Abstract
The human β-globin gene is expressed at high levels in erythroid cells and regulated by proximal and distal cis-acting DNA elements, including promoter, enhancer, and a locus control region (LCR). Transcription complexes are recruited not only to the globin gene promoters but also to the LCR. Previous studies have implicated the ubiquitously expressed transcription factor USF and the tissue-restricted activator NF-E2 in the recruitment of transcription complexes to the β-globin gene locus. Here we demonstrate that although USF is required for the efficient association of RNA polymerase II (Pol II) with immobilized LCR templates, USF and NF-E2 together regulate the association of Pol II with the adult β-globin gene promoter. Recruitment of Pol II to the LCR occurs in undifferentiated murine erythroleukemia cells, but phosphorylation of LCR-associated Pol II at serine 5 of the C-terminal domain is mediated by erythroid differentiation and requires the activity of NF-E2. Furthermore, we provide evidence showing that USF interacts with NF-E2 in erythroid cells. The data provide mechanistic insight into how ubiquitous and tissue-restricted transcription factors cooperate to regulate the recruitment and activity of transcription complexes in a tissue-specific chromatin domain.
Keywords: DNA-binding Protein, DNA Transcription, Gene Regulation, Hemoglobin, RNA Polymerase II, Transcription Enhancers, Transcription Regulation
Introduction
Eukaryotic transcription is regulated at many different levels, including the modification and remodeling of chromatin structure and perhaps the recruitment of genes to transcription factories (1–3). The DNA sequence in regulatory regions and the trans-acting DNA binding activities control accessibility and position of genes in the nucleus and mediate the recruitment and activity of transcription complexes. Genes that are expressed in a cell type-specific manner are often regulated by a combination of transcription factors that are expressed ubiquitously or in a more tissue-restricted manner (4).
The human β-globin gene locus provides an excellent example for the study of gene regulatory mechanisms during differentiation and development (5, 6). The five β-like globin genes are expressed in a developmental stage-specific manner exclusively in erythroid cells. The genes are regulated by proximal promoter and enhancer elements as well as by the locus control region (LCR),2 a powerful DNA-regulatory element located far upstream of the globin genes and composed of several DNase I-hypersensitive (HS) sites (7–9)(Fig. 1A). Many DNA binding proteins and co-regulators control chromatin structure and gene expression in the globin gene locus (10). However, among these proteins, there is not a single transcription factor that is exclusively expressed in erythroid cells. Thus, it is the combination, the abundance, and perhaps the erythroid-specific modification of transcription factors that turn on the globin genes exclusively in erythroid cells and at the correct developmental stage.
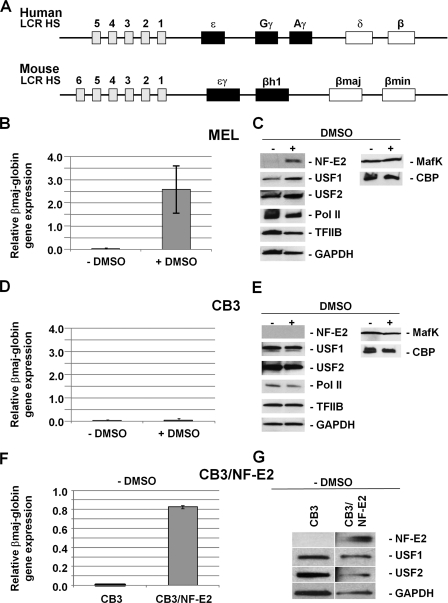
FIGURE 1.
DMSO mediated increase in USF, NF-E2 (p45), and β-globin expression in MEL but not CB3 cells. A, structure of the human and mouse β-globin gene loci. The β-globin gene loci consist of five (human) or four (mouse) genes that are expressed during development. The murine ϵγ- and βh1-globin genes are expressed in embryonic erythroid cells, and the βmin- and βmaj-globin genes are expressed in fetal and adult erythroid cells (70). The human ϵ-globin gene is expressed at the embryonic stage, the Gγ- and Aγ-globin genes are expressed at the fetal stage, and the δ- and β-globin genes are expressed at the adult stage. All of the genes are regulated by an LCR located far upstream of the genes and composed of several erythroid-specific HS sites. B, quantitative RT-PCR analysis of βmaj-globin gene expression in MEL cells incubated with or without 1.5% DMSO for 3 days. RNA was isolated from MEL cells, reverse transcribed, and subjected to qPCR using primers specific for the adult βmaj-globin gene. The results are shown as the relative expression normalized to transcription of the β-actin gene. Error bars, S.E. from three independent experiments. C, Western blotting analysis of NF-E2 (p45), USF1, USF2, Pol II, TFIIB, GAPDH, MafK, and CBP in MEL cells incubated with or without 1.5% DMSO for 3 days. Proteins from whole cell extracts (60 μg) were electrophoresed in 4–20% Ready Gels (Bio-Rad), transferred to nitrocellulose membranes, and incubated with antibodies as indicated. D, quantitative RT-PCR analysis of βmaj-globin gene expression in CB3 cells incubated with or without 1.5% DMSO for 3 days. RNA was isolated and analyzed as described in B. E, Western blotting analysis of NF-E2 (p45), USF1, USF2, Pol II, TFIIB, GAPDH, MafK, and CBP in CB3 cells incubated with or without 1.5% DMSO for 3 days. Proteins were processed and analyzed as described in C. F, quantitative RT-PCR analysis of βmaj-globin gene expression in CB3 and CB3/NF-E2 cells. RNA was processed and analyzed as described in B. G, Western blotting analysis of NF-E2 (p45), USF1, USF2, and GAPDH expression in CB3 and CB3/NF-E2 cells. Proteins were processed and analyzed as described in C.
Among the many proteins that regulate expression of the adult β-globin gene are NF-E2 and USF (11–13). Both transcription factors interact with LCR HS sites and with the β-globin gene promoter (14–19). NF-E2 is a heterodimer composed of the hematopoietic specific subunit p45 and a ubiquitously expressed subunit p18 (small Maf protein) (20, 21). The p45 subunit provides a leucine zipper dimerization motif and both DNA binding and activation domains, whereas the p18 subunit only carries the leucine zipper and DNA binding motif. NF-E2 binds to a DNA sequence referred to as the Maf recognition element (MARE), which is located in LCR HS sites 2, 3, and 4 (22). NF-E2 also interacts directly or indirectly with the adult β-globin gene promoter, which lacks a consensus MARE sequence (18, 19). NF-E2 associates with co-regulators (e.g. MLL2 and CBP/p300) and components of the basal Pol II transcription apparatus (23, 24). Recently, the Brand and Groudine laboratories (25, 26) characterized proteins that interact with the small MafK protein during differentiation of murine erythroleukemia (MEL) cells. These studies demonstrated that MafK is part of a large protein complex whose composition changes during erythroid differentiation. The MafK protein complex contains different activities that modify chromatin structure, suggesting that NF-E2 plays a role in chromatin opening of the globin gene locus. Other studies have shown that NF-E2 (p45) is required for the efficient recruitment of Pol II to the adult β-globin gene promoter but not to the LCR (27). Several studies have shown that NF-E2 binding sites are required for full activity of globin gene-associated regulatory elements (28–31).
Despite all of the evidence pointing to an important role of NF-E2 (p45) in the regulation of globin gene expression, mice deficient for p45 reveal only a mild defect in erythropoiesis and globin gene expression (32). The mild reduction in globin gene expression in these mice may in part be due to the presence of two NF-E2-related proteins in erythroid cells, which may compensate for the lack of NF-E2 (22). There are no mice available that are depleted for NF-E2 and the two NF-E2-related proteins. Nonetheless, NF-E2-deficient MEL cells express very low levels of the globin genes, and it is currently not known why the NF-E2-related proteins fail to partially replace NF-E2 in this system (12). In addition, Kotkow and Orkin (33) demonstrated that expression of a dominant negative p18 (small Maf) protein in MEL cells reduces the activity of NF-E2 and represses globin gene expression. These data demonstrate that the MEL cell system is ideally suited to study the function of NF-E2 (p45).
The transcription factor USF is a dimeric helix-loop-helix protein normally composed of the closely related subunits USF1 and USF2, although homodimers can form as well (34). USF binds to classical E-box elements (CANNTG) and regulates transcription through the recruitment of co-regulator complexes (35–37). USF has been shown to interact with LCR element HS2 and with the adult β-globin gene promoter, and expression of a dominant negative mutant of USF in erythroid cells reduces the recruitment of Pol II to these cis-regulatory elements (13–15, 38). USF has also been shown to bind to and to contribute to the function of chromosomal barrier elements (39, 40). Recent work has demonstrated that USF interacts with the histone acetyltransferase CBP and with the histone methyltransferase Prmt1 (36, 37). The function of USF in regulating transcription and barrier activity is probably mediated at least in part by these co-factors.
We show here that Pol II, NF-E2, USF1, USF2, TFIIB, and the coactivator CBP are efficiently recruited to LCR element HS2, whereas only TFIIB and USF2 are recruited to the adult βmaj-globin gene promoter in undifferentiated MEL cells. Upon induction of erythroid differentiation, these proteins are also detectable at the adult βmaj-globin gene promoter. Pol II phosphorylated at serine 5 of the C-terminal domain (CTD) is first detectable at the LCR but not at the globin gene during differentiation of MEL cells. In MEL cells deficient for NF-E2 (p45), recruitment of Pol II at the βmaj-globin gene locus is reduced, consistent with previous data (27). Interestingly, however, there is a lack of serine 5 phosphorylated Pol II at LCR HS2 and the adult βmaj-globin gene promoter in NF-E2 deficient cells. Re-expression of NF-E2 in these cells leads to increased recruitment of Pol II and phosphorylation of Pol II at the βmaj-globin gene promoter. We further demonstrate that USF2 interacts with Pol II in both undifferentiated and in differentiated MEL cells. In contrast, USF1 only interacts with Pol II in differentiated MEL cells. Finally, we demonstrate that although USF is required for the recruitment of Pol II to the LCR, NF-E2 appears to dissociate Pol II from the LCR in a β-globin promoter-dependent manner and together with USF mediates the recruitment of Pol II to the adult β-globin gene promoter.
EXPERIMENTAL PROCEDURES
Cell Culture and Protein Extracts
MEL cells were grown in Dulbecco's modified Eagle's medium (Cellgro) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin antibiotic mixture. CB3 and CB3/NF-E2 cells were grown in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum, 200 mm l-glutamine, and 1% penicillin/streptomycin antibiotic mixture. For DMSO-induced cells, 1 × 105 cells/ml MEL or CB3 cells were incubated with 1.5% DMSO for 72 h. All cells were grown in 5% CO2 at 37 °C.
Nuclear extracts used in co-immunoprecipitation experiments were prepared as described by Leach et al. (16). Whole cell extracts used in the in vitro dissociation assay were prepared as described previously (41). Whole cell extracts used in Western blotting experiments were prepared using radioimmunoprecipitation assay buffer (50 mm Tris-HCl (pH 7.4), 100 mm NaCl, 10 mm EDTA, 0.25% sodium deoxycholate, 1% Nonidet P-40, 0.1% SDS, and protease inhibitor mixture; Roche Applied Science). The cDNAs of NF-E2 (p45 tethered to MafG) (16, 42) and A-USF (13) were subcloned into pET-28a(+) and pET-19b vector (Novagen), respectively, and the recombinant proteins were expressed in Escherichia coli according to the manufacturer's protocol.
Western Blotting
Western blotting experiments were performed as described by Leach et al. (16). A total of 60 μg of whole cell extracts, unless otherwise noted, were loaded onto 4–20% Ready gel (Bio-Rad). The proteins were visualized by ECL Plus chemiluminescence (Amersham Biosciences). The following antibodies were used: GAPDH (FL-335; sc-25778), NF-E2 (C-19; sc-291), NF-E2 p18 (MafK) (C-16; sc-477), USF1 (C-20; sc-229), USF2 (C-20; sc-862), TFIIB (C-18; sc-225), CBP (A-22; sc-369), and goat anti-mouse IgG-horseradish peroxidase (sc-2005) (all purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA)), goat anti-rabbit IgG-horseradish peroxidase (Kirkegaard & Perry Laboratories), and anti-rabbit IgG Trueblot (eBioscience). These antibodies were used for all of the Western blotting and for the co-immunoprecipitation experiments. The concentrations of the antibodies used followed the manufacturers' guidelines.
Chromatin Immunoprecipitation (ChIP)
The ChIP assay was performed essentially as described by Leach et al. with minor modifications (16). After preclearing the diluted cell lysate with Protein A-Sepharose beads, 2 μg of the appropriate antibody was used for each ChIP sample. For ChIP assays using mouse IgM antibodies, Dynabeads® rat anti-mouse IgM (a gift from Scott R. Jamison (Invitrogen)) was used instead of Protein A-Sepharose beads.
The antibodies used were the same as those described for the Western blotting experiments except for the following antibodies: normal mouse IgM (Santa Cruz Biotechnology, Inc.), rabbit IgG (Bethyl Laboratories), RNA polymerase II, clone CTD4H8, 05-623 (Upstate); RNA polymerase II 8WG16 monoclonal antibody and RNA polymerase II H14 (Ser(P)-5-Pol II) monoclonal antibody (Covance), and RNA polymerase II Ser(P)-2-specific antibody (Abcam, catalog no. ab5095). Samples were analyzed by quantitative real-time PCR using myiQ (Bio-Rad). The murine βmaj and HS2 primers used for ChIP samples were described by Crusselle-Davis et al. (13). GAPDH and the HS3/2 flanking region were analyzed using primers described by Levings et al. (43). The p values were determined by three independent experiments and calculated by using the Microsoft Excel t test function. p < 0.05 and p < 0.1 are considered to be statistically significant.
Co-immunoprecipitation (Co-IP) and GST Pull-down Assay
For each co-IP experiment, 1 mg of nuclear extract from MEL or CB3 cells was used. The nuclear extracts were precleared by incubating with anti-rabbit IgG beads (eBioscience) for 30 min. The precipitation was achieved by incubating 2.5 μg of appropriate antibody with the precleared cell nuclear extract for 2.5 h on a spinning wheel at 4 °C. The protein complexes were captured by incubating the antibody containing cell nuclear extracts with anti-rabbit IgG beads for 2 h. Then the beads were collected and washed three times with radioimmunoprecipitation assay buffer. All of the incubations were performed at 4 °C on a spinning wheel. The immunoprecipitated protein complexes were eluted with Laemmli buffer (Bio-Rad) at 95 °C for 10 min and then loaded onto 4–20% Ready Gel (Bio-Rad) and analyzed by Western blotting. The antibodies used for co-IP are the same as those used for Western blotting experiments, except that Pol II (N-20) sc-899 (Santa Cruz Biotechnology, Inc.) was used for the co-IP experiments.
The cDNAs encoding full-length or C-terminal truncated human USF1 (hUSF1) were inserted into the pGEX-5X-1 vector (Promega) as described by Huang et al. (36). The USF1-N construct was generated by removing a BlpI/XhoI fragment, encoding amino acids 202–310, from pGEX-5X-1-USF1 (36). The USF1-LZ− construct was generated by removing a NheI/SacI fragment, encoding amino acids 262–291, from pGEX-5X-1-USF1 (36). GST and wild-type and mutant GST-hUSF1 fusion proteins were expressed in and purified from E. coli. Equal amounts of the proteins were coupled to glutathione-Sepharose 4 fast flow beads (GE Healthcare, catalog no. 17-5132-01) according to the manufacturer's instructions. Equal amounts of protein-coupled beads were incubated with recombinant NF-E2 (16) in 300 μl of pull-down binding buffer (50 mm Tris-HCl, pH 8.0, 2 mm EDTA, 150 mm NaCl, 0.1% Nonidet P-40, 0.1 μg/μl BSA, 1 mm dithiothreitol, 1 mm phenylmethylsulfonyl fluoride) for 1 h at 4 °C. The beads were washed 3–5 times with 1 ml of pull-down binding buffer (without BSA), collected, boiled with Laemmli sample buffer (Bio-Rad), and loaded onto 4–20% Ready gels (Bio-Rad), followed by a Western blotting assay with antibody against NF-E2 (C-19; Santa Cruz Biotechnology, Inc., catalog no. sc-291).
RNA Isolation, Reverse Transcription, and Real-time PCR
RNA was isolated by using the guanidine thiocyanate method (44) and reverse transcribed by using iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer's protocol. Quantitative PCR (qPCR) was performed using MyiQ (Bio-Rad), and reactions were carried out using iQ SYBR Green super mix (Bio-Rad). Real-time PCR analysis of cDNA was performed using the following cycles: 95 °C for 5 min, 40 cycles of 94 °C for 30 s, 60 °C for 30 s, 72 °C for 1 min, and final extension at 72 °C for 5 min. β-Actin was used as endogenous control for RT-qPCR. The qPCR primers used for amplifying the murine βmaj-globin gene were the same as previously described by Levings et al. (43). The RT primers for β-actin were as follows: US, 5′- GTGGGCCGCTCTAGGCACCA-3′; DS, 5′-TGGCCTTAGGGTGCAGGGGG-3′.
In Vitro Transfer/Dissociation Assay
The in vitro Pol II transfer/dissociation analysis was performed as described by Vieira et al. (45) with minor modifications. Briefly, a plasmid containing the wild-type human β-globin LCR was linearized and immobilized on streptavidin-coated magnetic beads, as described by Leach et al. (46). 1.5 μg of the immobilized LCR was incubated with 500 μg of MEL whole cell extracts for 30 min at 30 °C, and then unbound material was removed from the beads. 1 μg of wild-type or mutant human β-globin gene construct was added in experiments in which dissociation was assayed in the presence of DNA templates. The three β-globin gene mutants, pRSβINImut (INImut), pRSβ+60E-boxmutb (+60Eboxmut), and pRSβMAREa (NF-E2mut1), as well as a β-globin gene construct lacking a 1.2-kbp fragment including the promoter (β−), were described previously by Leach et al. (16) and Vieira et al. (45). After incubating beads-LCR-protein complexes with β-globin gene constructs for 30 min at 30 °C, the tubes were placed on a magnetic device, and the supernatant was collected from each tube, and subjected to Western blotting analysis. In some experiments, 60 ng of recombinant NF-E2, A-USF, or BSA was added with or without the β-globin gene construct during the dissociation reaction. The experiments were repeated at least three times, and the results were reproducible.
To quantitate the efficiency of the transfer of Pol II from the LCR to the β-globin gene template, we modified the above described procedures as follows. After incubating the LCR-coupled streptavidin beads with MEL nuclear extracts and removing unbound material, each sample was equally divided in half. One half of each sample was boiled with Laemmli sample buffer (Bio-Rad), loaded onto 4–20% Ready gel (Bio-Rad), and then analyzed by Western blotting using an antibody against Pol II (clone CTD4H8, 05-623, Upstate). The other half of each sample was subjected to an in vitro transfer assay as described previously (45). The supernatants, either containing or lacking the β-globin template, were collected at the end of the assay and cross-linked with 0.5% formaldehyde at room temperature for 10 min. Then 0.125 m glycine was added to each sample, incubating at room temperature for 5 min to stop the cross-linking reactions. All samples were dialyzed against ChIP dilution buffer and then subjected to IP analysis using the ChIP assay protocol described previously with antibodies against Pol II (clone CTD4H8, 05-623, Upstate) and rabbit IgG (Bethyl Laboratories). Precipitation of the β-globin gene template was monitored by quantitative real-time PCR using primers located downstream of the β-globin gene promoter: US, 5′-ATTGCATCAGTGTGGAAGTC-3′; DS, 5′-ATTGCCCTGAAAGAAAGAGATTAG-3′.
RESULTS
In the course of our studies we used three different cell lines, MEL, CB3, and CB3/NF-E2 cells. MEL cells are murine erythroleukemia cells that are arrested at a proerythroblast stage and can be differentiated by a variety of chemical inducers, including DMSO. DMSO-mediated induction of MEL cell differentiation has been widely used to study gene regulatory mechanisms in the globin gene loci (25, 27). Fig. 1B shows that incubation of MEL cells for 3 days with 1.5% DMSO led to a more than 50-fold increase in expression of the adult βmaj-globin gene. The second cell line, CB3, is also derived from MEL cells but lacks expression of NF-E2 (p45), due to viral insertion into the p45 gene locus (47). CB3 cells failed to express the adult β-globin gene upon exposure to DMSO (Fig. 1D). Finally, CB3/NF-E2 cells are CB3 cells stably transfected with an expression construct for p45, the large subunit of NF-E2 (12). In these cells, expression of p45 is under control of the EF1α promoter. The CB3/NF-E2 cells expressed relatively high levels of the adult βmaj-globin gene in the absence of DMSO induction (Fig. 1F) but failed to increase globin gene expression in response to DMSO (data not shown). We compared the expression of several proteins previously implicated in globin gene regulation in uninduced and DMSO-induced MEL (Fig. 1C) and CB3 (Fig. 1E) cells by Western blotting analysis. The data demonstrate that the expression of transcription factors USF1, USF2, and NF-E2 (p45) increased during differentiation of MEL cells. In contrast, expression of Pol II, TFIIB, the p45 dimerization partner MafK, CBP, and GAPDH remained similar between uninduced and induced MEL cells. The results obtained from CB3 cells were somewhat different in that these cells did not express NF-E2 (p45), as expected, and did not reveal an increase in USF expression upon exposure to DMSO. In contrast to CB3 cells, CB3/NF-E2 cells expressed NF-E2 (p45) (Fig. 1G); however, the USF protein levels were comparable between CB3 and CB3/NF-E2 cells.
We next examined the interaction of the above mentioned proteins with the globin gene locus in uninduced and DMSO-induced MEL and CB3 cells (Figs. 2 and 3). We focused our attention on two elements that have previously been shown to be the major sites of interactions for both NF-E2 and USF, namely LCR element HS2 and the adult βmaj-globin gene promoter (13, 18). Both of these elements harbor multiple E-box motifs, and HS2 also contains two consensus MARE elements. NF-E2 has also been shown to interact with the adult βmaj-globin gene promoter, although it lacks a consensus MARE sequence (18, 19). As a negative control, we examined the interaction of the proteins with a DNA element located between HS2 and HS3. None of the proteins examined here interacted significantly with the negative control region in uninduced or induced MEL or CB3 cells (13, 37, 43, 45) (supplemental Fig. S1). Fig. 2A demonstrates that NF-E2 (p45), MafK, USF1, USF2, CBP, and TFIIB were already associated with LCR element HS2 in uninduced MEL cells. Only USF2, MafK, and TFIIB were significantly enriched at the adult βmaj-globin gene promoter in uninduced MEL cells, whereas all of the other factors were not enriched. Upon induction of MEL cell differentiation, there was a 2–5-fold increase in the association of all of the factors with LCR element HS2 and a 5–10-fold increase in the interaction of all factors with the adult βmaj-globin gene promoter. Only upon induction of differentiation did NF-E2 (p45), USF1, and CBP reveal significant enrichment at the adult βmaj-globin gene promoter.
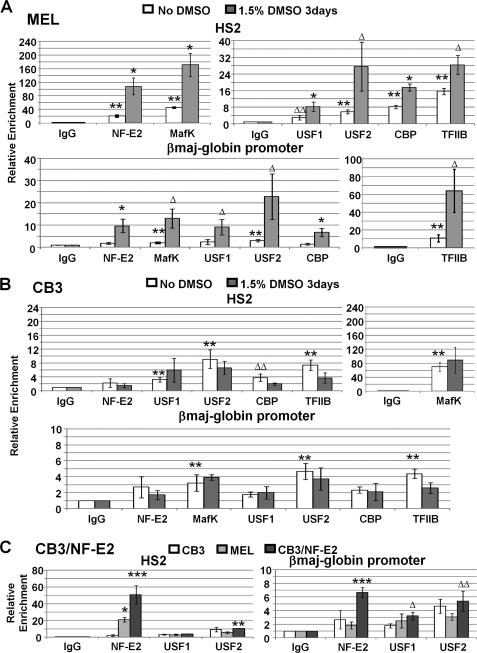
FIGURE 2.
Lack of NF-E2 (p45) impairs the assembly of protein complexes at LCR HS2 and at the adult βmaj-globin gene promoter. A, ChIP analysis of protein chromatin interactions in LCR HS2 and the adult βmaj-globin gene promoter in MEL cells incubated with or without 1.5% DMSO for 3 days. After cross-linking MEL cells with 1% formaldehyde, chromatin was isolated, fragmented by sonication, and subjected to immunoprecipitation with antibodies against NF-E2 (p45), MafK, USF1, USF2, CBP, and TFIIB. Reactions with the IgG antibody served as a negative control. The DNA was purified from the precipitate and subjected to qPCR using primers specific for LCR HS2 and the adult βmaj-globin gene promoter as indicated. Error bars, S.E. of three independent experiments (**, sample versus IgG, p < 0.05; ΔΔ, sample versus IgG, 0.05 < p < 0.1; *, DMSO versus no DMSO, p < 0.05; Δ, DMSO versus no DMSO, 0.05 < p < 0.1). B, ChIP analysis of protein chromatin interactions in LCR HS2 and the adult βmaj-globin gene promoter in CB3 cells incubated with or without 1.5% DMSO for 3 days. DNA was isolated from immunoprecipitated material and analyzed as described in A. Error bars, S.E. of three independent experiments (symbols are as in A). C, comparative ChIP analysis of protein chromatin interactions in uninduced CB3, MEL, and CB3/NF-E2 cells. Cross-linked chromatin was precipitated with IgG or antibodies against NF-E2 (p45), USF1, or USF2, and DNA was analyzed as described in A. Error bars, S.E. of three independent experiments (*, CB3/NF-E2 versus CB3, p < 0.05; Δ, CB3/NF-E2 versus CB3, 0.05 < p < 0.1; **, CB3/NF-E2 versus MEL, p < 0.05; ΔΔ, CB3/NF-E2 versus MEL, 0.05 < p < 0.1; ***, CB3/NF-E2 versus CB3 and MEL p < 0.05).
FIGURE 3.
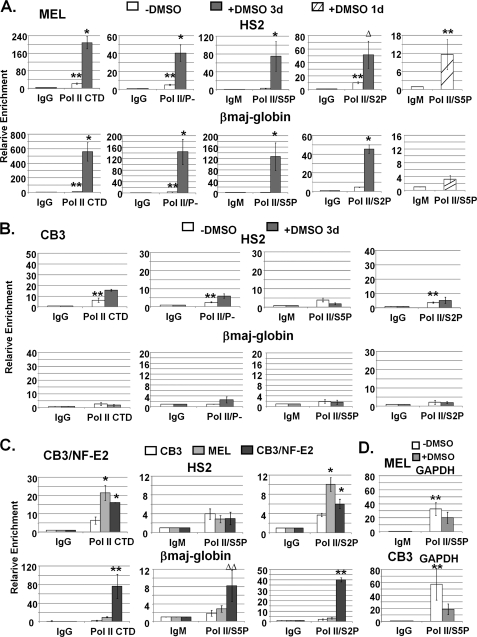
Efficient recruitment and phosphorylation of Pol II at the β-globin gene locus requires NF-E2 (p45). A, ChIP analysis of Pol II interactions in the β-globin gene locus in MEL cells incubated with or without 1.5% DMSO for 1 or 3 days. Chromatin was isolated from cross-linked MEL cells, fragmented by sonication, and immunoprecipitated with antibodies specific for the Pol II CTD (Pol II/CTD), for unphosphorylated Pol II (Pol II/P−), or for Pol II phosphorylated at serine 5 (Pol II/S5P) or serine 2 (Pol II/S2P) of the CTD. IgG or IgM antibodies were used in these experiments as negative controls. DNA was isolated from the precipitates and subjected to qPCR with DNA primers specific for LCR HS2 or the adult βmaj-globin gene promoter as indicated. Error bars, S.E. from three independent experiments (**, sample versus IgG, p < 0.05; *, DMSO versus no DMSO, p < 0.05; Δ, DMSO versus no DMSO, 0.05 < p < 0.1). B, ChIP analysis of Pol II interactions in the β-globin gene locus in CB3 cells incubated with or without 1.5% DMSO for 3 days. Chromatin precipitation and DNA analysis by qPCR was performed as described in A. Error bars, S.E. from three independent experiments (**, as described in A). C, comparative ChIP analysis of Pol II interactions in uninduced CB3, MEL, and CB3/NF-E2 cells. Chromatin precipitation and DNA analysis by qPCR were performed as described in A. Error bars, S.E. from three independent experiments (*, CB3/NF-E2 versus CB3, p < 0.05; **, CB3/NF-E2 versus CB3 and MEL, p < 0.05; ΔΔ, CB3/NF-E2 versus CB3 and MEL, 0.05 < p < 0.1). D, ChIP analysis of Ser-5-phosphorylated Pol II at the GAPDH promoter in undifferentiated and DMSO-induced MEL and CB3 cells (as indicated). Cells were grown in the absence or presence of DMSO (1.5% for 3 days). DNA was isolated from immunoprecipitated material and analyzed by qPCR as described in A. Error bars, S.E. from three independent experiments (**, as described in A).
The protein interaction profile of the examined proteins with the βmaj-globin gene locus was similar between uninduced CB3 and MEL cells, except for NF-E2 (p45), which was not enriched at HS2 and the β-globin gene promoter in CB3 cells (Fig. 2, compare A and B). The proteins MafK, USF1, USF2, CBP, and TFIIB revealed significant enrichment at LCR element HS2 and were either absent or detected at lower levels at the adult βmaj-globin gene promoter compared with MEL cells. These data demonstrate that NF-E2 is not required for the initial low level recruitment of USF to the globin gene locus. The recruitment of MafK to the LCR in CB3 and uninduced MEL cells is probably mediated in part by BACH1, which is replaced during differentiation by p45 (48). In contrast to MEL cells, none of the protein/chromatin interactions increased upon exposure of CB3 cells to DMSO (Fig. 2B).
We next analyzed the interaction of NF-E2 and USF with the β-globin gene locus in CB3/NF-E2 cells, in which expression of p45 has been restored by stable transfection with a p45-expressing DNA construct. The data demonstrate that NF-E2 efficiently interacted with LCR HS2 and with the adult βmaj-globin gene promoter in CB3 cells expressing p45 (Fig. 2C). Interestingly, NF-E2 interacted more efficiently with the βmaj-globin gene promoter in CB3/NF-E2 cells compared with uninduced MEL cells. This result demonstrates that NF-E2 is capable of interacting with the adult βmaj-globin gene promoter in uninduced cells when expressed at high levels. In uninduced MEL cells, we only observed significant interactions of NF-E2 with the LCR and not with the adult βmaj-globin gene promoter (Fig. 2A). Re-expression of p45 did not lead to increased interactions of USF1 and USF2 with LCR HS2 and the globin promoter (Fig. 2C).
Previous work by Johnson et al. (27) demonstrated that NF-E2 (p45) is required for the recruitment of Pol II to the adult β-globin gene promoter but not to LCR element HS2. Pol II is recruited to DNA in its unphosphorylated form, is first phosphorylated at Ser-5 in the CTD during transcription initiation, and is subsequently phosphorylated at Ser-2 in the CTD to allow efficient elongation (49). We examined the interaction of total Pol II (Pol II/CTD), unphosphorylated Pol II (Pol II/P−), Ser-5-phosphorylated Pol II (Pol II/S5P), and Ser-2-phosphorylated Pol II (Pol II/S2P) with the LCR element HS2 and with the adult βmaj-globin gene in uninduced and DMSO-induced MEL and CB3 cells (Fig. 3, A and B). We also analyzed the interaction of the different forms of Pol II with a negative control region located between HS2 and HS3 (supplemental Fig. S2) and found that Pol II does not efficiently interact with this region of the globin gene locus. The data demonstrate that Pol II was recruited to LCR element HS2 but not as efficiently to the adult βmaj-globin gene promoter in uninduced MEL cells (Fig. 3A). Interestingly, there was no Ser-5-phosphorylated Pol II associated with LCR element HS2 or with the βmaj-globin gene promoter in uninduced MEL cells. A positive control experiment demonstrated that Ser-5-phosphorylated Pol II was efficiently recruited to the GAPDH gene in uninduced MEL cells (Fig. 3D). We observed a significant enrichment for the Ser-2-phosphorylated form of Pol II at LCR element HS2 but not at the βmaj-globin gene promoter in uninduced cells. The low levels of Ser-2-phosphorylated Pol II at LCR element HS2 indicates ongoing transcription within the LCR in uninduced MEL cells. This is consistent with previous analysis of intergenic transcription in the β-globin gene locus (50, 51). The fact that we did not detect Ser-5-phosphorylated Pol II in HS2 in undifferentiated MEL cells suggests that intergenic transcription in these cells initiates upstream of HS2. After induction of differentiation, we detected a strong increase in the association of Pol II, including unphosphorylated Pol II as well as Ser-5- and Ser-2-phosphorylated forms of Pol II, with both LCR HS2 and with the adult βmaj-globin gene promoter (Fig. 3A). We found that the Ser-5-phosphorylated form of Pol II significantly associated with LCR HS2 but not with the βmaj-globin gene promoter in MEL cells that were induced by 1.5% DMSO for only 24 h. This result suggests that elongation-competent Pol II complexes are first assembled at the LCR.
In CB3 cells, low but significant levels of Pol II were associated with LCR element HS2 but not with the adult βmaj-globin gene promoter (Fig. 3B), consistent with previous observations made by Johnson et al. (27). There was no increase in the association of Pol II with the globin locus-associated elements upon incubation of CB3 cells with DMSO. Importantly, in contrast to MEL cells, Ser-5- or Ser-2-phosphorylated forms of Pol II were not detectable at LCR element HS2 or at the adult β-globin gene promoter in CB3 cells either before or after incubation with DMSO (Fig. 3B), demonstrating that NF-E2 (p45) is required for the phosphorylation of Pol II in the β-globin gene locus. Similar to what we observed at HS2, Pol II was also recruited to HS3, and phosphorylation of Pol II at HS3 was also dependent on NF-E2 (p45) and erythroid differentiation (data not shown).
We next addressed the question of whether re-expression of p45 in NF-E2-deficient CB3 cells would restore the association and phosphorylation of Pol II at the β-globin gene locus. Re-expression of NF-E2 (p45) led to a 4-fold increase in the association of Pol II with LCR HS2 when compared with CB3 cells (Fig. 3C). The levels of Pol II bound at HS2 in these cells were comparable with those detected in uninduced MEL cells. However, the association of Pol II with the adult βmaj-globin gene promoter was increased about 40-fold compared with CB3 cells and was much higher than in uninduced MEL cells. These data show that NF-E2 is important for the efficient recruitment of Pol II to the βmaj-globin gene. We did not observe an increase in Ser-5-phosphorylated Pol II at the LCR in CB3 cells expressing p45. In contrast, there was a significant increase of both Ser-5- and Ser-2-phosphorylated Pol II at the adult βmaj-globin gene promoter.
Because our data (13) and data previously published by Johnson et al. (27) suggest that USF and NF-E2 are both required for the recruitment of Pol II to the adult β-globin gene promoter, we examined whether these proteins interact in erythroid cells (Fig. 4A). In co-IP experiments, we found that USF1 and USF2 interact with NF-E2 (p45) in uninduced MEL cells with comparable efficiency. There appeared to be an increase in interactions between USF2 and NF-E2 relative to interactions between USF1 and NF-E2 after induction of differentiation (Fig. 4A). We performed reciprocal experiments in which we immunoprecipitated with a p45 antibody and performed the Western blotting with USF antibodies, and the results confirmed the interactions between USF and NF-E2 (data not shown). We also observed interactions of USF2 with USF1, NF-E2, and Pol II in DMSO-induced MEL cells. There was a reproducible but weak signal for Pol II in USF2-precipitated material from uninduced cells (compare the signal in the USF2 lane with the signal in the IgG lane (Fig. 4A)), suggesting that USF2 but not USF1 interacts with Pol II in undifferentiated MEL cells. The more efficient co-IP of USF with Pol II in differentiated cells could be due to the fact that there is an increase in expression of USF during differentiation (52) or that there is an additional activity induced upon induction that mediates interactions between USF and Pol II. This activity could in fact be the increased expression of NF-E2. It is also interesting to note that in undifferentiated MEL cells, USF1 homodimers appeared to be more abundant than USF1/USF2 heterodimers. This was not the case in differentiated MEL cells, in which we detected efficient interactions of USF2 with both USF1 and NF-E2 (p45). Furthermore, USF2 but not USF1 interacts efficiently with the co-activator CBP in MEL cells (Fig. 4A). There was a weak signal for TFIIB in the USF2-precipitated material in both undifferentiated as well as differentiated MEL cells (upon longer exposure; data not shown). In contrast to MEL cells, we did not detect interactions between USF and Pol II in CB3 cells, although interactions between USF2 and CBP were detectable (data not shown). We did not detect USF, NF-E2, or CBP in samples precipitated with antibodies against Pol II. This is probably because only a small fraction of nuclear Pol II will associate with these proteins at any given time. All of the protein-protein interaction data here have been reproduced (data not shown).
FIGURE 4.
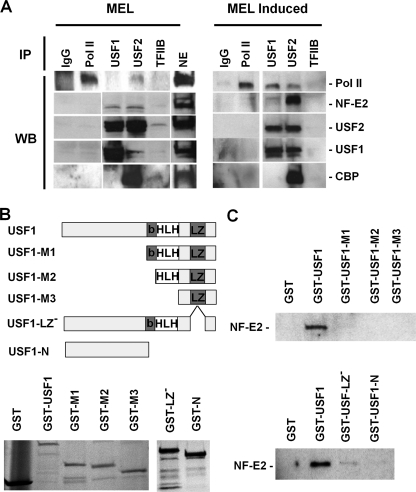
Interactions of USF1 and USF2 with NF-E2 (p45) and Pol II during differentiation of MEL cells. A, co-immunoprecipitation experiments were performed by first subjecting nuclear extracts from MEL cells incubated with or without 1.5% DMSO for 3 days to immunoprecipitation with antibodies specific for IgG, Pol II (N-20), USF1, USF2, and TFIIB. The immunoprecipitated material was electrophoresed using 4–20% Ready Gels (Bio-Rad) and transferred to nitrocellulose membranes. The nitrocellulose membranes were incubated with antibodies against Pol II, NF-E2 (p45), USF1, USF2, and CBP, as indicated, and subjected to ECL Plus chemiluminescence (Amersham Biosciences). B, generation and expression of USF1/GST fusion proteins in E. coli. The cDNA encoding full-length or truncated USF1 was ligated into the pGEX-5X-1 vector. Fusion proteins were expressed in and purified from E. coli and analyzed by SDS-PAGE. The following USF1-derived proteins were purified: full-length USF1 protein (USF1); deletion of the N-terminal half (USF-M1); deletion of the N terminus and the basic region (USF1-M2); deletion of the N terminus, the basic region, and the helix-loop-helix domain (M3); deletion of the leucine zipper (USF-1 LZ−); and deletion of the C terminus as well as the basic region, the HLH domain, and the LZ domain (USF1-N). C, interaction of USF1 with NF-E2. Equal amounts of GST fusion proteins were incubated with His-tagged NF-E2. After washing, proteins were eluted from the GST-beads, electrophoresed using SDS-PAGE, and subjected to Western blotting analysis using NF-E2 (p45)-specific antibodies. WB, Western blot.
NF-E2 and USF could be part of a large protein complex, and thus the interaction could be either direct or mediated by other proteins. We carried out GST pull-down assays using GST-tagged recombinant USF1 and recombinant His-tagged NF-E2 (p45 tethered to MafG). The results demonstrated that USF1 directly interacts with NF-E2. Because both proteins contain a leucine-zipper interaction domain, we analyzed the interaction of different USF1 truncation mutants lacking the N terminus, including the USR (USF1-M1), the N terminus plus basic region (USF1-M2), or the N terminus plus basic region and helix-loop-helix domain (USF1-M3), the C terminus USF1-N), or the leucine zipper (USF1-LZ−), as indicated in Fig. 4, B and C. We detected efficient interactions only between full-length USF1 and NF-E2, demonstrating that both C terminus and N terminus of USF1 harbor elements important for the interaction with NF-E2. It is possible that the USF1 deletion mutants are impaired in correct folding, and this could impact the ability of USF1 to interact with NF-E2. However, it may be important to note that deletion of the leucine zipper domain still allowed interactions between the two proteins, albeit with much lower efficiency compared with full-length USF1 (Fig. 4C). This result demonstrates that the USF1 leucine zipper is not the critical determinant for the specific interaction between USF1 and NF-E2.
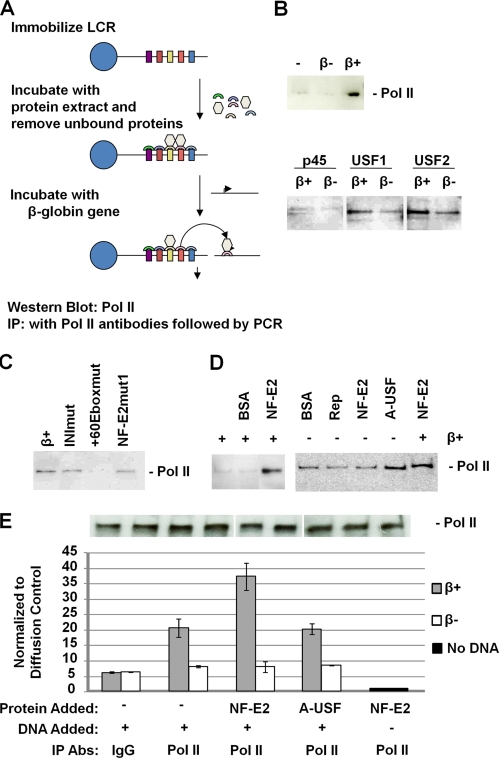
We next examined the mechanism(s) by which NF-E2 and USF could regulate LCR-mediated recruitment of Pol II to the adult β-globin gene promoter using a protein dissociation/transfer assay (Fig. 5A). We previously established a method for the analysis of Pol II transfer from the LCR to the adult β-globin gene promoter (45). A plasmid containing all five HS sites from the human β-globin LCR is linearized, biotinylated, and immobilized on streptavidin-coated magnetic beads. The immobilized LCR is then incubated with protein extracts from MEL cells. We previously demonstrated that under the applied conditions, Pol II is recruited to LCR elements HS2 and HS3 (46). After removing all material not bound to the LCR, the immobilized protein-DNA complex is incubated with DNA templates containing the adult β-globin promoter or mutants thereof. In the initial experiments described here, we incubated the immobilized LCR construct with protein extracts from MEL cells. Unbound material was removed, and the LCR-protein complex was then incubated in the presence of a β-globin gene construct either containing (β+) or lacking (β−) the β-globin gene promoter. After incubation for 30 min at 30 °C, material not bound to the LCR was removed and subjected to Western blotting analysis using NF-E2-, USF-, or Pol II-specific antibodies as shown in Fig. 5B. This experimental setup allowed us to monitor β-globin promoter-dependent dissociation of proteins from the LCR.
FIGURE 5.
USF and NF-E2 regulate the recruitment and dissociation of Pol II to and from immobilized LCR templates. A, scheme of the experimental strategy. A linearized and biotinylated plasmid containing the human β-globin LCR was immobilized on streptavidin-coated magnetic beads as described by Vieira et al. (45). The LCR was then incubated with whole cell extracts from MEL cells. Unbound material was removed, and the LCR-protein complex was washed several times and incubated with different DNA templates in the presence or absence of recombinant NF-E2 (p45) tethered to MafG (16, 42) or dominant negative USF (A-USF) (13). Proteins that dissociate from the LCR after the incubation step were analyzed using Western blotting analysis. Transfer of proteins to the β-globin gene promoter was analyzed by immunoprecipitation (IP) followed by quantitative PCR. B, β-globin promoter-mediated dissociation of Pol II, NF-E2 (p45), USF1, and USF2, from the LCR in the presence of a plasmid containing the β-globin gene with (β+) or without (β−) its promoter. Proteins and DNA were removed from the immobilized LCR after incubation for 30 min at 30 °C. C, analysis of the effect of β-globin promoter mutations on the dissociation of Pol II. DNA plasmids containing the wild-type β-globin promoter (β+) or the promoter with mutations in the initiator (INImut), the +60 E-box (+60Eboxmut), or the partial MARE sequence (NF-E2mut1) were incubated for 30 min with the immobilized LCR-protein complex in the presence of recombinant NF-E2. Dissociated proteins were removed and analyzed by Western blotting experiments using an antibody specific for Pol II. D, effect of NF-E2 (p45) and A-USF on the dissociation of Pol II from the immobilized LCR. The immobilized LCR-protein complex was incubated for 30 min with BSA, AAV Rep (Rep), NF-E2, or A-USF in the absence or presence of a plasmid containing the wild-type β-globin gene promoter (β+). Dissociated proteins were removed from the LCR and analyzed by Western blotting experiments using an antibody specific for Pol II. E, quantitative PCR analysis of Pol II transfer from immobilized LCR templates to the β-globin gene promoter. Immobilized LCR-protein complexes were incubated with or without a plasmid containing the β-globin gene with (β+) or without (β−) its promoter region for 30 min at 30 °C. Unbound material was subjected to immunoprecipitation using IgG- or Pol II-specific antibodies. The DNA was isolated from the precipitate and subjected to quantitative real-time PCR using primers specific for the β-globin gene. The experiment was repeated, and the error bars represent S.E. An aliquot was taken from immobilized LCR-protein complexes in each transfer dissociation assay and analyzed by Western blotting using Pol II-specific antibodies (shown above the graph).
The data demonstrate that there was an increase in the dissociation of USF1, USF2, NF-E2 (p45), and Pol II from the LCR in the presence of the adult β-globin gene promoter (Fig. 5B), suggesting that the promoter efficiently competes for the binding of these proteins. We observed a reproducible decrease in the dissociation of Pol II from the immobilized LCR if β-globin templates were used that carry mutations in an E-box located 60 bp downstream of the transcription initiation sites (+60Eboxmut; Fig. 5C). We previously demonstrated that this E-box interacts with USF and is required for the efficient in vitro transcription of the β-globin gene (16). A mutation of a partial MARE sequence (NF-E2mut1) in the downstream promoter region did not affect β-globin promoter-mediated dissociation of Pol II from the LCR. Expression of a dominant negative mutant of USF (A-USF) in MEL cells reduced recruitment of Pol II to LCR HS2 and to the adult β-globin gene promoter (13). Interestingly, the addition of A-USF increased the dissociation of Pol II from the LCR even in the absence of a β-globin gene promoter, whereas BSA, AAV Rep protein, or NF-E2 were all unable to do so (Fig. 5D). We have shown previously that NF-E2 increases the efficiency of transfer of Pol II from the LCR to the adult β-globin gene promoter (45). We show here that NF-E2 only facilitated dissociation of Pol II from the LCR in the presence of the adult β-globin gene promoter (Fig. 5D). The fact that the USF-binding site is required for efficient dissociation/transfer suggests that USF may be required to stabilize NF-E2 binding at the promoter, which lacks a consensus MARE.
We next verified some of the results from the in vitro dissociation experiment using a quantitative assay in which we performed the incubation experiments as described above but analyzed the transfer of Pol II from the LCR to the β-globin gene promoter by cross-linking and subsequent IP with Pol II-specific antibodies. The DNA was isolated from the precipitated material and analyzed by quantitative PCR using primers that amplify a fragment from the β-globin gene (β+) or the mutant β-globin gene lacking the promoter (β−). Pol II was transferred from the LCR to the β-globin gene in a promoter-dependent manner, and NF-E2, but not A-USF, facilitated the transfer of Pol II to the β-globin gene (Fig. 5E). To control for the initial amount of Pol II recruited to the LCR, we performed Western blotting experiments. The data demonstrate that all samples analyzed quantitatively had about the same amount of Pol II recruited to the LCR before dissociation/transfer was analyzed. The fact that A-USF did not affect recruitment of Pol II to the β-globin gene promoter is probably due to the fact that it increases dissociation of Pol II from the LCR but that it will at the same time reduce recruitment of Pol II to the β-globin gene promoter.
DISCUSSION
Previous studies have shown that β-globin LCR HS sites recruit transcription complexes and that the LCR is required for the association of the β-globin gene locus with transcription factories (45, 50, 53, 54). Furthermore, long intergenic noncoding transcripts originate from within or upstream of the LCR and are detectable throughout the globin gene locus in a developmental stage-specific manner (51). During differentiation of erythroid cells, transcription complexes and other activities first associate with the LCR before they are detectable at the globin gene promoters (43, 55, 56). These data suggest that the LCR is the primary attachment site for the recruitment of transcription complexes and these complexes are delivered to the globin gene locus by a tracking, linking, or looping mechanism (57). Not consistent with this model, however, is the observation that, even in the absence of the LCR, Pol II is efficiently recruited to the adult β-globin gene promoter, although it exhibits defects in elongation of transcription (58). Sawado et al. (58) discussed the possibility that the LCR provides activities for the efficient elongation of Pol II at the β-globin gene promoter. If Pol II is first recruited to the LCR, another possibility is that elongation-competent transcription complexes are assembled at the LCR and transferred to high affinity globin gene promoters. In other words, close proximity to the LCR would favor recruitment of elongation-active transcription complexes to the adult β-globin gene promoter.
To further elucidate how the LCR and interacting proteins mediate recruitment and activity of Pol II in the β-globin gene locus, we analyzed MEL cells expressing or not expressing NF-E2 (p45). NF-E2 (p45) has previously been shown to be required for the recruitment of Pol II to the adult β-globin gene promoter but not to the LCR. We demonstrate here that Pol II is efficiently recruited to LCR HS2 but not to the β-globin gene promoter in undifferentiated MEL cells (Fig. 3). Recruitment of Pol II to LCR HS2 is inefficient in the absence of NF-E2 (p45). Perhaps more importantly, however, is the observation that in the absence of NF-E2 (p45), there is no phosphorylated Pol II detectable at LCR HS2 or the βmaj-globin gene promoter. This result suggests that NF-E2 (p45) is not only important for the efficient recruitment of Pol II to the globin gene locus but plays an important role in converting Pol II into an elongation-competent form, as has been discussed previously by Sawado et al. (18, 58). Re-expression of p45 in NF-E2-deficient CB3 cells caused a strong increase in the recruitment of Pol II at the βmaj-globin gene promoter. Expression of p45 also led to the phosphorylation of Pol II at the promoter but not at LCR HS2. This result is interesting and somewhat contrasts with the findings in MEL cells showing that Ser-5-phosphorylated Pol II is first detectable at the LCR during DMSO-induced differentiation. Expression of relatively high levels of p45 in uninduced CB3 cells probably causes the local remodeling of the chromatin structure at the βmaj-globin gene promoter, consistent with findings from the Brand and Bresnick laboratories (26, 59). According to the transfer model, the open chromatin structure at the promoter would lead to the efficient transfer of Ser-5-phosphorylated Pol II to the promoter. Alternatively, the NF-E2 (p45)-induced opening of the chromatin structure at the adult globin gene promoter may bypass the need for LCR-mediated Pol II recruitment. In this respect, it will be interesting to examine proximity between the LCR and the adult β-globin gene promoter in the CB3/NF-E2 cells. CB3 cells reveal reduced levels of GATA-1 binding to the βmaj-globin gene (60). Previous work has shown that GATA-1 is required for mediating proximity between the LCR and the β-globin gene promoter (61). Therefore, proximity between the LCR and the β-globin gene is probably not established in CB3 cells.
We detected low levels of unphosphorylated Pol II binding at the adult β-globin promoter in uninduced MEL cells, which could be recruited to the βmaj-globin gene in an LCR-dependent or -independent manner in undifferentiated cells. We observed that, along with Pol II, USF2, MafK, and TFIIB are already associated with LCR HS2 and with the adult β-globin gene promoter in undifferentiated MEL cells, whereas USF1, CBP, and NF-E2 (p45) only associate with HS2 in undifferentiated cells. Using co-IP, we found that after differentiation of MEL cells, there is an increased interaction of USF2 with NF-E2 (p45), USF1, and Pol II (Fig. 4). This is accompanied by an increased recruitment of all of these proteins to the β-globin gene locus. Previous studies had already shown that the NF-E2 activity increases during differentiation of erythroid cells (62). We suggest that increased expression of USF and NF-E2 facilitate the formation of large protein complexes that regulate Pol II recruitment and activity in the β-globin gene locus.
We propose that partial elongation-incompetent transcription complexes are first assembled at the LCR in undifferentiated MEL cells. This is in part mediated by USF2, CBP, and TFIIB. Our previous data showing that expression of a dominant negative mutant of USF in undifferentiated MEL cells reduces the recruitment of Pol II to LCR HS2 (13) are consistent with the hypothesis that USF participates in the recruitment of Pol II to the LCR in undifferentiated MEL cells. During differentiation, an increase in expression of NF-E2 (p45), USF, and other proteins leads to the efficient recruitment of additional activities to the LCR, including those that convert Pol II from a transcriptionally inert to a transcriptionally competent form. The assembly of elongation-competent transcription complexes is accompanied by a conformational change in the globin gene locus that brings the adult β-globin gene in close proximity to the LCR (Fig. 6) (63). The presence of high affinity basal promoter elements in the adult β-globin gene promoter facilitates the transfer of elongation competent transcription complexes to the promoter. The transfer is mediated at least in part by NF-E2. However, the association of NF-E2 with the β-globin gene promoter is probably stabilized through its interaction with USF and the presence of USF binding sites. The in vitro Pol II transfer experiments revealed that NF-E2 is able to dissociate Pol II from the LCR and that this process requires the presence of a β-globin promoter template. The transfer of Pol II to the promoter also required a binding site for USF (Fig. 5C). These data are consistent with our findings from MEL cells and further demonstrate that USF and NF-E2 cooperate to mediate the transfer/recruitment of Pol II to the adult β-globin promoter. The function of NF-E2 in vivo is probably more complex, involving the remodeling of chromatin structure at the adult β-globin gene promoter, which would further facilitate recruitment of the transcription complex.
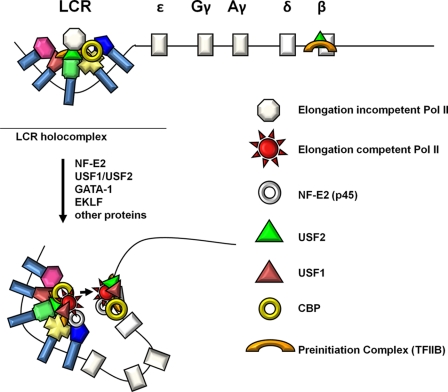
FIGURE 6.
Model of NF-E2 and USF mediated assembly and transfer of elongation competent transcription complexes in the β-globin gene locus. Incomplete elongation-incompetent Pol II transcription complexes are first recruited to the LCR. This is mediated in part by USF2, its associated co-factor CBP, TFIIB, and other proteins. After erythroid differentiation expression of NF-E2 (p45) and USF increases and these proteins efficiently associate with the LCR. This leads to the recruitment or assembly of transcriptionally competent Pol II complexes and phosphorylation of the Pol II CTD. The differentiation of erythroid cells is also accompanied by a conformational change in the globin locus that juxtaposes the adult globin gene with the LCR. This facilitates the transfer of elongation-competent transcription complexes from the LCR to the adult globin gene.
The maintenance of proximity between the LCR and promoter guarantees the continued loading of elongation-competent transcription complexes to the adult β-globin gene promoter (Fig. 6). It is also possible, however, that the conformational change bringing the β-globin gene into close proximity to the LCR precedes the assembly of active transcription complexes. Our data do not clearly distinguish between these two possibilities. The only piece of evidence arguing for LCR-mediated assembly of elongation-competent transcription complexes is the observation that Ser-5-phosphorylated Pol II is first detectable at LCR HS2 during the differentiation of MEL cells.
In contrast to other hematopoietic specific transcription factors, such as GATA-1, Fog-1, EKLF, and NL1, NF-E2 (p45) is not critical for mediating proximity between the LCR and the adult β-globin gene or for the formation of an active chromatin hub (61, 64–66). However, our data suggest that NF-E2 and USF function in the context of the active chromatin hub and perhaps mediate the assembly of elongation-competent transcription complexes at the LCR and at the adult β-globin gene promoter. We have characterized proteins that associate with USF1 in HeLa cells.3 Interestingly, three of these proteins are implicated in the recruitment and activity of Pol II. These proteins are TAF4 and -6 (TBP-associated factors 4 and 6) as well as the elongation factor EFIA2. In this respect, it is interesting to note that USF1 only associates with Pol II and the β-globin gene locus after differentiation of erythroid cells.
Many proteins contribute to expression of the globin gene locus, and NF-E2 and USF function within a cascade of events that regulate accessibility and location of the globin genes in the nucleus (10). It will be increasingly important to determine how the different transcription factors function together to mediate extremely high level transcription of the β-like globin genes during erythroid differentiation and development. Another protein that acts at the adult β-globin gene is EKLF, which recruits chromatin remodeling complexes to the promoter but also contacts components of the transcription initiation complex (67–69). Future studies will address if and how EKLF communicates with USF and NF-E2 in regulating expression of the adult β-globin gene.
Supplementary Material
Acknowledgments
We thank our colleagues in the Huang and Bungert laboratories for support and helpful discussions. We thank Drs. Michael Kilberg and Steven Sugrue (both at the University of Florida) for antibodies. We also thank the laboratories of Drs. Rolf Renne, Michael Kilberg, and Jianrong Lu (University of Florida), particularly Dr. Jianhong Hu, Dr. Nan Su, and Tong Lin, for materials and advice.
This work was supported, in whole or in part, by National Institutes of Health Grants DK052356 (to J. B.) and HL091929 (to S. H.). This work was also supported by American Heart Association Grant AHAGRNT2260915 (to J. B.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
S. Huang, unpublished data.
- LCR
- locus control region
- HS
- DNase I-hypersensitive site
- MARE
- Maf recognition element
- CBP
- CREB-binding protein
- CREB
- cAMP-response element-binding protein
- Pol
- polymerase
- MEL
- murine erythroleukemia
- CTD
- C-terminal domain
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- IP
- immunoprecipitation
- ChIP
- chromatin immunoprecipitation
- GST
- glutathione S-transferase
- BSA
- bovine serum albumin
- qPCR
- quantitative PCR
- RT
- reverse transcription, βmaj- and βmin-globin, β-major and β-minor globin, respectively.
REFERENCES
- 1.Felsenfeld G., Groudine M. (2003) Nature 421, 448–453 [DOI] [PubMed] [Google Scholar]
- 2.Chakalova L., Debrand E., Mitchell J. A., Osborne C. S., Fraser P. (2005) Nat. Rev. Genet. 6, 669–677 [DOI] [PubMed] [Google Scholar]
- 3.Sutherland H., Bickmore W. A. (2009) Nat. Rev. Genet. 10, 457–466 [DOI] [PubMed] [Google Scholar]
- 4.Loose M., Swiers G., Patient R. (2007) Curr. Opin. Hematol. 14, 307–314 [DOI] [PubMed] [Google Scholar]
- 5.Stamatoyannopoulos G. (2005) Exp. Hematol. 33, 259–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liang S., Moghimi B., Yang T. P., Strouboulis J., Bungert J. (2008) J. Cell. Biochem. 105, 9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. (1987) Cell 51, 975–985 [DOI] [PubMed] [Google Scholar]
- 8.Forrester W. C., Takegawa S., Papayannopoulou T., Stamatoyannopoulos G., Groudine M. (1987) Nucleic Acids Res. 15, 10159–10177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuan D., Solomon W., Li Q., London I. M. (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 6384–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim S. I., Bresnick E. H. (2007) Oncogene 26, 6777–6794 [DOI] [PubMed] [Google Scholar]
- 11.Andrews N. C. (1998) Int. J. Biochem. Cell Biol. 30, 429–432 [DOI] [PubMed] [Google Scholar]
- 12.Bean T. L., Ney P. A. (1997) Nucleic Acids Res. 25, 2509–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crusselle-Davis V. J., Vieira K. F., Zhou Z., Anantharaman A., Bungert J. (2006) Mol. Cell. Biol. 26, 6832–6843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bresnick E. H., Felsenfeld G. (1993) J. Biol. Chem. 268, 18824–18834 [PubMed] [Google Scholar]
- 15.Elnitski L., Miller W., Hardison R. (1997) J. Biol. Chem. 272, 369–378 [DOI] [PubMed] [Google Scholar]
- 16.Leach K. M., Vieira K. F., Kang S. H., Aslanian A., Teichmann M., Roeder R. G., Bungert J. (2003) Nucleic Acids Res. 31, 1292–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forsberg E. C., Downs K. M., Bresnick E. H. (2000) Blood 96, 334–339 [PubMed] [Google Scholar]
- 18.Sawado T., Igarashi K., Groudine M. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 10226–10231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang S. H., Vieira K., Bungert J. (2002) Nucleic Acids Res. 30, e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Igarashi K., Kataoka K., Itoh K., Hayashi N., Nishizawa M., Yamamoto M. (1994) Nature 367, 568–572 [DOI] [PubMed] [Google Scholar]
- 21.Andrews N. C., Kotkow K. J., Ney P. A., Erdjument-Bromage H., Tempst P., Orkin S. H. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 11488–11492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motohashi H., Shavit J. A., Igarashi K., Yamamoto M., Engel J. D. (1997) Nucleic Acids Res. 25, 2953–2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blobel G. A. (2002) J. Leukoc. Biol. 71, 545–556 [PubMed] [Google Scholar]
- 24.Amrolia P. J., Ramamurthy L., Saluja D., Tanese N., Jane S. M., Cunningham J. M. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 10051–10056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brand M., Ranish J. A., Kummer N. T., Hamilton J., Igarashi K., Francastel C., Chi T. H., Crabtree G. R., Aebersold R., Groudine M. (2004) Nat. Struct. Mol. Biol. 11, 73–80 [DOI] [PubMed] [Google Scholar]
- 26.Demers C., Chaturvedi C. P., Ranish J. A., Juban G., Lai P., Morle F., Aebersold R., Dilworth F. J., Groudine M., Brand M. (2007) Mol. Cell. 27, 573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson K. D., Christensen H. M., Zhao B., Bresnick E. H. (2001) Mol. Cell. 8, 465–471 [DOI] [PubMed] [Google Scholar]
- 28.Loyd M. R., Okamoto Y., Randall M. S., Ney P. A. (2003) Blood 102, 4223–4228 [DOI] [PubMed] [Google Scholar]
- 29.Ney P. A., Sorrentino B. P., McDonagh K. T., Nienhuis A. W. (1990) Genes Dev. 4, 993–1006 [DOI] [PubMed] [Google Scholar]
- 30.Stamatoyannopoulos J. A., Goodwin A., Joyce T., Lowrey C. H. (1995) EMBO J. 14, 106–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong Q. H., McDowell J. C., Dean A. (1996) Mol. Cell. Biol. 16, 6055–6064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shivdasani R. A., Rosenblatt M. F., Zucker-Franklin D., Jackson C. W., Hunt P., Saris C. J., Orkin S. H. (1995) Cell 81, 695–704 [DOI] [PubMed] [Google Scholar]
- 33.Kotkow K. J., Orkin S. H. (1995) Mol. Cell. Biol. 15, 4640–4647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gregor P. D., Sawadogo M., Roeder R. G. (1990) Genes Dev. 4, 1730–1740 [DOI] [PubMed] [Google Scholar]
- 35.Meisterernst M., Roy A. L., Lieu H. M., Roeder R. G. (1991) Cell 66, 981–993 [DOI] [PubMed] [Google Scholar]
- 36.Huang S., Li X., Yusufzai T. M., Qiu Y., Felsenfeld G. (2007) Mol. Cell. Biol. 27, 7991–8002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crusselle-Davis V. J., Zhou Z., Anantharaman A., Moghimi B., Dodev T., Huang S., Bungert J. (2007) FEBS J. 274, 6065–6073 [DOI] [PubMed] [Google Scholar]
- 38.Liang S. Y., Moghimi B., Crusselle-Davis V. J., Lin I. J., Rosenberg M. H., Li X., Strouboulis J., Huang S., Bungert J. (2009) Mol. Cell. Biol. 29, 5900–5910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.West A. G., Huang S., Gaszner M., Litt M. D., Felsenfeld G. (2004) Mol. Cell. 16, 453–463 [DOI] [PubMed] [Google Scholar]
- 40.Gallagher P. G., Nilson D. G., Steiner L. A., Maksimova Y. D., Lin J. Y., Bodine D. M. (2009) Blood 113, 1547–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bungert J., Kober I., Düring F., Seifart K. H. (1992) J. Mol. Biol. 223, 885–898 [DOI] [PubMed] [Google Scholar]
- 42.Hung H. L., Kim A. Y., Hong W., Rakowski C., Blobel G. A. (2001) J. Biol. Chem. 276, 10715–10721 [DOI] [PubMed] [Google Scholar]
- 43.Levings P. P., Zhou Z., Vieira K. F., Crusselle-Davis V. J., Bungert J. (2006) FEBS J. 273, 746–755 [DOI] [PubMed] [Google Scholar]
- 44.Chomczynski P., Sacchi N. (1987) Anal. Biochem. 162, 156–159 [DOI] [PubMed] [Google Scholar]
- 45.Vieira K. F., Levings P. P., Hill M. A., Crusselle V. J., Kang S. H., Engel J. D., Bungert J. (2004) J. Biol. Chem. 279, 50350–50357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leach K. M., Nightingale K., Igarashi K., Levings P. P., Engel J. D., Becker P. B., Bungert J. (2001) Mol. Cell. Biol. 21, 2629–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu S. J., Rowan S., Bani M. R., Ben-David Y. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 8398–8402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun J., Brand M., Zenke Y., Tashiro S., Groudine M., Igarashi K. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 1461–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sims R. J., 3rd, Belotserkovskaya R., Reinberg D. (2004) Genes Dev. 18, 2437–2468 [DOI] [PubMed] [Google Scholar]
- 50.Tuan D., Kong S., Hu K. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 11219–11223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miles J., Mitchell J. A., Chakalova L., Goyenechea B., Osborne C. S., O'Neill L., Tanimoto K., Engel J. D., Fraser P. (2007) PLoS ONE 2, e630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin I. J., Zhou Z., Crusselle-Davis V. J., Moghimi B., Gandhi K., Anantharaman A., Pantic D., Huang S., Jayandharan G., Zhong L., Srivastava A., Bungert J. (2009) J. Biol. Chem. 284, 20130–20135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson K. D., Grass J. A., Park C., Im H., Choi K., Bresnick E. H. (2003) Mol. Cell. Biol. 23, 6484–6493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ragoczy T., Bender M. A., Telling A., Byron R., Groudine M. (2006) Genes Dev. 20, 1447–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bottardi S., Aumont A., Grosveld F., Milot E. (2003) Blood 102, 3989–3997 [DOI] [PubMed] [Google Scholar]
- 56.Bottardi S., Ross J., Pierre-Charles N., Blank V., Milot E. (2006) EMBO J. 25, 3586–3595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levings P. P., Bungert J. (2002) Eur. J. Biochem. 269, 1589–1599 [DOI] [PubMed] [Google Scholar]
- 58.Sawado T., Halow J., Bender M. A., Groudine M. (2003) Genes Dev. 17, 1009–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chaturvedi C. P., Hosey A. M., Palii C., Perez-Iratxeta C., Nakatani Y., Ranish J. A., Dilworth F. J., Brand M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 18303–18308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson K. D., Grass J. A., Boyer M. E., Kiekhaefer C. M., Blobel G. A., Weiss M. J., Bresnick E. H. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 11760–11765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vakoc C. R., Letting D. L., Gheldof N., Sawado T., Bender M. A., Groudine M., Weiss M. J., Dekker J., Blobel G. A. (2005) Mol. Cell 17, 453–462 [DOI] [PubMed] [Google Scholar]
- 62.Nagai T., Igarashi K., Akasaka J., Furuyama K., Fujita H., Hayashi N., Yamamoto M., Sassa S. (1998) J. Biol. Chem. 273, 5358–5365 [DOI] [PubMed] [Google Scholar]
- 63.Palstra R. J., Tolhuis B., Splinter E., Nijmeijer R., Grosveld F., de Laat W. (2003) Nat. Genet. 35, 190–194 [DOI] [PubMed] [Google Scholar]
- 64.Drissen R., Palstra R. J., Gillemans N., Splinter E., Grosveld F., Philipsen S., de Laat W. (2004) Genes Dev. 18, 2485–2490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song S. H., Hou C., Dean A. (2007) Mol. Cell 28, 810–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kooren J., Palstra R. J., Klous P., Splinter E., von Lindern M., Grosveld F., de Laat W. (2007) J. Biol. Chem. 282, 16544–16552 [DOI] [PubMed] [Google Scholar]
- 67.Armstrong J. A., Bieker J. J., Emerson B. M. (1998) Cell 95, 93–104 [DOI] [PubMed] [Google Scholar]
- 68.Bieker J. J. (2001) J. Biol. Chem. 276, 34355–34358 [DOI] [PubMed] [Google Scholar]
- 69.Sengupta T., Cohet N., Morlé F., Bieker J. J. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 4213–4218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bulger M., Groudine M. (1999) Genes Dev. 13, 2465–2477 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.