Abstract
The mechanism underlying the protective effect of sphingosine kinase 1 (SphK1) in inflammatory injury is not clear. We demonstrated using SphK1-null mice (SphK1−/−) the crucial role of SphK1 in suppressing lipopolysaccharide-induced neutrophil oxidant production and sequestration in lungs and mitigating lung inflammatory injury. This effect of SphK1 was independent of the production of sphingosine 1-phosphate, the product of SphK1 activity. The anti-inflammatory effect of SphK1 in the lipopolysaccharide model was mediated through SphK1 interaction with JNK. SphK1 stabilization of JNK in turn inhibited JNK binding to the JNK-interacting protein 3 (JIP3) and thus abrogated the activation of NADPH oxidase and oxidant generation and resultant NF-κB activation. Therefore, SphK1-mediated down-regulation of JNK activity serves to dampen inflammation and tissue injury.
Keywords: Signal Transduction/Protein Kinases/MAP, Lung, MAP Kinases (MAPKs), NF-kappa B, Reactive Oxygen Species (ROS), Lung Injury, NADPH Oxidase
Introduction
Lipopolysaccharide (LPS)3-induced acute lung injury is characterized by increased lung vascular permeability and edema formation with profound tissue damage (1). LPS triggers the local activation of macrophages and neutrophils in the lung interstitium and airways, which release inflammatory cytokines and chemokines, tumor necrosis factor-α, and MIP-2 (macrophage inflammatory protein-2) (2). These mediators in turn promote neutrophil sequestration by activating endothelial cell adhesion molecule expression (such as ICAM-1) and induce neutrophil transmigration into the interstitium and airspace, where neutrophils propagate inflammation through the release of reactive oxygen species (ROS) (3). NADPH oxidase-mediated ROS production thus has a pivotal role in mediating increased endothelial permeability and tissue injury (4).
Sphingosine 1-phosphate (S1P), the product of sphingosine kinase (SphK)-mediated phosphorylation of sphingosine, has been shown to enhance the vascular endothelial barrier through binding of S1P to endothelial differentiation gene family receptors (5, 6). SphK is the major kinase that phosphorylates sphingosine to generate S1P, and two isoforms of SphK have been identified, SphK1 and SphK2 (7). SphK1 is the predominant isoform responsible for the presence of S1P in the circulation (8). SphK1 is believed to act through S1P to modulate LPS-induced activation of MAPK proteins, phosphoinositide 3-kinase, and NF-κB in macrophages and inflammatory cascade in neutrophils (9–11). However, there is a controversy whether generation of S1P and high circulating concentration of S1P after sepsis can alone account for the endothelial barrier protective and anti-inflammatory effects of SIP (12, 13). This controversy arises in part because the G protein-coupled endothelial differentiation receptors are expected to be fully saturated at the high ambient circulating levels of SIP (13). In this study, we demonstrated that SphK1 can function independently of S1P to modulate LPS-induced lung inflammatory injury. We found that SphK1 prevented the LPS-induced neutrophil-endothelial adhesive interaction, neutrophil transmigration, and lung inflammatory injury through a reduction of JNK activation, thus preventing NADPH oxidase activation.
We used LPS in these studies to induce tissue inflammation because it has been shown to activate the MAPK JNK pathway (14–16). Inhibition of JNK decreased the severity of LPS-induced pulmonary neutrophil influx and inflammation (15). Our data show SphK1 can prevent LPS-induced JNK activation and JNK-mediated NADPH oxidase activation through blocking JNK interaction with the JNK-interacting protein 3 (JIP3). These observations identify a novel SphK1-mediated innate immune mechanism that may provide a crucial therapeutic target against inflammation and injury.
EXPERIMENTAL PROCEDURES
Mice, Cell Cultures, and Reagents
C57 black 6 (C57BL6) mice were obtained from Charles River Laboratory. SphK1−/− mice were a gift from Dr. Richard L. Proia (NIDDK, National Institutes of Health, Bethesda). SphK1−/− mice were backcrossed with C57BL6 mice for five generations to eliminate any background effects on the observed phenotypes. SphK1−/− and wild-type (WT) littermates were used in this study. All mice were housed in the University of Illinois Animal Care Facility in accordance with institutional and National Institutes of Health guidelines. Mouse neutrophils were isolated from murine bone marrow using Percoll density gradients as described previously (17). Neutrophil purity and viability as assessed by trypan blue staining were >95%. Mouse lung vascular endothelial cells (MLVEC) were isolated from WT and SphK1−/− mice as described previously (18). LPS from Escherichia coli 0111:B4 purchased from Sigma was used for all in vivo experiments. SKI, SP600125, and U0126 were purchased from Calbiochem. SphK1 cDNA was purchased from OpenBiosystems. Anti-SphK1 antibodies were purchased from Abgent and EMD. Anti-p-JNK, p-p38, and p-ERK antibodies were purchased from Cell Signaling. JNK protein was purchased from Millipore.
Determination of Lung Microvascular Permeability and Edema Formation
Microvascular liquid permeability and edema formation were quantified by measuring capillary filtration coefficient (Kf,c) and final lung wet-to-dry weight ratios, respectively. As described previously (18), mice were anesthetized with an intraperitoneal injection of ketamine (60 mg/kg), xylazine (2.5 mg/kg), and acepromazine (2.5 mg/kg) under an approved institutional animal protocol. The trachea was cannulated with a stainless steel tube for constant positive pressure ventilation (120 breaths/min). Heparin (50 units) was administered via the right internal jugular vein to prevent blood clotting. A thoracotomy was performed to expose the heart and lungs. An incision was made in the right ventricle for insertion of a pulmonary arterial catheter (polyethylene). The cannula was secured with suture around the pulmonary artery and aorta. The lungs were perfused via a peristaltic pump with modified Krebs-Henseleit solution and supplemented with 5 g/100 ml of bovine serum albumin (fraction V, 99% pure and endotoxin free; Sigma). The heart and lungs were rapidly excised and transferred en bloc to a perfusion device. Lung wet weight was monitored with an electronic beam balance and vascular pressures with transducers. The isolated lungs were then ventilated (120 breaths/min) and perfused at constant flow (2 ml/min), temperature (37 °C), and venous pressure (+2 cm H2O).
Lung Tissue Myeloperoxidase (MPO) Activity
Lung tissue MPO activity was measured as described previously (18). Briefly, lungs were homogenized in 1 ml of phosphate-buffered saline, pH 6, with 5% hexadecyltrimethylammonium bromide and 5 mm EDTA. The homogenates were sonicated, centrifuged at 40,000 × g for 20 min, and run through two freeze-thaw cycles. The samples were homogenized and centrifuged a second time. The supernatant was then collected and mixed 1:30 (v/v) with assay buffer (0.2 mg/ml o-dianisidine hydrochloride and 0.0005% H2O2). Absorbance change was measured at 460 nm for 3 min, and MPO activity was calculated as the change in absorbance over time. Neutrophil sequestration was quantified as MPO activity normalized by the final dry lung weight.
Measurement of S1P Concentration
S1P levels were measured in lungs and plasma of SphK1−/− and wild-type mice as described previously (19). Briefly, mice were injected with LPS intraperitoneally, and their lungs were collected at various time points following injection. S1P content was determined by liquid chromatography/tandem mass spectrometry with electrospray ionization using API 4000 Q-trap mass spectrometer equipped with turbo-V ion source. C17-S1P was used as an internal standard.
Measurement of ROS Production
Neutrophils in the presence or absence of LPS at 5 × 106 cells/ml were added to cultured MLVEC isolated from WT and SphK1−/− mice. In some groups, MLVEC were pretreated with various inhibitors as indicated in the figures and text. ROS generation in co-cultured medium was measured using luminol-enhanced chemiluminescence as described previously (20). Briefly, luminol was added to the co-culture medium to a final concentration of 50 μm, and horseradish peroxidase was added to a final concentration of 40 units/ml. ROS production was determined after cells were stimulated with LPS for the indicated times. The relative concentration of ROS in the culture medium was expressed as counts/s.
Immunoprecipitation and Immunoblotting
Activation of JNK, ERK, and p38 was assessed using antibodies that recognize the phosphorylated forms of JNK, ERK, and p38, and antibodies against nonphosphorylated JNK, ERK, and p38 were used to assess protein loading. Activation of p47Phox was assessed using antibodies that recognize p47Phox phosphorylation at Ser-473, and an antibody against nonphosphorylated p47Phox was used to assess protein loading.
Associations of proteins with other proteins were studied using a soluble and enriched membrane fraction. After homogenization of tissue in phosphate-buffered saline (containing 1% protease inhibitors), the samples were centrifuged for 15 min at 14,000 × g. The pellets were sonicated two times for 12 s in lysis buffer containing 100 mm Tris-HCl, pH 7.5, 1% Nonidet P-40, 5 mm EDTA, 5 mm EGTA, 150 mm NaCl, to which a protease inhibitor mixture (Sigma) was added. After centrifugation again for 15 min at 14,000 × g, the supernatants of enriched membrane fraction were collected for immunoprecipitation. Equal amounts of protein (200 μg/ml total protein) in supernatants were precleared using purified IgG followed by protein A-Sepharose beads. The precleared supernatants were recovered by centrifugation and incubated overnight at 4 °C with primary antibodies, followed by incubations with protein A-Sepharose beads for 2 h. The beads were collected by centrifugation (10,000 × g, 5 min using an Eppendorf refrigerated microcentrifuge at 4 °C), washed extensively (5 × 10 min), solubilized, and run on 4–20% SDS-PAGE. After transfer to nitrocellulose, the membrane was then incubated with antibodies against proteins interested at 4 °C. After washing, horseradish peroxidase-coupled secondary antibodies were then used to probe the membranes. The membranes were developed as described in the manufacturer's instructions.
Measurement of SphK Activity
Lungs were isolated 6 h after injection of SKI (50 mg/kg, intraperitoneal) and LPS (10 mg/kg, intraperitoneal) and were homogenized at 4 °C in buffer, including 20 mm Tris-HCl, pH 7.5, 20% glycerol, 1 mm β-mercaptoethanol, 1 mm EDTA, 1 mm EGTA, 1 mm Na3VO4, 15 mm NaF, 0.5 mm 4-deoxypyridixine, 40 mm glycerophosphate, 1 mm phenylmethylsulfonyl fluoride, and proteinase inhibitor. Insoluble material was removed by centrifugation at 15,000 rpm for 30 min. Protein concentration was measured, and 20 μg was used to analyze the SphK activity in a reaction that was performed at 37 °C in solution containing 20 μm sphingosine, 5 mm MgCl2, 500 μm ATP (10 μCi of 32P). Sphingosine kinase 1 (0.5 μg) was further added in the positive control group. The reaction was terminate by adding 1 μl of 6 n HCl. The sample were centrifuged and applied to a silica gel TLC plate. TLC plates were developed in solution (butanol/ethanol/acetic acid/water = 80:20:10:20), air-dried, and visualized by autoradiography.
Liposome-mediated Gene Transfer in Mice
The mouse SphK1 gene was cloned from the recently cloned murine SphK1 cDNA. The PCR products were digested and subcloned into the pEGFP-C1 vector for transfection. The SphK1 dominant-negative mutant (G82D), which has been shown to block SphK1 activity and its functions (21), was generated by site-directed mutagenesis with the QuikChange mutagenesis kit (Stratagene), as directed by the manufacturer. All constructs were confirmed by DNA sequencing. Cell transfection was performed using commercially available transfection reagents and machinery (Amaxa). For in vivo studies, the expression plasmid was delivered using liposome-mediated gene transfer system as described previously (22). The complex consisting of SphK1 activity expression plasmid or the mutant plasmid and the liposomes were injected into the retro-orbital venous plexus.
Statistical Analysis
Statistical comparisons were made using two-tailed Student's t test. Experimental values were reported as the means ± S.E. Differences in mean values were considered significant at p < 0.05.
RESULTS
SphK1 Suppresses LPS-induced Lung Inflammatory Injury
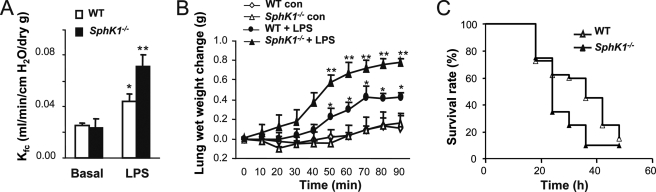
Using the LPS-induced lung inflammatory injury model, we first determined the role of SphK1. Kf,c and lung wet weight gain were measured to assess lung vascular permeability and edema formation, respectively. Injection of LPS (intraperitoneal) led to a significant increase of lung vascular permeability and edema formation in WT mice compared with basal condition (Fig. 1, A and B). Strikingly, these increases were augmented greater than 2-fold in the SphK1−/− mice (Fig. 1, A and B), indicating that SphK1 has a protective role in preventing lung vascular hyper-permeability and edema formation in response to LPS. LPS-challenged SphK1−/− mice also had decreased survival rate compared with LPS-challenged WT mice (p = 0.03, log-rank test; see Fig. 1C).
FIGURE 1.
SphK1 prevents LPS-induced lung vascular hyper-permeability and edema formation. A, changes of microvessel permeability (measured as capillary filtration coefficient, Kf,c) in WT and SphK1−/− lungs in the presence and absence of LPS challenge (10 mg/kg, intraperitoneal). *, increase (p < 0.05) in Kf,c from basal value; **, increased (p < 0.05) Kf,c in SphK1−/− group compared with WT after LPS challenge. Results are mean of five independent experiments; bars indicate means ± S.E. B, time-dependent changes of pulmonary edema formation as measured by increase in wet weight of lungs of mice treated same as above. Lung wet weight was determined at the times indicated. *, increase (p < 0.05) in lung wet weight from basal value; **, increased (p < 0.05) lung wet weight in SphK1−/− group compared with WT after LPS challenge. Results are mean of 5 independent experiments; bars indicate means ± S.E. con, control. C, survival in mice after LPS (30 mg/kg, intraperitoneal) in WT and SphK1−/− mice (n = 20 each). Differences in mortality were assessed by log-rank test (p < 0.05).
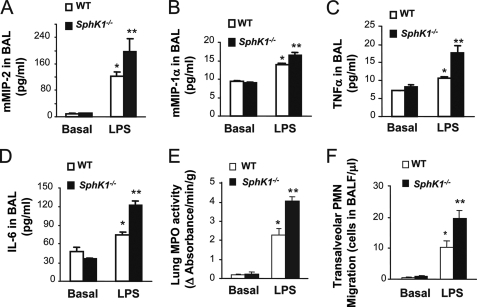
Lung inflammatory injury induced by LPS is characterized by rapid sequestration of neutrophils in response to inflammatory chemokines and cytokines released in the lungs (1). Hence, we determined whether there was also an augmented release of chemokines and cytokines in lungs of SphK1−/− mice compared with WT. We observed that concentrations of two chemokines, MIP-2 and MIP-1α, were indeed significantly greater in bronchoalveolar lavage fluid of SphK1−/− mice after LPS stimulation compared with WT (Fig. 2, A and B). We also observed increased release of two cytokines, tumor necrosis factor-α and interleukin-6, in bronchoalveolar lavage fluid of SphK1−/− mice compared with WT (Fig. 2, C and D). We next examined LPS-induced changes in lung tissue MPO activity, used as a marker for neutrophil sequestration (23, 24). Although basal MPO activity in SphK1−/− lungs was the same as in WT, LPS induced a significantly greater MPO activity in SphK1−/− mouse lungs than in WT (Fig. 2E). There was also consistently greater LPS-induced neutrophil recruitment into the airspace in SphK1−/− mice compared with WT (Fig. 2F).
FIGURE 2.
SphK1 prevents LPS-induced generation of inflammatory chemokines and cytokines and lung neutrophil sequestration. Lung tissue chemokines, MIP-2 (A) and MIP-1α (B), cytokines, tumor necrosis factor-α (TNF-α) (C), and interleukin-6 (IL-6) (D) were measured by enzyme-linked immunosorbent assay at 3 or 6 h after LPS challenge in WT and SphK1−/− mice. *, increased (p < 0.05) lung tissue chemokines compared with control value (without LPS challenge); **, increased (p < 0.05) in lung tissue chemokines or cytokines compared with WT. Results are mean of six experiments; bars indicate mean ± S.E. E, lung neutrophil sequestration as measured by MPO activity. Mice were challenged with LPS (10 mg/kg, intraperitoneal). * denotes increase (p < 0.05) compared with unchallenged; ** denotes increase (p < 0.05) in SphK1−/− mice compared with WT mice post-LPS stimulation. Results are mean of four independent experiments; bars indicate means ± S.E. F, neutrophil migration into airspace of WT or SphK1−/− mice. All mice were challenged for 6 h with LPS (10 μg/ml, LPS was dissolved in 0.9% saline and mice inhaled LPS for 30 min). *, compared with no LPS stimulation (basal value, p < 0.05); **, increased (p < 0.05) in SphK1−/− group compared with WT group post-LPS stimulation. Results are mean values of six experiments; bars show mean ± S.E. BAL, bronchoalveolar lavage; PMN, polymorphonuclear leukocytes.
SphK1 Suppresses Neutrophil Adhesion to Endothelial Cells and Neutrophil Transmigration
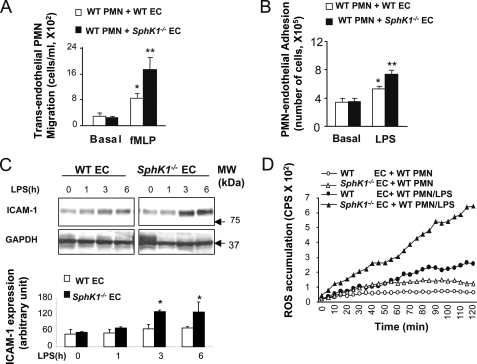
To determine the function of SphK1 in regulating neutrophil-endothelial interaction, we studied neutrophil adhesion to cultured endothelial cells and the transmigration of neutrophils. Neutrophil transendothelial migration was analyzed using the Transwell assay with chambers separated by a monolayer of cultured WT or SphK1−/− MLVEC. We applied LPS-primed WT or SphK1−/− neutrophils to the upper chamber and the chemoattractant fMLP to the lower chamber, and we determined the rate of cells migrating across the endothelial barrier after 4 h of incubation. Transendothelial migration of WT neutrophils across WT MLVEC was 4-fold greater after fMLP stimulation compared with only LPS activation of neutrophils. In contrast, neutrophils had greater migration across SphK1−/− MLVEC in response to fMLP compared with the migration of WT neutrophils across WT MLVEC (Fig. 3A). The increased neutrophil transmigration across SphK1−/− MLVEC was coupled to increased neutrophil adhesion to endothelial cells (Fig. 3B). Thus, SphK1 expression in endothelial cells played a fundamental role in mediating neutrophil-endothelial adhesion and transendothelial neutrophil migration.
FIGURE 3.
SphK1 suppresses neutrophil adhesion to endothelial cells and neutrophil transmigration and ROS production. A, migration of LPS-primed neutrophil across endothelial cells. *, compared with no fMLP stimulation (basal value, p < 0.05); **, increased (p < 0.05) in SphK1−/− neutrophils compared with WT post-LPS stimulation. Results are mean of four independent experiments; bars indicate means ± S.E. B, neutrophil adhesion to endothelial cells. *, compared with no LPS stimulation, (p < 0.05, n = 4 separate experiments); **, increased (p < 0.05) adhesion compared with adhesion of WT neutrophils to WT MLVEC post-LPS stimulation. Results are mean of four independent experiments. Bars indicate means ± S.E. C, upper panel, representative time course of ICAM-1 expression after LPS (1 μg/ml) challenge of MLVEC. Endothelial cells (EC) challenged without or with LPS were homogenized, electrophoresed, and blotted with anti-ICAM-1 antibody to determine time-dependent ICAM-1 expression; lower panel, quantification of ICAM-1 expression. D, neutrophil ROS generation after LPS stimulation (1 μg/ml) determined based on luminol-ECL. cps, counts/s of light emitted. The figure shows representative curves from one of three independent experiments that produced similar results. PMN, polymorphonuclear leukocytes.
Neutrophil adhesion to endothelial cells is mediated through β2 integrins on neutrophils that interact with ICAM-1 on the endothelial cell surface (25–27). We found the LPS-induced expression of ICAM-1 was greater in SphK1−/− MLVEC compared with WT (Fig. 3C, p < 0.05). Increased neutrophil adhesion to the endothelium also induces augmented release of reactive oxygen species from both neutrophils and endothelial cells (3, 28–30). We observed LPS-induced oxidant production was greater with WT neutrophils adherent to SphK1−/− endothelial cells compared with WT neutrophils adherent to WT endothelial cells (Fig. 3D). Taken together, deletion of SphK1 caused greater chemokine production and oxidant production as well as ICAM-1 expression in endothelial cells, which contributed to the significantly greater lung neutrophil sequestration in SphK1−/− lungs compared with WT.
SphK1 Deficiency Has Little Effect on S1P Production in Response to LPS
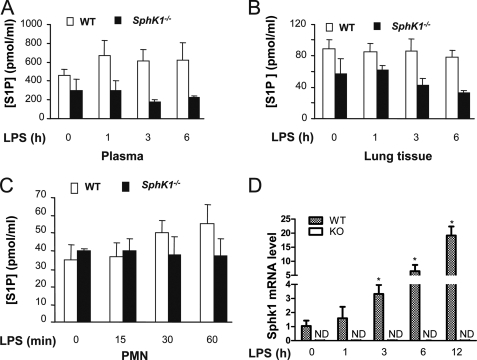
SphK1 has been identified as the predominant kinase isoform generating S1P that functions through S1P receptors to down-regulate the inflammatory signaling cascade and lymphocyte trafficking (7, 8). Here, we addressed the effects of SphK1 deficiency in mice. We first examined changes in S1P concentrations in response to LPS. We noted that basal S1P concentrations in plasma and lungs were lower in SphK1−/− mice compared with WT (Fig. 4, A and B), indicating the SphK1 is required to maintain the constitutive S1P plasma concentration (31). Basal concentrations of S1P in WT and knock-out neutrophils, however, were not significantly different (Fig. 4C). Surprisingly, LPS stimulation did not significantly increase S1P concentrations in plasma, lungs, and neutrophils in both WT and SphK1−/− mice (Fig. 4, A–C), whereas LPS markedly increased SphK1 gene expression in WT lungs (Fig. 4D). Thus, although LPS induced SphK1 expression, the S1P concentration was not significantly increased in response to LPS.
FIGURE 4.
LPS-induced S1P generation and SphK1 transcription in WT and SphK1−/−mice. A–C, S1P concentrations in plasma (A), lungs (B), and neutrophils (C) of WT and SphK1−/− mice. Plasma, lungs, and neutrophils were isolated from WT or SphK1−/− mice challenged with or without LPS challenge (10 mg/kg, intraperitoneal) for the indicated times. D, total RNA was isolated from lungs, using TRIzol reagent (Invitrogen), and then treated with DNase. Total RNA (2 μg) was reverse-transcribed using a SuperScript preamplification kit (Invitrogen). Reverse transcription-PCR profile was compared with glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The experiment was repeated three times. Results are mean of four independent experiments. Bars indicate mean ± S.E. KO, knock-out; PMN, polymorphonuclear leukocytes; ND, not detectable.
Inhibition of SphK Activity Does Not Recapitulate the Phenotype Seen in SphK1−/− Mice
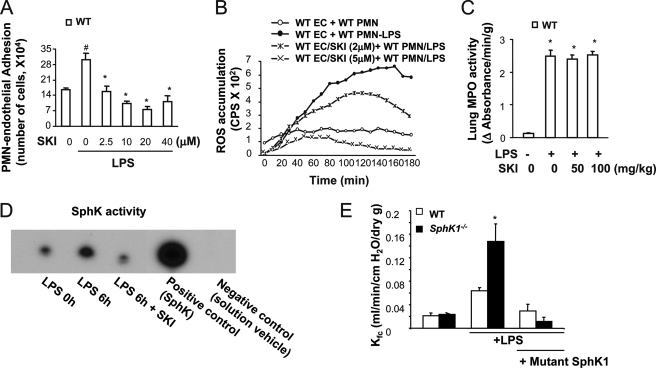
To address whether the phenotype of SphK1−/− mice described above can be mimicked by pharmacological inhibition of SphK1 activity, we used the selective SphK inhibitor SKI (32) to study neutrophil-endothelial interactions. In contrast to enhanced neutrophil adhesion to endothelial cells from SphK1−/− mice, blocking SphK activity with SKI failed to increase neutrophil adhesion to endothelial cells (Fig. 5A). Similar results were obtained on measuring endothelial adhesion-dependent neutrophil oxidant production, i.e. LPS-induced ROS production did not increase in neutrophils adherent to SKI-treated endothelial cells compared with control (no drug treatment) endothelial cells (Fig. 5B). Injection of SKI (intraperitoneal) in WT mice also had no significant effect on LPS-induced lung neutrophil sequestration compared with SphK1−/− mice (Fig. 5C). Injection of SKI (50 mg/kg, intraperitoneal) was effective, however, in blocking SphK activity (Fig. 5D). Thus, inhibition of SphK activity in WT endothelial cells and in mice did not recapitulate the phenotype seen in SphK1−/− mice of augmented neutrophil adhesion to endothelial cells, greater neutrophil oxidant production, and increased lung neutrophil sequestration.
FIGURE 5.
Effects of inhibition of SphK on neutrophil adhesion to endothelial cells, neutrophil oxidant generation, and neutrophil sequestration and lung vascular permeability. A, neutrophil adhesion to WT MLVEC in the presence or absence of the SphK inhibitor SKI. # denotes increase (p < 0.05) compared with unchallenged control cells; * denotes decrease (p < 0.05) compared with non-SKI, but with LPS-treated cells. Results are means of four independent experiments; bars indicate means ± S.E. B, superoxide generation in the presence and absence of SKI. The figure shows representative curves from one of three independent experiments that produced similar results. C, lung neutrophil sequestration as measured by MPO activity in the presence and absence of SKI. * denotes increase (p < 0.05) compared with unchallenged control cells. Results are mean of four independent experiments; bars indicate mean ± S.E. D, examination of SphK activity. SphK inhibitor SKI prevented the activation of SphK induced by LPS. E, change of microvessel permeability (measured as Kf,c) in WT and SphK1−/− lungs from mice transduced with the mutant SphK1 plasmid in the presence and absence of LPS challenge (10 mg/kg, intraperitoneal). PMN, polymorphonuclear leukocytes; EC, endothelial cells.
To address whether SphK1 is sufficient to mediate the down-regulation of lung inflammation and injury induced by LPS, we next carried out an in vivo experiment in which the mice were administered a dominant-negative mutant (G82D) plasmid of SphK1 using the liposome method (33). In WT and SphK1−/− mice challenged with LPS 46 h after mutant SphK1 plasmid administration, we observed a significant reduction in lung vascular permeability in the mutant SphK1 plasmid-expressing SphK1−/−mice compared with vector plasmid-treated SphK1−/− controls (Fig. 5E). These results show that SphK1 is indispensable for the down-regulation of neutrophil-endothelial interaction and lung vascular hyper-permeability induced by LPS.
SphK1 Functions by Suppressing LPS-induced JNK Activation and JNK Association with JIP3
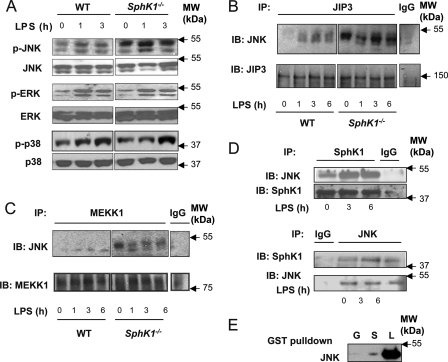
To address the SphK-dependent mechanism preventing LPS-induced lung inflammation, we focused on MAPKs known to be activated during LPS-induced acute lung injury (34). Studies have also shown the critical role of Sphk1 in mediating LPS-induced MAPK activation in macrophages (9). Thus, we addressed the possibility that SphK1 deletion interferes with the MAPK pathway upon LPS stimulation. We observed that loss of function of SphK1 has no effect on the LPS-induced activation of p38 MAPK and ERK as assessed by phosphorylation-specific antibodies (Fig. 6A). However, LPS significantly increased the activation of JNK in these cells (Fig. 6A). As JNK activation by LPS is mediated through the JNK-interacting protein 3 (JIP3) (16), we next investigated the role of JIP3 in the response. We observed a markedly greater association of JNK and JIP3 in the SphK1−/− cells compared with WT (Fig. 6B). A similar result was obtained in the interaction between JNK and MEKK1 (Fig. 6C).
FIGURE 6.
SphK1 modulates JNK activation and interaction of JNK with JIP3. A, phosphorylation of MAPKs JNK, ERK, and p38 in lungs of WT or SphK1−/− mice. Mice were stimulated with LPS (10 mg/kg) for the times indicated. Equal protein loading was verified by membrane probing with anti-p38, -JNK, and -ERK antibodies. B and C, association of JNK with JIP3 (B) and MEKK1 (C) in WT and SphK1−/− lungs. Whole lung lysates were prepared after LPS stimulation, and equal amounts of protein were immunoprecipitated (IP) with anti-JIP3 and anti-MEKK1 antibodies and immunoblotted (IB) with antibodies against JNK and MEKK1. D, co-immunoprecipitation of JNK and SphK1 in WT lungs. Whole lung lysates were prepared after LPS stimulation, and equal amounts of protein were immunoprecipitated with anti-JNK or anti-SphK1 antibody and immunoblotted with antibody against SphK1 or JNK. E, interaction between JNK and SphK1. Purified JNK protein was incubated with purified GST (G) or GST-SphK1 (S) and blotted with anti-JNK antibody, respectively. L, JNK loading control.
To explore further how SphK1 prevented JNK association with JIP3 and MEKK1, we determined the binding interaction of SphK1 to JNK and whether the binding could compete with binding of JNK to JIP3. We observed that LPS indeed increased the interaction between JNK and SphK1 as evident by immunoprecipitation analysis using either anti-SphK1 or anti-JNK antibody (Fig. 6D). In addition, SphK1 was bound directly to JNK as assessed by protein interaction in vitro (Fig. 6E). Thus, SphK1 interacts with JNK and thereby prevents JNK associated with JIP3 and abrogates JNK activation.
Inhibition of JNK Activation Suppresses LPS-induced Neutrophil-Endothelial Interaction in SphK−/− Mice
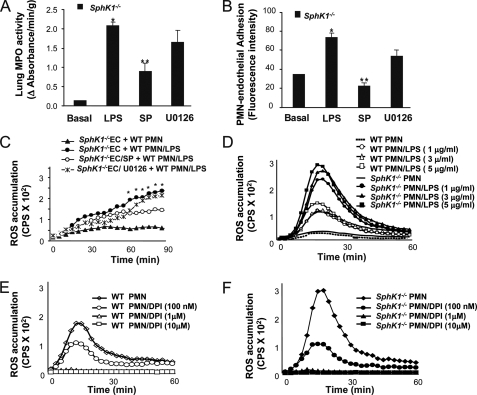
We next determined whether the increased JNK activation was responsible for the greater neutrophil binding to endothelial cells and lung neutrophil sequestration. Here, we assessed lung neutrophil sequestration and neutrophil adhesion in SphK1−/− mice in the presence of the JNK inhibitor SP600125. SP600125 significantly reduced LPS-induced lung neutrophil sequestration seen in SphK1−/− mice (Fig. 7A), whereas the ERK inhibitor, U0126, had no significant effect (Fig. 7A). Similar results were obtained with neutrophil adhesion to endothelial cells, i.e. the JNK inhibitor, SP600125, but not the ERK inhibitor reduced neutrophil adhesion to SphK1−/− MLVEC (Fig. 7B). These data demonstrate that the augmented activation of JNK in SphK1−/− mice mediates the greater lung neutrophil sequestration and oxidant production induced by LPS.
FIGURE 7.
JNK mediates lung neutrophil sequestration, neutrophil adhesion, and NADPH oxidase activation following the deletion of SphK1. A, lung neutrophil sequestration as measured by MPO activity in the presence and absence of JNK and ERK inhibitors. * denotes increase (p < 0.05) compared with unchallenged control mice; ** denotes decrease (p < 0.05) in SP600125 (SP)-treated SphK1−/− mice compared with non-SP600125-treated SphK1−/− mice post-LPS stimulation. Results are the mean of four independent experiments; bars indicate mean ± S.E. B, neutrophil adhesion to WT MLVEC in the presence or absence of JNK and ERK inhibitors. * denotes increase (p < 0.05) compared with no LPS; ** denotes decrease (p < 0.05) to SP600125-treated SphK1−/− endothelial cells (EC) compared with no SP600125-treated SphK1−/− endothelial cells post-LPS stimulation. Results are mean of four independent experiments. Bars indicate mean ± S.E. C, superoxide generation in the presence and absence of JNK and ERK inhibitors. *, p < 0.05 compared with “SphK1−/−EC/SP + WT PMN/LPS” group. D–F, dose dependence of LPS-induced ROS production (D) and diphenyleneiodonium inhibition of ROS generation after LPS challenge in WT neutrophils (E) or SphK1−/− neutrophils (F). Diphenyleneiodonium (DPI) (100 nm, 1 μm, and 10 μm) was added 30 min prior to LPS stimulation. ROS generation by Sphk1−/− and WT neutrophil were stimulated with LPS. PMN, polymorphonuclear leukocytes.
JNK Activation Signals NADPH Oxidase
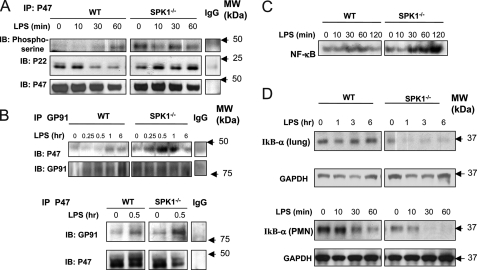
Because we observed that LPS-induced oxidant production was greater in WT neutrophils adherent to SphK1−/− endothelial cells compared with WT neutrophils adherent to WT endothelial cells (Fig. 3D) and inhibition of JNK activation suppressed LPS-induced increased neutrophil-endothelium interaction in SphK−/− mice, we addressed whether the greater production of ROS was mediated through enhanced activation of JNK in SphK−/− mice. As shown in Fig. 7C, SP600125 (JNK inhibitor) significantly reduced LPS-induced oxidant production by neutrophils adherent to SphK1−/− MLVEC, whereas the ERK inhibitor, U0126, had little effect (Fig. 7C). Thus, the increased activation of JNK in SphK1−/− mice mediates the augmented oxidant production induced by LPS. We followed up by determining the mechanism of the increased ROS production mediated by the JNK activation. LPS stimulated ROS production in neutrophils in a dose-dependent manner (Fig. 7D), and diphenyleneiodonium (inhibitor of NADPH oxidase) prevented ROS release from in both WT and SphK1−/− neutrophils in a dose-dependent manner (Fig. 7, D–F), indicating increased ROS release was mediated by the activation of NADPH oxidase. NADPH oxidase is composed of membrane-bound gp91Phox and p22Phox subunits as well as cytosolic subunits p47Phox, p67Phox, and monomeric GTPase Rac (4). NADPH oxidase activation involves the translocation of cytosolic oxidase components (p47Phox, p67Phox, and Rac) to the plasma membrane and phosphorylation of p47Phox (4). We found that phosphorylation of p47Phox was increased in SphK1−/− neutrophils (Fig. 8A), and the formation of p47Phox with p22Phox complex and of p47Phox with gp91Phox complex was also increased in SphK1−/− lungs in response to LPS relative to controls (Fig. 8, A and B). These findings show that JNK regulates NADPH oxidase activation through the phosphorylation of p47Phox and membrane translocation of p47Phox. Importantly, the expression of the redox-sensitive pro-inflammatory transcription factor NF-κB, downstream of NADPH oxidase activation, was also up-regulated in SphK1−/− cells, whereas its negative regulator, IκBα, was down-regulated as compared with WT (Fig. 8, C and D).
FIGURE 8.
SphK1 down-regulates the activation of NADPH oxidase and NF-κB. A, immunoprecipitation (IP) of p47Phox and immunoblotting (IB) with antibodies against phosphoserine, p22Phox, and p47Phox. The activations of p47Phox and its associations with p22Phox were augmented in neutrophil from SphK1−/− mice. B, immunoprecipitation of gp91Phox and p47Phox and immunoblotting with antibodies against p47Phox and gp91Phox. There are LPS stimulation-dependent associations of gp91Phox with p47Phox in lung from SphK1−/− mice. C, augmented LPS-induced NF-κB activation in neutrophils from SphK1−/− mice. Neutrophils were stimulated with LPS for the indicated times. Nuclear extracts from neutrophils were used for EMSA analysis. D, augmented LPS-induced IκB-α degradation in lungs and neutrophils from SphK1−/− mice. The expression of IκB-α was determined by Western blotting. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression is shown as control.
DISCUSSION
Our results have identified a novel kinase-independent pathway by which SphK1 modulates LPS-induced inflammation and injury. We showed SphK1 prevents LPS-induced lung inflammatory injury through blocking the binding and recruitment of neutrophils to the vascular endothelium as well as reducing neutrophil adhesion-dependent oxidant production. This anti-inflammatory function of SphK1 is the result of the direct interaction of SphK1 with JNK (as assessed by in vitro assays), which prevented JNK activation through the binding to the protein JIP3.
The primary data in support of this conclusion are as follows. (i) LPS stimulation fails to significantly increase S1P production in the plasma, lungs, and neutrophils of both WT and SphK1−/− mice. (ii) SKI, the SphK inhibitor, fails to increase neutrophil adhesion to endothelial cells, neutrophil oxidant production, and lung neutrophil sequestration that was seen in the SphK1−/− mice. (iii) Expression of the dominant-negative mutant of SphK1(G82D) (21, 35) prevented the augmented increase in lung vascular permeability seen in SphK1−/− mice in response to LPS. (iv) LPS augments the interaction between JNK and SphK1. (v) SphK1 binding to JNK prevents JNK binding to JIP3 and in turn abrogates the activation of NADPH oxidase and generation of ROS responsible for inflammation.
Upon LPS stimulation, JIP3 is known to be recruited to toll-like receptor 4 (TLR4) and recruits MEKK1, which facilitates the activation of JNK. In addition, SphK1 may also be recruited to the membrane after LPS stimulation where it binds TRAF6 (9, 36). TRAF6 was shown to be associated with TLR4 through MyD88 and interleukin 1 receptor-associated kinase (37). Thus, recruitment of both JNK and SphK1 to the TLR4 complex after LPS stimulation raises the possibility, which we addressed here, that SphK1 functions to suppress JNK activation. We observed that the heightened LPS-induced interaction between SphK1 and JNK neutralizes the inflammatory activity of JNK thus preventing its ability to bind to JIP3.
How are our data consistent with the model described in Fig. 9? First, we observed that SphK1 directly interacted with JNK, and this interaction increased on stimulation with LPS. Second, deletion of SphK1 resulted in increased phosphorylation of JNK and increased association of JNK and JIP3 and the activation of JNK signaling. Third, we observed that JNK inhibition significantly reduced the LPS-induced lung neutrophil sequestration seen in the SphK1−/− mice and neutrophil adhesion to SphK1−/− MLVEC. Thus, the mechanism of SphK1 mediated protection against LPS-induced lung inflammation and injury involves SphK1-induced suppression of the interaction of JNK with its activating protein JIP3, which block the JNK signaling cascade.
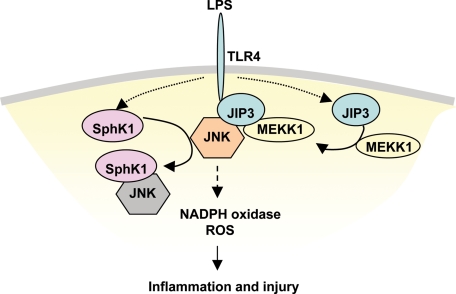
FIGURE 9.
Model of SphK1-mediated protection against LPS-induced lung inflammation and injury. Upon LPS stimulation, JIP3, which mediates JNK activation by LPS (16), is recruited to TLR4 and recruits MEKK1. This in turn facilitates the activation of JNK. LPS promotes increased interaction of SphK1 with JNK and thereby competitively suppresses JNK interaction with its activating protein JIP3, thus blocking JNK activation. Reduced activation of JNK thus leads to reduced downstream activation of NADPH oxidase and prevention of LPS-induced inflammation and injury.
Interestingly, the deletion of SphK1 also resulted in increased activation of NADPH oxidase. These results demonstrate that NADPH oxidase activation occurs downstream of JNK activation. In this context, NADPH oxidase-mediated production of ROS could serve as a positive feedback augmenting the JNK activation and amplifying the inflammation cascade (38–40). Although it is not clear how JNK regulates NADPH oxidase activation (41, 42), our results clearly show that down-regulation of NADPH oxidase activity occurred secondary to the reduced JNK activation. Hence, the ability of SphK1 to sequester JNK and block NADPH oxidase activation plays a pivotal role in dampening LPS-induced inflammation and injury.
The two forms of sphingosine kinases (SphKs), SphK1 and SphK2, have received considerable attention because of their poorly understood functions that are distinct from their catalytic function needed for the production of S1P (43–46). Although SphKs may play a role in mediating inflammation seen in asthma (43–47), SphK1 also has a protective function in LPS- and platelet-activating factor-induced lung inflammation (35, 48, 49). This function may be independent of the S1P production. Studies have demonstrated that plasma S1P generation can decrease the basal endothelial permeability and mitigate the increase in permeability in response to mediators such as platelet-activating factor (49). The present data show that SphK1-mediated prevention of inflammation and injury induced by LPS is independent of S1P production because SIP production did not increase as would be expected if S1P is to play a crucial anti-inflammatory role in the present studies. Instead, the interaction of SphK1 with JNK was crucial in the inhibition of JNK activation and thereby in preventing the activation of NADPH oxidase and LPS-induced lung inflammatory injury.
The JNK → NADPH oxidase signaling pathway has been linked to activation of transcription factor NF-κB and de novo synthesis of adhesion molecules and chemokines (50–52). The activation of this pathway is consistent with our observation of increased expression of ICAM-1 and chemokines MIP-1 and MIP-2 in the SphK1−/− mice.
Thus, the present findings have uncovered the potentially important immunomodulatory role of SphK1 that is independent of its catalytic activity. We showed that SphK1-mediated sequestration of JNK could serve as a therapeutic target for the prevention of LPS-induced lung inflammatory injury.
Acknowledgments
We thank R. L. Proia (National Institutes of Health) for providing SphK1−/− mice and W. Wu and Y. Li for useful suggestions and reading the manuscript. We also thank G. Liu, C. Gilbert, D. Visintine, and D. Salvi for technical assistance, and we thank A. Skobeleva (University of Chicago) for technical assistance with mass spectrometric measurements of S1P content in plasma and tissues.
This work was supported, in whole or in part, by National Institutes of Health Grant P01 HL77806. This work was also supported by a Parker B. Francis Fellowship in Pulmonary Research.
- LPS
- lipopolysaccharide
- fMLP
- formyl-methionyl-leucyl-phenylalanine
- MEKK1
- MAPK kinase kinase 1
- MLVEC
- mouse lung vascular endothelial cells
- MPO
- myeloperoxidase
- SKI
- SphK inhibitor
- S1P
- sphingosine 1-phosphate
- WT
- wild type
- JNK
- c-Jun N-terminal kinase
- ROS
- reactive oxygen species.
REFERENCES
- 1.Reutershan J., Ley K. (2004) Crit. Care 8, 453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodman R. B., Strieter R. M., Martin D. P., Steinberg K. P., Milberg J. A., Maunder R. J., Kunkel S. L., Walz A., Hudson L. D., Martin T. R. (1996) Am. J. Respir. Crit. Care Med. 154, 602–611 [DOI] [PubMed] [Google Scholar]
- 3.Wang Q., Doerschuk C. M. (2002) Antioxid. Redox. Signal. 4, 39–47 [DOI] [PubMed] [Google Scholar]
- 4.Frey R. S., Ushio-Fukai M., Malik A. (2009) Antioxid. Redox. Signal. 11, 791–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McVerry B. J., Peng X., Hassoun P. M., Sammani S., Simon B. A., Garcia J. G. (2004) Am. J. Respir. Crit. Care Med. 170, 987–993 [DOI] [PubMed] [Google Scholar]
- 6.Peng X., Hassoun P. M., Sammani S., McVerry B. J., Burne M. J., Rabb H., Pearse D., Tuder R. M., Garcia J. G. (2004) Am. J. Respir. Crit. Care Med. 169, 1245–1251 [DOI] [PubMed] [Google Scholar]
- 7.Oskeritzian C. A., Milstien S., Spiegel S. (2007) Pharmacol. Ther. 115, 390–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Venkataraman K., Thangada S., Michaud J., Oo M. L., Ai Y., Lee Y. M., Wu M., Parikh N. S., Khan F., Proia R. L., Hla T. (2006) Biochem. J. 397, 461–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu W., Mosteller R. D., Broek D. (2004) Mol. Cell. Biol. 24, 7359–7369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yadav M., Clark L., Schorey J. S. (2006) J. Immunol. 176, 5494–5503 [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim F. B., Pang S. J., Melendez A. J. (2004) J. Biol. Chem. 279, 44802–44811 [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharya J. (2004) Am. J. Respir. Crit. Care Med. 170, 928–929 [DOI] [PubMed] [Google Scholar]
- 13.Takabe K., Paugh S. W., Milstien S., Spiegel S. (2008) Pharmacol. Rev. 60, 181–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arndt P. G., Suzuki N., Avdi N. J., Malcolm K. C., Worthen G. S. (2004) J. Biol. Chem. 279, 10883–10891 [DOI] [PubMed] [Google Scholar]
- 15.Arndt P. G., Young S. K., Lieber J. G., Fessler M. B., Nick J. A., Worthen G. S. (2005) Am. J. Respir. Crit. Care Med. 171, 978–986 [DOI] [PubMed] [Google Scholar]
- 16.Matsuguchi T., Masuda A., Sugimoto K., Nagai Y., Yoshikai Y. (2003) EMBO J. 22, 4455–4464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J., Gao X. P., Ramchandran R., Zhao Y. Y., Vogel S. M., Malik A. B. (2008) Nat. Immunol. 9, 880–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garrean S., Gao X. P., Brovkovych V., Shimizu J., Zhao Y. Y., Vogel S. M., Malik A. B. (2006) J. Immunol. 177, 4853–4860 [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y., Kalari S. K., Usatyuk P. V., Gorshkova I., He D., Watkins T., Brindley D. N., Sun C., Bittman R., Garcia J. G., Berdyshev E. V., Natarajan V. (2007) J. Biol. Chem. 282, 14165–14177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao X. P., Zhu X., Fu J., Liu Q., Frey R. S., Malik A. B. (2007) J. Biol. Chem. 282, 6116–6125 [DOI] [PubMed] [Google Scholar]
- 21.Pitson S. M., Moretti P. A., Zebol J. R., Xia P., Gamble J. R., Vadas M. A., D'Andrea R. J., Wattenberg B. W. (2000) J. Biol. Chem. 275, 33945–33950 [DOI] [PubMed] [Google Scholar]
- 22.Miyawaki-Shimizu K., Predescu D., Shimizu J., Broman M., Predescu S., Malik A. B. (2006) Am. J. Physiol. Lung Cell Mol. Physiol. 290, L405–L413 [DOI] [PubMed] [Google Scholar]
- 23.Tate R. M., Repine J. E. (1983) Am. Rev. Respir. Dis. 128, 552–559 [DOI] [PubMed] [Google Scholar]
- 24.Malik A. B., Lo S. K. (1996) Pharmacol. Rev. 48, 213–229 [PubMed] [Google Scholar]
- 25.Albelda S. M., Smith C. W., Ward P. A. (1994) FASEB J. 8, 504–512 [PubMed] [Google Scholar]
- 26.Ley K. (1996) Cardiovasc. Res. 32, 733–742 [PubMed] [Google Scholar]
- 27.Smith C. W. (1993) Semin. Hematol. 30, 45–55 [PubMed] [Google Scholar]
- 28.Hordijk P. (2003) Cell. Biochem. Biophys. 38, 305–322 [DOI] [PubMed] [Google Scholar]
- 29.Gautam N., Herwald H., Hedqvist P., Lindbom L. (2000) J. Exp. Med. 191, 1829–1839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abraham E. (2003) Crit. Care Med. 31, S195–S199 [DOI] [PubMed] [Google Scholar]
- 31.Allende M. L., Sasaki T., Kawai H., Olivera A., Mi Y., van Echten-Deckert G., Hajdu R., Rosenbach M., Keohane C. A., Mandala S., Spiegel S., Proia R. L. (2004) J. Biol. Chem. 279, 52487–52492 [DOI] [PubMed] [Google Scholar]
- 32.French K. J., Schrecengost R. S., Lee B. D., Zhuang Y., Smith S. N., Eberly J. L., Yun J. K., Smith C. D. (2003) Cancer Res. 63, 5962–5969 [PubMed] [Google Scholar]
- 33.Zhou M. Y., Lo S. K., Bergenfeldt M., Tiruppathi C., Jaffe A., Xu N., Malik A. B. (1998) J. Clin. Invest. 101, 2427–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Togbe D., Schnyder-Candrian S., Schnyder B., Doz E., Noulin N., Janot L., Secher T., Gasse P., Lima C., Coelho F. R., Vasseur V., Erard F., Ryffel B., Couillin I., Moser R. (2007) Int. J. Exp. Pathol. 88, 387–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wadgaonkar R., Patel V., Grinkina N., Romano C., Liu J., Zhao Y., Sammani S., Proia R., Garcia J. G., Natarajan V. (2009) Am. J. Physiol. Lung Cell Mol. Physiol. 296, L603–L613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryu J., Kim H. J., Chang E. J., Huang H., Banno Y., Kim H. H. (2006) EMBO J. 25, 5840–5851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barton G. M., Medzhitov R. (2003) Science 300, 1524–1525 [DOI] [PubMed] [Google Scholar]
- 38.Luo J. L., Kamata H., Karin M. (2005) J. Clin. Invest. 115, 2625–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ventura J. J., Cogswell P., Flavell R. A., Baldwin A. S., Jr., Davis R. J. (2004) Genes Dev. 18, 2905–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen H. M., Liu Z. G. (2006) Free Radic. Biol. Med. 40, 928–939 [DOI] [PubMed] [Google Scholar]
- 41.Papa S., Bubici C., Zazzeroni F., Pham C. G., Kuntzen C., Knabb J. R., Dean K., Franzoso G. (2006) Cell Death Differ. 13, 712–729 [DOI] [PubMed] [Google Scholar]
- 42.Kawasumi M., Hashimoto Y., Chiba T., Kanekura K., Yamagishi Y., Ishizaka M., Tajima H., Niikura T., Nishimoto I. (2002) Neurosignals 11, 236–250 [DOI] [PubMed] [Google Scholar]
- 43.Hait N. C., Oskeritzian C. A., Paugh S. W., Milstien S., Spiegel S. (2006) Biochim. Biophys. Acta 1758, 2016–2026 [DOI] [PubMed] [Google Scholar]
- 44.Beaven M. A. (2007) Immunity 26, 271–273 [DOI] [PubMed] [Google Scholar]
- 45.Olivera A. (2008) Prostaglandins Other Lipid Mediat. 86, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosen H., Goetzl E. J. (2005) Nat. Rev. Immunol. 5, 560–570 [DOI] [PubMed] [Google Scholar]
- 47.Olivera A., Mizugishi K., Tikhonova A., Ciaccia L., Odom S., Proia R. L., Rivera J. (2007) Immunity 26, 287–297 [DOI] [PubMed] [Google Scholar]
- 48.Tauseef M., Kini V., Knezevic N., Brannan M., Ramchandaran R., Fyrst H., Saba J., Vogel S. M., Malik A. B., Mehta D. (2008) Circ. Res. 103, 1164–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Camerer E., Regard J. B., Cornelissen I., Srinivasan Y., Duong D. N., Palmer D., Pham T. H., Wong J. S., Pappu R., Coughlin S. R. (2009) J. Clin. Invest. 119, 1871–1879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis R. J. (2000) Cell 103, 239–252 [DOI] [PubMed] [Google Scholar]
- 51.Chen C. C., Chow M. P., Huang W. C., Lin Y. C., Chang Y. J. (2004) Mol. Pharmacol. 66, 683–693 [DOI] [PubMed] [Google Scholar]
- 52.Roebuck K. A., Carpenter L. R., Lakshminarayanan V., Page S. M., Moy J. N., Thomas L. L. (1999) J. Leukocyte Biol. 65, 291–298 [DOI] [PubMed] [Google Scholar]