Abstract
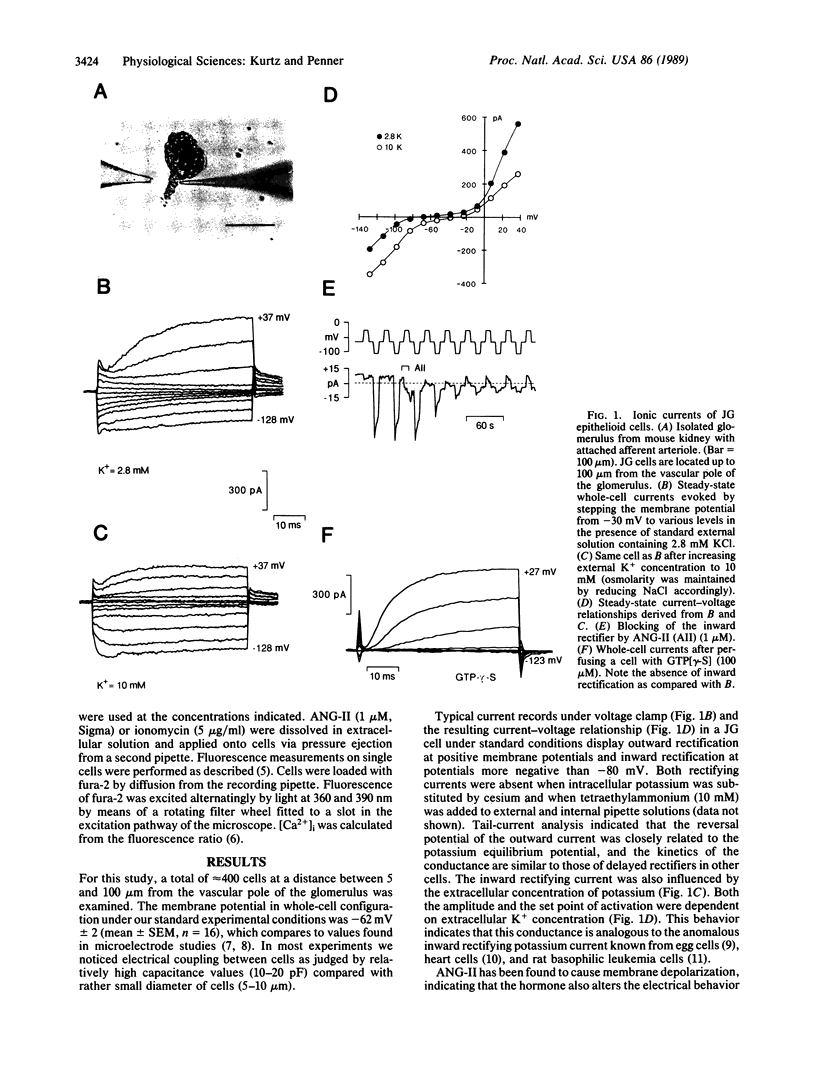
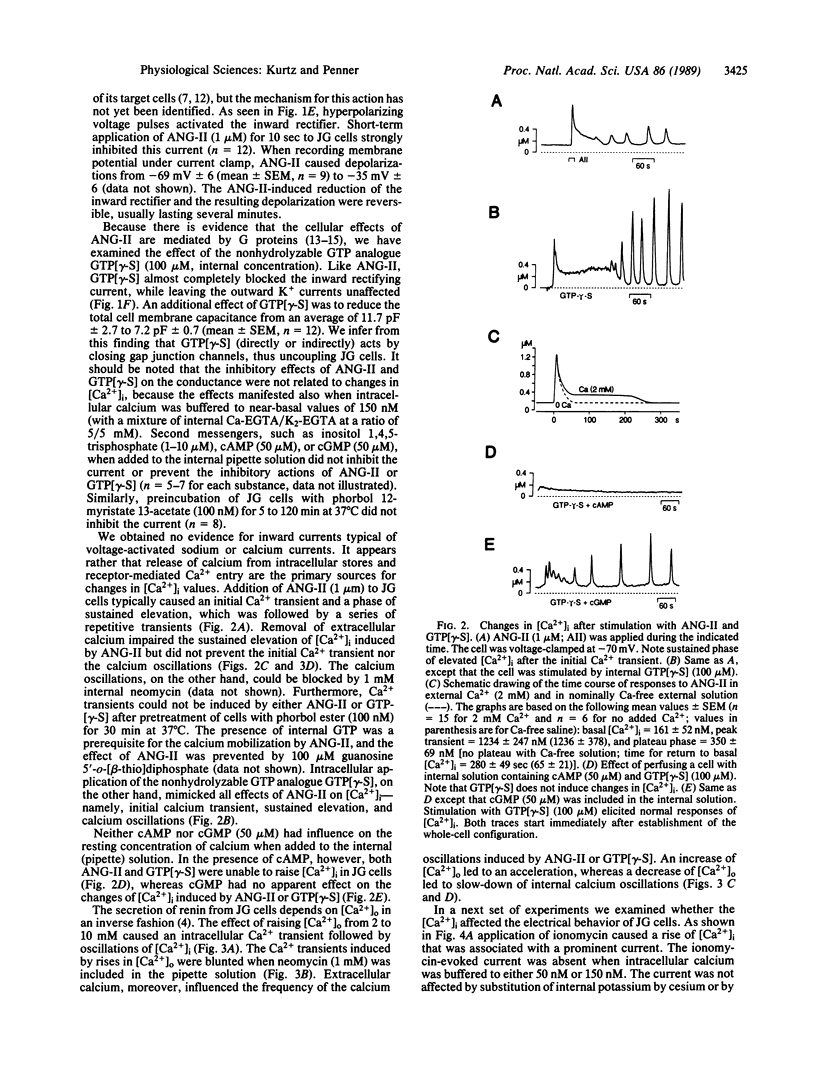
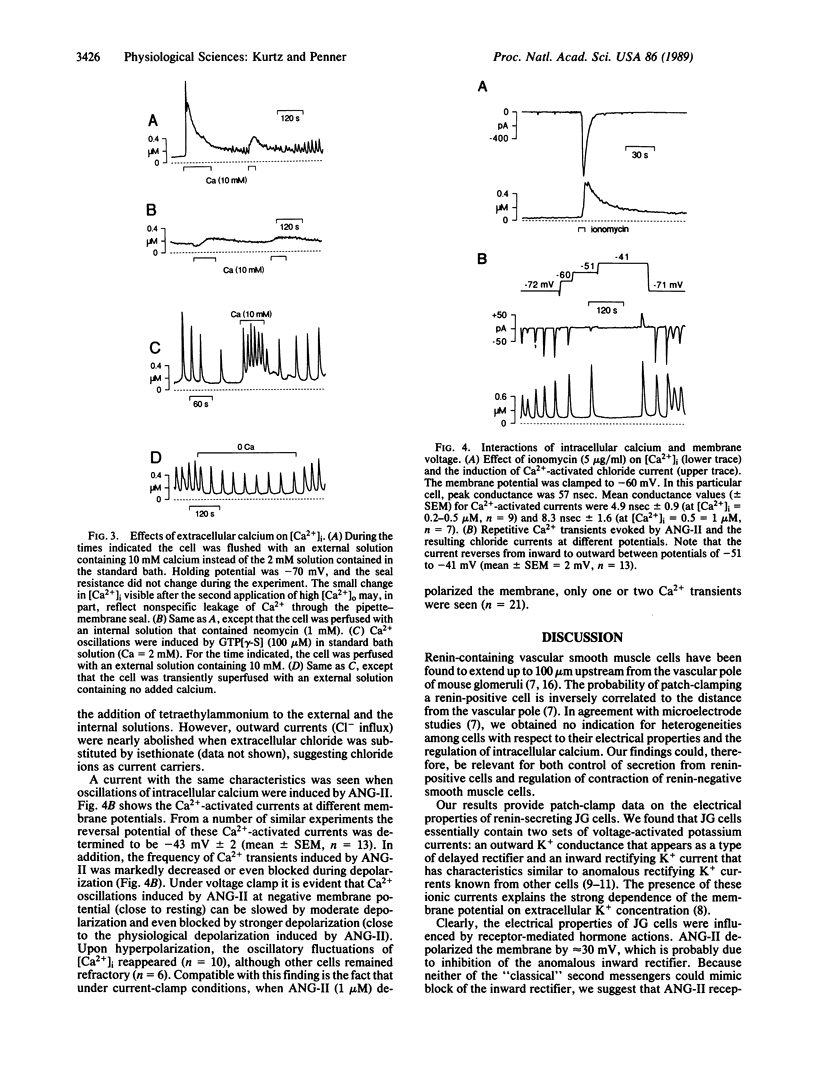
Simultaneous patch-clamp and fura-2 measurements were used to investigate the electrical properties and receptor-mediated changes of intracellular calcium in renal juxtaglomerular cells. Here we report the presence of voltage-activated inward and outward rectifying potassium currents and the inhibition of the anomalous inward rectifying potassium current by angiotensin II (ANG-II). This action of ANG-II was mimicked by guanosine 5'-[gamma-thio]triphosphate but not by cAMP, cGMP, inositol 1,4,5-trisphosphate, or phorbol ester, suggesting that ANG-II inhibits the potassium channel directly by means of a guanine nucleotide-binding regulatory protein or by means of an unusual type of second messenger. Blocking of the inward rectifier was paralleled by membrane depolarization, but we obtained no evidence for calcium entry due to voltage-gated calcium channels in juxtaglomerular cells. Instead, under voltage clamp, ANG-II and guanosine 5'-[gamma-thio]triphosphate induced release of calcium from intracellular stores followed by a sustained phase of transmembrane calcium influx and oscillations of intracellular Ca2+ concentrations. Changes in intracellular Ca2+ concentrations were found to depend on the extracellular Ca concentration--i.e., the sustained elevation was abolished in absence of extracellular Ca, and the frequency of repetitive calcium release was directly related to the extracellular concentration of calcium. Moreover, an elevation of extracellular Ca concentration by itself induced release of intracellular calcium in the absence of other stimuli. Changes in intracellular Ca2+ concentrations were accompanied by prominent calcium-activated chloride currents, and this mechanism is inferred to be responsible for the inhibitory role of calcium in renin secretion. Intracellular application of cAMP but no cGMP inhibited ANG-II and guanosine 5'-[gamma-thio]triphosphate induced calcium mobilization in juxtaglomerular cells, being consistent with the facilitatory effects of elevated cAMP levels of renin release. The frequency of ANG-II induced oscillations was also markedly attenuated at depolarized membrane potentials suggesting effective negative feedback control of ANG-II-induced depolarization on repetitive Ca2+ transients induced by the hormone.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barajas L. Anatomy of the juxtaglomerular apparatus. Am J Physiol. 1979 Nov;237(5):F333–F343. doi: 10.1152/ajprenal.1979.237.5.F333. [DOI] [PubMed] [Google Scholar]
- Bührle C. P., Nobiling R., Taugner R. Intracellular recordings from renin-positive cells of the afferent glomerular arteriole. Am J Physiol. 1985 Aug;249(2 Pt 2):F272–F281. doi: 10.1152/ajprenal.1985.249.2.F272. [DOI] [PubMed] [Google Scholar]
- Bührle C. P., Scholz H., Hackenthal E., Nobiling R., Taugner R. Epithelioid cells: membrane potential changes induced by substances influencing renin secretion. Mol Cell Endocrinol. 1986 Apr;45(1):37–47. doi: 10.1016/0303-7207(86)90080-8. [DOI] [PubMed] [Google Scholar]
- Churchill P. C., Churchill M. C. Isoproterenol-stimulated renin secretion in the rat: second messenger roles of Ca and cyclic AMP. Life Sci. 1982 Apr 12;30(15):1313–1319. doi: 10.1016/0024-3205(82)90694-4. [DOI] [PubMed] [Google Scholar]
- Churchill P. C. Second messengers in renin secretion. Am J Physiol. 1985 Aug;249(2 Pt 2):F175–F184. doi: 10.1152/ajprenal.1985.249.2.F175. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Howell T. W., Gomperts B. D. Two G-proteins act in series to control stimulus-secretion coupling in mast cells: use of neomycin to distinguish between G-proteins controlling polyphosphoinositide phosphodiesterase and exocytosis. J Cell Biol. 1987 Dec;105(6 Pt 1):2745–2750. doi: 10.1083/jcb.105.6.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman M. C. Membrane potential of juxtaglomerular cells. Nature. 1976 Apr 8;260(5551):542–544. doi: 10.1038/260542a0. [DOI] [PubMed] [Google Scholar]
- Frederiksen O., Leyssac P. P., Skinner S. L. Sensitive osmometer function of juxtaglomerular cells in vitro. J Physiol. 1975 Nov;252(3):669–679. doi: 10.1113/jphysiol.1975.sp011164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Habener J. F., Rosenblatt M., Potts J. T., Jr Parathyroid hormone: biochemical aspects of biosynthesis, secretion, action, and metabolism. Physiol Rev. 1984 Jul;64(3):985–1053. doi: 10.1152/physrev.1984.64.3.985. [DOI] [PubMed] [Google Scholar]
- Hackenthal E., Aktories K., Jakobs K. H. Pertussis toxin attenuates angiotensin II-induced vasoconstriction and inhibition of renin release. Mol Cell Endocrinol. 1985 Sep;42(2):113–117. doi: 10.1016/0303-7207(85)90098-x. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Rosenthal N. P. Potassium current and the effect of cesium on this current during anomalous rectification of the egg cell membrane of a starfish. J Gen Physiol. 1976 Jun;67(6):621–638. doi: 10.1085/jgp.67.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima I., Shibata H., Ogata E. Pertussis toxin blocks angiotensin II-induced calcium influx but not inositol trisphosphate production in adrenal glomerulosa cell. FEBS Lett. 1986 Aug 18;204(2):347–351. doi: 10.1016/0014-5793(86)80841-9. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Della Bruna R., Pfeilschifter J., Taugner R., Bauer C. Atrial natriuretic peptide inhibits renin release from juxtaglomerular cells by a cGMP-mediated process. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4769–4773. doi: 10.1073/pnas.83.13.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz A. Intracellular control of renin release--an overview. Klin Wochenschr. 1986 Sep 15;64(18):838–846. doi: 10.1007/BF01725556. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Pfeilschifter J., Bauer C. Is renin secretion governed by the calcium permeability of the juxtaglomerular cell membrane? Biochem Biophys Res Commun. 1984 Oct 30;124(2):359–366. doi: 10.1016/0006-291x(84)91561-4. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Pfeilschifter J., Hutter A., Bührle C., Nobiling R., Taugner R., Hackenthal E., Bauer C. Role of protein kinase C in inhibition of renin release caused by vasoconstrictors. Am J Physiol. 1986 Apr;250(4 Pt 1):C563–C571. doi: 10.1152/ajpcell.1986.250.4.C563. [DOI] [PubMed] [Google Scholar]
- Leyssac P. P. Further studies on oscillating tubulo-glomerular feedback responses in the rat kidney. Acta Physiol Scand. 1986 Feb;126(2):271–277. doi: 10.1111/j.1748-1716.1986.tb07814.x. [DOI] [PubMed] [Google Scholar]
- Lindau M., Fernandez J. M. A patch-clamp study of histamine-secreting cells. J Gen Physiol. 1986 Sep;88(3):349–368. doi: 10.1085/jgp.88.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y., Nakajima S., Inoue M. Pertussis toxin-insensitive G protein mediates substance P-induced inhibition of potassium channels in brain neurons. Proc Natl Acad Sci U S A. 1988 May;85(10):3643–3647. doi: 10.1073/pnas.85.10.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. J Physiol. 1988 Jan;395:193–214. doi: 10.1113/jphysiol.1988.sp016914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E. F., Scarpa A. Rapid mobilization of cellular Ca2+ in bovine parathyroid cells evoked by extracellular divalent cations. Evidence for a cell surface calcium receptor. J Biol Chem. 1987 Apr 15;262(11):5188–5196. [PubMed] [Google Scholar]
- Orellana S. A., Solski P. A., Brown J. H. Phorbol ester inhibits phosphoinositide hydrolysis and calcium mobilization in cultured astrocytoma cells. J Biol Chem. 1985 May 10;260(9):5236–5239. [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Penner R. Multiple signaling pathways control stimulus-secretion coupling in rat peritoneal mast cells. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9856–9860. doi: 10.1073/pnas.85.24.9856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Conductance properties of single inwardly rectifying potassium channels in ventricular cells from guinea-pig heart. J Physiol. 1984 Feb;347:641–657. doi: 10.1113/jphysiol.1984.sp015088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skøtt O. Do osmotic forces play a role in renin secretion? Am J Physiol. 1988 Jul;255(1 Pt 2):F1–10. doi: 10.1152/ajprenal.1988.255.1.F1. [DOI] [PubMed] [Google Scholar]
- Taugner R., Marin-Grez M., Keilbach R., Hackenthal E., Nobiling R. Immunoreactive renin and angiotensin II in the afferent glomerular arterioles of rats with hypertension due to unilateral renal artery constriction. Histochemistry. 1982;76(1):61–69. doi: 10.1007/BF00493286. [DOI] [PubMed] [Google Scholar]
- Uhing R. J., Prpic V., Jiang H., Exton J. H. Hormone-stimulated polyphosphoinositide breakdown in rat liver plasma membranes. Roles of guanine nucleotides and calcium. J Biol Chem. 1986 Feb 15;261(5):2140–2146. [PubMed] [Google Scholar]
- Watson S. P., Lapetina E. G. 1,2-Diacylglycerol and phorbol ester inhibit agonist-induced formation of inositol phosphates in human platelets: possible implications for negative feedback regulation of inositol phospholipid hydrolysis. Proc Natl Acad Sci U S A. 1985 May;82(9):2623–2626. doi: 10.1073/pnas.82.9.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright F. S., Briggs J. P. Feedback control of glomerular blood flow, pressure, and filtration rate. Physiol Rev. 1979 Oct;59(4):958–1006. doi: 10.1152/physrev.1979.59.4.958. [DOI] [PubMed] [Google Scholar]



