., Smith T. J. (2003) Endocr. Rev. 24, 802–835 [DOI] [PubMed] [Google Scholar]
- 2.Lehmann G. M., Garcia-Bates T. M., Smith T. J., Feldon S. E., Phipps R. P. (2008) PPAR Res. 2008, 895901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldon S. E., O'loughlin C. W., Ray D. M., Landskroner-Eiger S., Seweryniak K. E., Phipps R. P. (2006) Am. J. Pathol. 169, 1183–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith T. J., Hoa N. (2004) J. Clin. Endocrinol. Metab. 89, 5076–5080 [DOI] [PubMed] [Google Scholar]
- 5.Kuriyan A. E., Phipps R. P., Feldon S. E. (2008) Curr. Opin. Ophthalmol. 19, 499–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang D., Liang J., Noble P. W. (2007) Annu. Rev. Cell Dev. Biol. 23, 435–461 [DOI] [PubMed] [Google Scholar]
- 7.Makkonen K. M., Pasonen-Seppänen S., Törrönen K., Tammi M. I., Carlberg C. (2009) J. Biol. Chem. 284, 18270–18281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campo G. M., Avenoso A., Campo S., D'Ascola A., Traina P., Calatroni A. (2009) Br. J. Biomed. Sci. 66, 28–36 [DOI] [PubMed] [Google Scholar]
- 9.Guo N., Kanter D., Funderburgh M. L., Mann M. M., Du Y., Funderburgh J. L. (2007) J. Biol. Chem. 282, 12475–12483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marzoll A., Nagy N., Wördehoff L., Dai G., Fries S., Lindner V., Grosser T., Fischer J. W. (2009) J. Cell Mol. Med. 13, 3713–3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer J. W., Schrör K. (2007) Thromb. Haemost. 98, 287–295 [PubMed] [Google Scholar]
- 12.Goetzl E. J., An S., Smith W. L. (1995) FASEB J. 9, 1051–1058 [DOI] [PubMed] [Google Scholar]
- 13.Herlong J. L., Scott T. R. (2006) Immunol. Lett. 102, 121–131 [DOI] [PubMed] [Google Scholar]
- 14.Boie Y., Sawyer N., Slipetz D. M., Metters K. M., Abramovitz M. (1995) J. Biol. Chem. 270, 18910–18916 [DOI] [PubMed] [Google Scholar]
- 15.Nagata K., Hirai H., Tanaka K., Ogawa K., Aso T., Sugamura K., Nakamura M., Takano S. (1999) FEBS Lett. 459, 195–199 [DOI] [PubMed] [Google Scholar]
- 16.Kostenis E., Ulven T. (2006) Trends Mol. Med. 12, 148–158 [DOI] [PubMed] [Google Scholar]
- 17.Forman B. M., Tontonoz P., Chen J., Brun R. P., Spiegelman B. M., Evans R. M. (1995) Cell 83, 803–812 [DOI] [PubMed] [Google Scholar]
- 18.Kliewer S. A., Lenhard J. M., Willson T. M., Patel I., Morris D. C., Lehmann J. M. (1995) Cell 83, 813–819 [DOI] [PubMed] [Google Scholar]
- 19.Tamori Y., Masugi J., Nishino N., Kasuga M. (2002) Diabetes 51, 2045–2055 [DOI] [PubMed] [Google Scholar]
- 20.Xue L., Barrow A., Pettipher R. (2009) Clin. Exp. Immunol. 156, 126–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis R. A., Soter N. A., Diamond P. T., Austen K. F., Oates J. A., Roberts L. J., 2nd (1982) J. Immunol. 129, 1627–1631 [PubMed] [Google Scholar]
- 22.Lauer S. A., Silkiss R. Z., McCormick S. A. (2008) Ophthal. Plast. Reconstr. Surg. 24, 257–261 [DOI] [PubMed] [Google Scholar]
- 23.Boschi A., Daumerie C., Spiritus M., Beguin C., Senou M., Yuksel D., Duplicy M., Costagliola S., Ludgate M., Many M. C. (2005) Br. J. Ophthalmol. 89, 724–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith T. J., Parikh S. J. (1999) Endocrinology 140, 3518–3525 [DOI] [PubMed] [Google Scholar]
- 25.Smith T. J., Sempowski G. D., Wang H. S., Del Vecchio P. J., Lippe S. D., Phipps R. P. (1995) J. Clin. Endocrinol. Metab. 80, 2620–2625 [DOI] [PubMed] [Google Scholar]
- 26.Baglole C. J., Reddy S. Y., Pollock S. J., Feldon S. E., Sime P. J., Smith T. J., Phipps R. P. (2005) Methods Mol. Med. 117, 115–127 [DOI] [PubMed] [Google Scholar]
- 27.Lee H. G., Cowman M. K. (1994) Anal. Biochem. 219, 278–287 [DOI] [PubMed] [Google Scholar]
- 28.Ohya T., Kaneko Y. (1970) Biochim. Biophys. Acta 198, 607–609 [DOI] [PubMed] [Google Scholar]
- 29.Noble P. W. (2002) Matrix Biol. 21, 25–29 [DOI] [PubMed] [Google Scholar]
- 30.Pettipher R., Hansel T. T., Armer R. (2007) Nat. Rev. Drug Discov. 6, 313–325 [DOI] [PubMed] [Google Scholar]
- 31.Nagata K., Hirai H. (2003) Prostaglandins Leukot. Essent. Fatty Acids 69, 169–177 [DOI] [PubMed] [Google Scholar]
- 32.Sturino C. F., O'Neill G., Lachance N., Boyd M., Berthelette C., Labelle M., Li L., Roy B., Scheigetz J., Tsou N., Aubin Y., Bateman K. P., Chauret N., Day S. H., Lévesque J. F., Seto C., Silva J. H., Trimble L. A., Carriere M. C., Denis D., Greig G., Kargman S., Lamontagne S., Mathieu M. C., Sawyer N., Slipetz D., Abraham W. M., Jones T., McAuliffe M., Piechuta H., Nicoll-Griffith D. A., Wang Z., Zamboni R., Young R. N., Metters K. M. (2007) J. Med. Chem. 50, 794–806 [DOI] [PubMed] [Google Scholar]
- 33.Leblanc Y., Roy P., Dufresne C., Lachance N., Wang Z., O'Neill G., Greig G., Denis D., Mathieu M. C., Slipetz D., Sawyer N., Tsou N. (2009) Bioorg. Med. Chem. Lett. 19, 2125–2128 [DOI] [PubMed] [Google Scholar]
- 34.Royer J. F., Schratl P., Carrillo J. J., Jupp R., Barker J., Weyman-Jones C., Beri R., Sargent C., Schmidt J. A., Lang-Loidolt D., Heinemann A. (2008) Eur J. Clin. Invest. 38, 663–671 [DOI] [PubMed] [Google Scholar]
- 35.Kabashima K., Narumiya S. (2003) Prostaglandins Leukot. Essent. Fatty Acids 69, 187–194 [DOI] [PubMed] [Google Scholar]
- 36.Cheng K., Wu T. J., Wu K. K., Sturino C., Metters K., Gottesdiener K., Wright S. D., Wang Z., O'Neill G., Lai E., Waters M. G. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 6682–6687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshimura-Uchiyama C., Iikura M., Yamaguchi M., Nagase H., Ishii A., Matsushima K., Yamamoto K., Shichijo M., Bacon K. B., Hirai K. (2004) Clin. Exp. Allergy 34, 1283–1290 [DOI] [PubMed] [Google Scholar]
- 38.Hirai H., Tanaka K., Yoshie O., Ogawa K., Kenmotsu K., Takamori Y., Ichimasa M., Sugamura K., Nakamura M., Takano S., Nagata K. (2001) J. Exp. Med. 193, 255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris B. J., Adams D. J., Beveridge D. J., van der Weyden L., Mangs H., Leedman P. J. (2004) Acta Physiol. Scand. 181, 369–373 [DOI] [PubMed] [Google Scholar]
- 40.Metzger H., Lindner E. (1981) Arzneimittelforschung 31, 1248–1250 [PubMed] [Google Scholar]
- 41.Weinberg E., Zeldich E., Weinreb M. M., Moses O., Nemcovsky C., Weinreb M. (2009) J. Cell. Biochem. 108, 207–215 [DOI] [PubMed] [Google Scholar]
- 42.Kim S. H., Shin T. Y. (2009) Toxicol. In Vitro 23, 1215–1219 [DOI] [PubMed] [Google Scholar]
- 43.Margulis A., Nocka K. H., Brennan A. M., Deng B., Fleming M., Goldman S. J., Kasaian M. T. (2009) J. Immunol. 183, 1739–1750 [DOI] [PubMed] [Google Scholar]
- 44.Kanaoka Y., Urade Y. (2003) Prostaglandins Leukot. Essent. Fatty Acids 69, 163–167 [DOI] [PubMed] [Google Scholar]
- 45.Aritake K., Kado Y., Inoue T., Miyano M., Urade Y. (2006) J. Biol. Chem. 281, 15277–15286 [DOI] [PubMed] [Google Scholar]
- 46.Bartalena L., Tanda M. L. (2009) N. Engl. J. Med. 360, 994–1001 [DOI] [PubMed] [Google Scholar]
- 47.Hwang C. J., Afifiyan N., Sand D., Naik V., Said J., Pollock S. J., Chen B., Phipps R. P., Goldberg R. A., Smith T. J., Douglas R. S. (2009) Invest. Ophthalmol. Vis. Sci. 50, 2262–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feldon S. E., Park D. J., O'Loughlin C. W., Nguyen V. T., Landskroner-Eiger S., Chang D., Thatcher T. H., Phipps R. P. (2005) Invest. Ophthalmol. Vis. Sci. 46, 3913–3921 [DOI] [PubMed] [Google Scholar]
- 49.Lehmann G. M., Feldon S. E., Smith T. J., Phipps R. P. (2008) Thyroid 18, 959–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gianoukakis A. G., Jennings T. A., King C. S., Sheehan C. E., Hoa N., Heldin P., Smith T. J. (2007) Endocrinology 148, 54–62 [DOI] [PubMed] [Google Scholar]
- 51.Kaback L. A., Smith T. J. (1999) J. Clin. Endocrinol. Metab. 84, 4079–4084 [DOI] [PubMed] [Google Scholar]
- 52.van Steensel L., Paridaens D., Schrijver B., Dingjan G. M., van Daele P. L., van Hagen P. M., van den Bosch W. A., Drexhage H. A., Hooijkaas H., Dik W. A. (2009) Invest. Ophthalmol. Vis. Sci. 50, 3091–3098 [DOI] [PubMed] [Google Scholar]
- 53.Kahaly G., Förster G., Hansen C. (1998) Thyroid 8, 429–432 [DOI] [PubMed] [Google Scholar]
- 54.Smith T. J., Bahn R. S., Gorman C. A., Cheavens M. (1991) J. Clin. Endocrinol. Metab. 72, 1169–1171 [DOI] [PubMed] [Google Scholar]
- 55.Wang H. S., Tung W. H., Tang K. T., Wong Y. K., Huang G. J., Wu J. C., Guo Y. J., Chen C. C. (2005) J. Cell. Biochem. 95, 256–267 [DOI] [PubMed] [Google Scholar]
- 56.Camenisch T. D., Spicer A. P., Brehm-Gibson T., Biesterfeldt J., Augustine M. L., Calabro A., Jr., Kubalak S., Klewer S. E., McDonald J. A. (2000) J. Clin. Invest. 106, 349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Itano N., Sawai T., Yoshida M., Lenas P., Yamada Y., Imagawa M., Shinomura T., Hamaguchi M., Yoshida Y., Ohnuki Y., Miyauchi S., Spicer A. P., McDonald J. A., Kimata K. (1999) J. Biol. Chem. 274, 25085–25092 [DOI] [PubMed] [Google Scholar]
- 58.Schwartz J. I., Liu F., Stroh M., Gipson A., Johnson-Levonas A. O., Lasseter K. C., Lai E., Wagner J. A. (2009) Am. J. Ther. 16, 215–223 [DOI] [PubMed] [Google Scholar]
- 59.Ulven T., Kostenis E. (2006) Curr. Top. Med. Chem. 6, 1427–1444 [DOI] [PubMed] [Google Scholar]
- 60.Hufnagel T. J., Hickey W. F., Cobbs W. H., Jakobiec F. A., Iwamoto T., Eagle R. C. (1984) Ophthalmology 91, 1411–1419 [DOI] [PubMed] [Google Scholar]
- 61.Many M. C., Costagliola S., Detrait M., Denef F., Vassart G., Ludgate M. C. (1999) J. Immunol. 162, 4966–4974 [PubMed] [Google Scholar]
- 62.Costagliola S., Many M. C., Denef J. F., Pohlenz J., Refetoff S., Vassart G. (2000) J. Clin. Invest. 105, 803–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamada M., Li A. W., West K. A., Chang C. H., Wall J. R. (2002) Autoimmunity 35, 403–413 [DOI] [PubMed] [Google Scholar]
- 64.Toole B. P. (2004) Nat. Rev. Cancer 4, 528–539 [DOI] [PubMed] [Google Scholar]
- 65.Phipps R. P., Borrello M. A., Blieden T. M. (1997) J. Periodontal Res. 32, 159–165 [DOI] [PubMed] [Google Scholar]
- 66.Borrello M. A., Phipps R. P. (1996) Cell. Immunol. 173, 198–206 [DOI] [PubMed] [Google Scholar]
- 67.Hagood J. S., Lasky J. A., Nesbitt J. E., Segarini P. (2001) Chest 120, Suppl. 1, 64S–66S [DOI] [PubMed] [Google Scholar]
- 68.Khoo T. K., Coenen M. J., Schiefer A. R., Kumar S., Bahn R. S. (2008) Thyroid 18, 1291–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baglole C. J., Bushinsky S. M., Garcia T. M., Kode A., Rahman I., Sime P. J., Phipps R. P. (2006) Am. J. Physiol. Lung Cell Mol. Physiol. 291, L19–L29 [DOI] [PubMed] [Google Scholar]
- 70.de la Motte C., Nigro J., Vasanji A., Rho H., Kessler S., Bandyopadhyay S., Danese S., Fiocchi C., Stern R. (2009) Am. J. Pathol. 174, 2254–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao F., Koenitzer J. R., Tobolewski J. M., Jiang D., Liang J., Noble P. W., Oury T. D. (2008) J. Biol. Chem. 283, 6058–6066 [DOI] [PMC free article] [PubMed] [Google Scholar]