Abstract
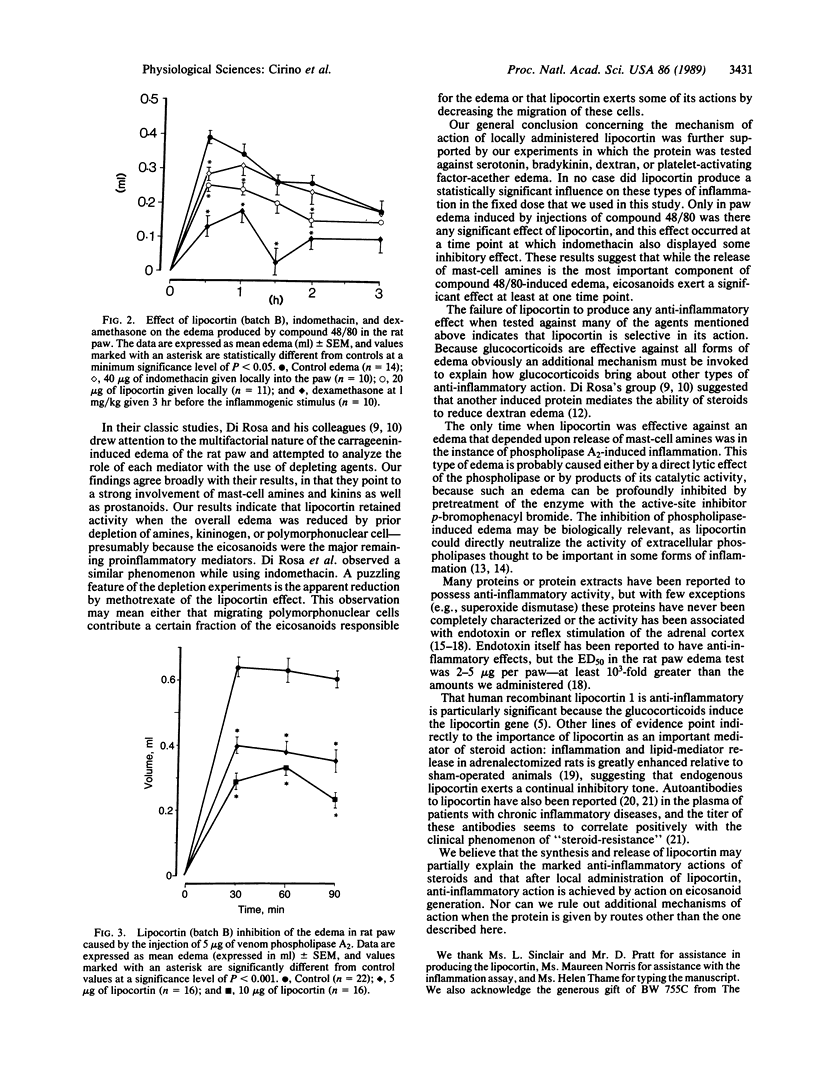
Human recombinant lipocortin 1 has been tested for anti-inflammatory activity in a conventional model of acute inflammation. Microgram amounts of the protein, locally administered, inhibited edema of the rat paw when induced by subplantar injections of carrageenin: the ED50 was 10-20 micrograms per paw, and inhibition (maximum of 60-70%) was not dependent upon an intact adrenal cortex. Doses of lipocortin that produced approximately 50% inhibition in the carrageenin test were inactive against edema elicited by bradykinin, serotonin, platelet-activating factor-acether, or dextran, whereas edema caused by Naja mocambique venom phospholipase A2 was strongly inhibited by lipocortin. The protein inhibited edema when rats were pretreated with agents that depleted mast-cell amines, kininogen, or polymorphonuclear leukocytes prior to initiation of the carrageenin edema but had no inhibitory action when rats were pretreated with the dual cyclooxygenase/lipoxygenase inhibitor BW 755C. These results demonstrate that human recombinant lipocortin has potent local anti-inflammatory activity, probably through selectively interfering with eicosanoid generation. Lipocortin is relatively ineffective against edema caused by mast-cell degranulation or kinins, except when degranulation is caused by phospholipase A2.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwell G. J., Carnuccio R., Di Rosa M., Flower R. J., Langham C. S., Parente L., Persico P., Russel-Smith N. C., Stone D. Glucocorticoids induce the formation and release of anti-inflammatory and anti-phospholipase proteins into the peritoneal cavity of the rat. Br J Pharmacol. 1982 May;76(1):185–194. doi: 10.1111/j.1476-5381.1982.tb09205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnuccio R., Di Rosa M., Guerrasio B., Iuvone T., Sautebin L. Vasocortin: a novel glucocorticoid-induced anti-inflammatory protein. Br J Pharmacol. 1987 Mar;90(3):443–445. doi: 10.1111/j.1476-5381.1987.tb11193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi E. Y., Henderson W. R., Klebanoff S. J. Phospholipase A2-induced rat mast cell secretion. Role of arachidonic acid metabolites. Lab Invest. 1982 Dec;47(6):579–585. [PubMed] [Google Scholar]
- Cirino G., Flower R. J., Browning J. L., Sinclair L. K., Pepinsky R. B. Recombinant human lipocortin 1 inhibits thromboxane release from guinea-pig isolated perfused lung. Nature. 1987 Jul 16;328(6127):270–272. doi: 10.1038/328270a0. [DOI] [PubMed] [Google Scholar]
- Cirino G., Flower R. J. Human recombinant lipocortin 1 inhibits prostacyclin production by human umbilical artery in vitro. Prostaglandins. 1987 Jul;34(1):59–62. doi: 10.1016/0090-6980(87)90262-0. [DOI] [PubMed] [Google Scholar]
- Di Rosa M., Flower R. J., Hirata F., Parente L., Russo-Marie F. Anti-phospholipase proteins. Prostaglandins. 1984 Oct;28(4):441–442. doi: 10.1016/0090-6980(84)90232-6. [DOI] [PubMed] [Google Scholar]
- Di Rosa M., Giroud J. P., Willoughby D. A. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol. 1971 May;104(1):15–29. doi: 10.1002/path.1711040103. [DOI] [PubMed] [Google Scholar]
- Di Rosa M., Papadimitriou J. M., Willoughby D. A. A histopathological and pharmacological analysis of the mode of action of nonsteroidal anti-inflammatory drugs. J Pathol. 1971 Dec;105(4):239–256. doi: 10.1002/path.1711050403. [DOI] [PubMed] [Google Scholar]
- Flower R. J., Parente L., Persico P., Salmon J. A. A comparison of the acute inflammatory response in adrenalectomised and sham-operated rats. Br J Pharmacol. 1986 Jan;87(1):57–62. doi: 10.1111/j.1476-5381.1986.tb10156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., del Carmine R., Nelson C. A., Axelrod J., Schiffmann E., Warabi A., De Blas A. L., Nirenberg M., Manganiello V., Vaughan M. Presence of autoantibody for phospholipase inhibitory protein, lipomodulin, in patients with rheumatic diseases. Proc Natl Acad Sci U S A. 1981 May;78(5):3190–3194. doi: 10.1073/pnas.78.5.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. A. Endogenous anti-inflammatory proteins. Biochem Pharmacol. 1977 Apr 15;26(8):693–698. doi: 10.1016/0006-2952(77)90210-6. [DOI] [PubMed] [Google Scholar]
- Parente L., Di Rosea M., Flower R. J., Ghiara P., Meli R., Persico P., Salmon J. A., Wood J. N. Relationship between the anti-phospholipase and anti-inflammatory effects of glucocorticoid-induced proteins. Eur J Pharmacol. 1984 Mar 23;99(2-3):233–239. doi: 10.1016/0014-2999(84)90246-2. [DOI] [PubMed] [Google Scholar]
- Parente L., Flower R. J. Hydrocortisone and 'macrocortin' inhibit the zymosan-induced release of lyso-PAF from rat peritoneal leucocytes. Life Sci. 1985 Apr 1;36(13):1225–1231. doi: 10.1016/0024-3205(85)90266-8. [DOI] [PubMed] [Google Scholar]
- Peers S. H. Dexamethasone inhibits platelet activating factor-induced inflammation in the paw but not the pleural cavity of rats. Eur J Pharmacol. 1988 May 20;150(1-2):131–135. doi: 10.1016/0014-2999(88)90758-3. [DOI] [PubMed] [Google Scholar]
- Pepinsky R. B., Sinclair L. K., Browning J. L., Mattaliano R. J., Smart J. E., Chow E. P., Falbel T., Ribolini A., Garwin J. L., Wallner B. P. Purification and partial sequence analysis of a 37-kDa protein that inhibits phospholipase A2 activity from rat peritoneal exudates. J Biol Chem. 1986 Mar 25;261(9):4239–4246. [PubMed] [Google Scholar]
- Smith M. J., Ford-Hutchinson A. W., Walker J. R. Anti-inflammatory activity of bacterial endotoxin. J Pharm Pharmacol. 1977 Nov;29(11):702–704. doi: 10.1111/j.2042-7158.1977.tb11441.x. [DOI] [PubMed] [Google Scholar]
- Vadas P., Pruzanski W. Role of secretory phospholipases A2 in the pathobiology of disease. Lab Invest. 1986 Oct;55(4):391–404. [PubMed] [Google Scholar]
- Wallner B. P., Mattaliano R. J., Hession C., Cate R. L., Tizard R., Sinclair L. K., Foeller C., Chow E. P., Browing J. L., Ramachandran K. L. Cloning and expression of human lipocortin, a phospholipase A2 inhibitor with potential anti-inflammatory activity. Nature. 1986 Mar 6;320(6057):77–81. doi: 10.1038/320077a0. [DOI] [PubMed] [Google Scholar]



