Abstract
Heat shock proteins (HSPs) have been implicated in the activation and survival of macrophages. This study examined the role of HSP70B′, a poorly characterized member of the HSP70 family, in response to oxidatively modified LDL (oxLDL) and immune complexes prepared with human oxLDL and purified human antibodies to oxLDL (oxLDL-IC) in monocytic and macrophage cell lines. Immunoblot analysis of cell lysates and conditioned medium from U937 cells treated with oxLDL alone revealed an increase in intracellular HSP70B′ protein levels accompanied by a concomitant increase in HSP70B′ extracellular levels. Fluorescence immunohistochemistry and confocal microscopy, however, demonstrated that oxLDL-IC stimulated the release of HSP70B′, which co-localized with cell-associated oxLDL-IC. In HSP70B′-green fluorescent protein-transfected mouse RAW 264.7 cells, oxLDL-IC-induced HSP70B′ co-localized with membrane-associated oxLDL-IC as well as the lipid moiety of internalized oxLDL-IC. Furthermore, the data demonstrated that HSP70B′ is involved in cell survival, and this effect could be mediated by sphingosine kinase 1 (SK1) activation. An examination of regularly implicated cytokines revealed a significant relationship between HSP70B′ and the release of the anti-inflammatory cytokine interleukin-10 (IL-10). Small interfering RNA knockdown of HSP70B′ resulted in a corresponding decrease in SK1 mRNA levels and SK1 phosphorylation as well as increased release of IL-10. In conclusion, these findings suggest that oxLDL-IC induce the synthesis and release of HSP70B′, and once stimulated, HSP70B′ binds to the cell-associated and internalized lipid moiety of oxLDL-IC. The data also implicate HSP70B′ in key cellular functions, such as regulation of SK1 activity and release of IL-10, which influence macrophage activation and survival.
Keywords: Cytokine, Heat Shock Protein, Inflammation, Low Density Lipoprotein (LDL), Macrophage, HSP70, HSP70B′, Oxidized LDL, Oxidized LDL Immune Complexes, Sphingosine Kinase
Introduction
Heat shock proteins (HSPs)2 belong to a group of more than 20 highly conserved stress proteins that are routinely employed by cells as cytoprotective agents against a variety of stress stimuli, including heat shock, oxidative and mechanical stress, and inflammation (1, 2). Evidence exists that HSP expression is higher at sites of atherosclerotic lesions than it is in normal tissue (3, 4). Elevated HSP70 levels are associated with atherosclerotic plaques, particularly in areas with abundant activated macrophages, and seem to coincide around sites of necrosis and lipid accumulation (5, 6).
Of particular interest in the development of atherogenesis is the role of oxidatively modified LDL (oxLDL). It is established that oxLDL particles are taken up by activated macrophages, resulting in lipid accumulation (7). oxLDL also triggers an immune response, initiating the production of predominantly proinflammatory IgG antibodies, which then form circulating complexes with oxLDL (oxLDL-IC) (8, 9). These immune complexes activate macrophages through the FcγRI receptor, resulting in the release of proinflammatory cytokines (interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α)) and the associated acceleration of foam cell formation (10–13). Whereas free oxLDL have been shown to be cytotoxic to monocytic cells (14–16), oxLDL complexed to IgG was found to promote survival (17–19).
Studies have demonstrated that circulating HSP60 is linked to cardiovascular disease (20). However, the role HSP70 family members play in the development of atherosclerotic plaques is still unclear. Increasing evidence suggests that HSPs may serve as cytokines themselves. Asea et al. (21) determined that activated macrophages secrete HSP70, which then binds to CD14 on the outer membrane, triggering the production and subsequent release of proinflammatory cytokines. This finding supports earlier experiments that induced cytokine production with the addition of exogenous HSP70 (22, 23).
Elevated oxLDL, a hallmark of increased risk of atherosclerosis, has been implicated as the initial factor in the HSP70-linked proinflammatory pathway of activated macrophages. Svensson et al. (24) demonstrated that high levels of oxLDL directly up-regulate and initiate release of HSP70 in macrophages, resulting in a corresponding increase in cytokine (IL-1β and IL-12) production. Whether exposure of human macrophages to elevated levels of oxLDL-IC elicits HSP70 regulation has not been previously examined.
We have recently shown that one member of the HSP70 family, HSP70B′ (also known as HSP70 protein 6; gene HSPA6), displayed a considerable increase in expression in response to oxLDL-IC but not oxLDL alone (25). Furthermore, evidence was provided that IL-1β secretion is HSP70B′-dependent, suggesting a novel, oxLDL-IC-dependent proinflammatory mechanism involving the little known HSP70B′ species (25). The HSP70B′ gene is unique to the human genome, probably arising after the divergence of rodents and humans (26), and although HSP70B′ and HSP70 are over 80% homologous, differences are present in sequences coding for substrate-binding and activation sites, evidence of a unique cellular function (27). Until recently, sequence data and immunological reagents for HSP70B′ were unavailable, and due to the sequence homology, standard antibodies designed for the common HSP70A/B members most likely included the detection of HSP70B′. HSP70, while functionally inducible, is fairly ubiquitous at basal conditions in many different cell types, whereas HSP70B′ is strictly inducible (27–29). Our recent findings have led to the hypothesis that HSP70B′ expression could be induced by Fcγ-RI activation in human macrophages, resulting in increased proinflammatory cytokine release, prolonged foam cell survival, and thus the associated increased risk of atherosclerotic plaque development (18, 25). Herein, we show that HSP70B′ was induced and released in response to oxLDL-IC and that HSP70B′ could bind to the cell-associated and internalized lipid moiety of oxLDL-IC. The presented data also implicated HSP70B′ in cellular functions that underlie the ability of oxLDL-IC to promote prolonged activation of foam cells, such as regulation of sphingosine kinase 1 (SK1) activity and release of IL-10.
EXPERIMENTAL PROCEDURES
Cells
The human monocytic cell line U937 was obtained from the American Type Culture Collection (Manassas, VA) (ATCC CRL-1593.2). This line is a promonocytic lymphoma cell line, which originates from resident macrophages (30). Cells were maintained in Iscove's modified Dulbecco's medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin, and 50 μg/ml streptomycin at 37 °C and 5% CO2. Unless otherwise indicated, cells were seeded at 1.5 × 106 cells/ml and incubated in serum-free medium in the presence of 200 ng/ml interferon-γ (IFN-γ) (EMD, Bioscience, San Diego, CA) for 18 h prior to the addition of experimental treatments. RAW 264.7 cells are a macrophage-like, Abelson leukemia virus-transformed cell line derived from BALB/c mice. RAW 264.7 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, penicillin, and streptomycin at 37 °C and 5% CO2. For routine maintenance, cells were grown to 80% confluence and subcultured every 2 days.
Lipoprotein Isolation and Oxidation
LDL (d = 1.019–1.063 g/ml) was isolated from plasma of donors who were free from clinically apparent disease and oxidatively modified using Cu2+ as described previously (25, 31, 32). The degree of LDL oxidation was monitored continuously by fluorescence emission at 234 nm using a fluorescence spectrophotometer (SLM-AMINCO® Series 2; Spectronic Instruments, Rochester, NY) and stopped when the fluorescence reached a value of ≥1.1 fluorescence units (31, 32). LDL modification was verified by particle migration on the Paragon® electrophoresis system (Beckman Coulter, Fullerton, CA).
Preparation of Immune Complexes
oxLDL-IC were prepared with human oxLDL and purified human anti-oxLDL antibodies as described previously (13, 25, 33). Keyhole limpet hemocyanin immune complexes (KLH-IC) were used as a control immune complex because keyhole limpet hemocyanin has a molecular weight comparable with LDL and because it can engage Fcγ receptors similar to oxLDL-IC but does not contain lipoproteins. Human KLH-IC was prepared as described previously (13). Immune complexes were suspended in phosphate-buffered saline (PBS), and the concentrations of total protein were determined using the BCA protein assay (Pierce).
Detection of HSP70 and HSP70B′ in Cell Lysates and Conditioned Media
Cells were treated with oxLDL, oxLDL-IC, and KLH-IC (150 μg/ml) for 2, 6, 12, and 24 h. Cells were pelleted and lysed using Extraction Reagent, a Tris-based buffer (Assay Designs, Ann Arbor, MI). Protein concentrations in the extracts were determined by the BCA protein assay. Equal amounts (10 μg) of protein were electrophoresed in 4–12% NuPAGE® BisTris precast polyacrylamide gels (Invitrogen) under reducing conditions, transferred to nitrocellulose membranes, blocked in 5% nonfat milk in TBST (Tris-buffered saline, 0.1% Tween 20), and probed with mouse monoclonal antibodies against human HSP70 (BD Biosciences) and HSP70B′ (Assay Designs). Membranes were then exposed to horseradish peroxidase-conjugated anti-mouse-IgG and visualized using Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life Sciences).
Protein in the media was captured using StrataCleanTM resin (hydroxylated silica particles) (Stratagene) according to the manufacturer's instructions and then electrophoresed using 4–12% precast NuPAGE gels under non-reducing conditions. HSP70 and HSP70B′ were immunoblotted using monoclonal mouse anti-human antibodies as mentioned above.
Detection of HSPs in Detergent-insoluble Cell Membrane Fractions
To examine whether HSP70B′ is translocated/associated with the cell membrane in response to oxLDL-IC, the NE-PERTM Nuclear and Cytoplasmic Extraction Reagents kit (Pierce) was used. The kit was used according to the manufacturer's instructions with modification to accommodate the insolubility of oxLDL-IC bound to membranes. Briefly, treated cells (8 × 106) were pelleted by centrifugation at 400 × g for 3 min. After isolating the cytoplasmic extract fraction using the designated detergent-containing reagent, the insoluble pellet including the immune complex was resuspended in 100 μl of ice-cold nuclear extraction reagent, vortexed for 15 s every 10 min, for a total of 40 min, and then centrifuged at 16,000 × g for 10 min. The supernatant was isolated immediately and mixed with 5 μl of StrataCleanTM resin. Captured proteins were analyzed by immunoblot as mentioned above.
Fluorescent Labeling of oxLDL and oxLDL-IC
Fluorescent labeling of oxLDL was performed as described previously (34) with modification. Briefly, oxLDL (1.0 ml, 1.0 mg of protein) was mixed with lipoprotein-deficient serum (1.0 ml) and then filter (0.22 μm)-sterilized. A 50-μl aliquot of DiI:1,1′-dioctadecyl-3,3,3′,3-tetramethylindocarbocyanine perchlorate (DiI) (Invitrogen), 3.0 mg/ml in dimethyl sulfoxide (Sigma), was added to the oxLDL/lipoprotein-deficient serum mixture. The mixture was gently mixed and incubated at 37 °C for 8 h. To isolate the labeled oxLDL, the density of the solution containing the fluorescent labeled LDL was raised to 1.225 g/ml with solid KBr, and the solution was loaded into a polymer ultracentrifuge tube (13-ml tube, Beckman). Tubes were then filled with a saline solution whose density was adjusted to 1.21 g/ml with solid KBr. The labeled LDL was then floated out of the mixture by ultracentrifugation with a Beckman SW41 Ti rotor at 41,000 rpm, 36 h, 4 °C. The labeled oxLDL floating at the top of the tube was aspirated, the density of this solution was raised to 1.225 g/ml, and the solution was again centrifuged at 41,000 rpm, 36 h, 4 °C. The top layer was aspirated, dialyzed against NaCl-EDTA solution (150 mm NaCl/300 μm EDTA, pH 8.6), and then filter-sterilized and stored at 4 °C. The concentration of protein in the labeled oxLDL was determined using the BCA protein assay. Labeled oxLDL was used to prepare oxLDL-IC as described above.
Fluorescent Labeling of Anti-HSP70B′ Antibody with Alexa Fluor® 488
Mouse monoclonal anti-human HSP70B′ antibody was labeled using the Alexa Fluor® 488 monoclonal antibody labeling kit (Invitrogen) according to the manufacturer's protocol.
Detection of HSP70B′ by Immunocytochemistry
Fluorescently labeled oxLDL (10 μg/ml) and oxLDL-IC (30 μg/ml) were incubated with cells for 5 h. Cells were then pelleted by centrifugation at 400 × g for 3 min and then fixed and permeabilized using the Cytofix/CytopermTM kit (Pharmingen, San Diego, CA) according to the manufacturer's instructions. Cells were probed with Alexa Fluor® 488-labeled monoclonal anti-human HSP70B′ and were mounted using the VECTASHIELD® HardSetTM with 4′,6-diamidino-2-phenylindole anti-fade mounting medium (Vector Laboratories, Burlingame, CA) and visualized using confocal microscopy (Zeiss LSM 510 laser-scanning confocal microscope, Carl Zeiss MicroImaging, Inc. (Thornwood, NY)). Fluorescent images were taken from representative fields of two independent experiments using the same gain and exposure settings, and the amount of green fluorescence was quantified using Adobe Photoshop CS2 to reveal the mean green channel intensity of individual cells as previously described (35).
Knockdown of HSP70 and HSP70B′
U937 cells were transfected with non-targeting or HSP70B′ ON-TARGETplus SMARTpool siRNAs (Dharmacon RNA Technologies, Chicago, IL) (D-001206-13 and L-019455-00) using the NucleofectorTM device (Amaxa Inc., Gaithersburg, MD) according to the manufacturer's instructions. Knockdown was verified using quantitative PCR (Q-PCR) analysis. 48 h post-transfection, cells were primed with IFN-γ and incubated in serum-free medium as described above or as indicated otherwise. Cells were then treated with oxLDL and oxLDL-IC (150 μg/ml) for 6, 12, 24, 48, and 72 h. To detect HSP70, HSP70B′, and SK1 in cell lysates, equal amounts of protein (20 μg) were separated on SDS-PAGE, transferred to nitrocellulose membranes, and probed with antibodies against HSP70 and HSP70B′ as described above. SK1 was probed with a polyclonal antibody against phosphorylated SK1 (a gift from Dr. Stuart Pitson, Centre for Cancer Biology, Adelaide, Australia).
Expression of GFP-tagged human HSP70 and HSP70B′
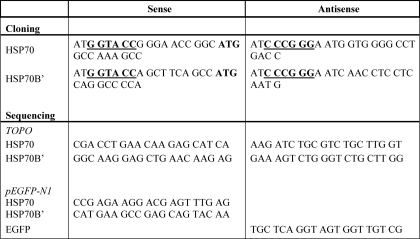
HSP70 and HSP70B′ cDNAs were purchased from OriGene (Rockville, MD) and used to generate a 1700-bp PCR amplicon using high fidelity Taq polymerase (Roche Applied Science) and custom designed primers (Table 1), with KpnI and ApaI restriction enzyme sites. The PCR products were cloned into a shuttle vector using the TOPO TA cloning kit (Invitrogen) and transformed in OneShot® Top10 competent cells (Invitrogen). The product was sequenced in both directions to verify the clone. The cloned HSP sequences were then cloned into the pEGFP-N1 expression vector (Clontech, Mountain View, CA), coding for an enhanced green fluorescent protein. Ligation of HSP70 or HSP70B′ sequences was confirmed by DNA sequencing. Transfection with LipoD293TM DNA in vitro transfection reagent (SignaGen Laboratories) was performed according to the manufacturer's instructions. Briefly, 1 μg of plasmid DNA and 3 μl of LipoD293TM reagent were each diluted into 50 μl of serum-free Dulbecco's modified Eagle's medium. The mixtures were then combined and incubated for 15 min at room temperature. RAW 264.7 cells (80–90% confluent) were incubated with the combined mixture under normal growing conditions for 18 h. Transfection reagent-containing medium was then replaced with complete medium and incubated for an additional 24 h prior to treatment. The amount of green fluorescence was quantified as described above. For live imaging, cells were maintained in serum-free treatment-containing medium in glass bottom 96-well plates (MatTek Corp., Ashland, MA) at 37 °C and 5% CO2 for the duration of the experiment.
TABLE 1.
Primers designed for cloning and DNA sequence verification
KpnI and ApaI restriction sequences for cloning primers for sense and antisense, respectively, are designated by boldface underlined letters. Boldface ATG is the methionine start site.
Cell Toxicity
Numbers of non-viable cells were determined using trypan blue. Cell survival was also assessed using the CyQUANT cell proliferation assay kit from Molecular Probes, Inc. (Eugene, OR), a highly sensitive, fluorescence-based assay for determining the number of cultured cells (36). Briefly, cells were rinsed with PBS and lysed, and the DNA was stained using the CyQUANT fluorescent dye. Fluorescence was measured using a GENios multidetection plate fluorescence reader (MTX Lab Systems, Vienna, VA). Cell numbers were extrapolated using a standard curve generated from fluorescence readings of known counts of U937 cells. Alternatively, RAW 264.7 cells were trypsinized, neutralized, and counted using the Nexcelom Auto T4 CellometerTM (Nexcelom Biosciences, Lawrence, MA).
Real-time Q-PCR
PCR primers were designed using the Beacon Designer 5 software (Premier Biosoft International, Palo Alto, CA). The forward and reverse primer sequences for HSP70B′ (CCCTAAGGCTTTCCTCTTGC and CATGAAGCCGAGCAGTACAA) and for SK1 (CTGGCAGCTTCCTTGAACCAT and TGTGCAGAGACAGCAGGTTCA) were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). IFN-γ-treated U937 cells were exposed to oxLDL-IC, oxLDL (150 μg/ml), or PBS vehicle for 8 h. The RNAeasy mini kit was used to isolate mRNA (Qiagen), and complementary DNA (cDNA) was synthesized using iScriptTM cDNA synthesis kit (Bio-Rad). Q-PCR was performed using the iCyclerTM real-time detection system (Bio-Rad) with a two-step method using iQTM SYBR Green Supermix (Bio-Rad). Amplification of glyceraldehyde-3-phosphate dehydrogenase was performed to standardize the amount of sample cDNA. Quantification was performed using the cycle threshold of receptor cDNA relative to that of glyceraldehyde-3-phosphate dehydrogenase cDNA in the same sample.
Cytokine Analysis
Conditioned medium was harvested and processed in duplicate with a custom Bio-Rad Bio-Plex human cytokine reagent kit for IL-1β, IL-6, IL-10, IL-12 (p70), and TNF-α according to the manufacturer's instructions (Bio-Rad). Briefly, conditioned media containing 0.5% BSA were incubated with anti-cytokine-conjugated beads, followed by incubation with biotinylated detection antibody. The reaction mixture was detected with streptavidin-phycoerythrin and analyzed using a BioPlex 200 machine (Bio-Rad). Unknown cytokine concentrations were calculated by BioPlex Manager Software using standard curves derived from a recombinant cytokine standard.
Statistics
Significant differences between two groups were evaluated by Student's t test and between more than two groups by one-way or two-way analysis of variance followed by Tukey's post hoc test for mean separation (p < 0.05). All data are expressed as mean ± S.D.
RESULTS
Differential Induction of HSP70 and HSP70B′ by oxLDL and oxLDL-IC
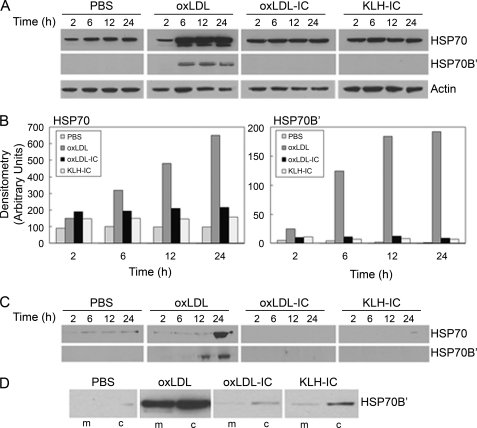
To determine the expression levels of HSP70 and HSP70B′ and the kinetics of this expression in response to oxLDL and oxLDL-IC in U937 monocytic cells, immunoblot analysis of HSP70 and HSP70B′ in cells and conditioned media was performed. Fig. 1, A and B, demonstrates that in total cell lysate, HSP70 levels increased in cells treated with oxLDL, whereas base-line levels remained unchanged in cells treated with oxLDL-IC, KLH-IC, or the PBS vehicle. Increases in HSP70 in response to oxLDL occurred at 6 h and continued through 24 h post-treatment. HSP70B′ expression levels, however, were undetectable in response to PBS or to either of the immune complex treatments but were significantly induced in response to oxLDL at 6–24 h post-treatment (Fig. 1, A and B).
FIGURE 1.
Immunoblot analysis of HSP70 and HSP70B′ expression in U937 cells in response to oxLDL and oxLDL-IC. A and B, total cell lysates; C, conditioned media; D, membrane/cytoplasmic fractions. A–C, cells (1.5 × 106 cells/ml) were treated with oxLDL, oxLDL-IC, KLH-IC (150 μg/ml), or PBS vehicle for 2, 6, 12, and 24 h. Equal protein (20 μg) was loaded in each lane and probed for cellular HSP70 and HSP70B′ (A); densitometry of HSP70 and HSP70B′ in A were normalized to actin and presented in B. Total protein secreted into the conditioned media was captured using StrataClean resin and subjected to immunoblot analysis (C). Results in A–C are representative of three independent experiments. D, cells (1.5 × 106 cells/ml) were treated with oxLDL, oxLDL-IC, KLH-IC (150 μg/ml), or PBS vehicle for 6 h and then lysed using the NE-PERTM nuclear and cytoplasmic extraction reagent kit to obtain membrane and cytoplasmic fractions. Equal protein (30 μg) was loaded in each lane and probed for HSP70B′. Results in D are representative of two independent experiments. m, membrane fractions; c, cytoplasmic fractions.
To determine whether HSP70 and HSP70B′ were secreted, conditioned media were collected and probed for HSP70 and HSP70B′ using immunoblot analysis. Fig. 1C shows that HSP70 was present in media collected from cells induced by oxLDL with a considerable increase at 24 h post-treatment. Interestingly, HSP70 was detected in media collected from cells exposed to the PBS vehicle but not from cells exposed to either of the immune complex treatments. In contrast, HSP70B′ was only detected in the conditioned media of oxLDL-treated cells.
To examine whether HSP70B′ was translocated to the cell membrane and therefore not detected in the whole cell lysate of cells induced with oxLDL-IC or KLH-IC, the cytoplasmic and membrane fractions were isolated and probed for HSP70B′. HSP70B′ was detected at higher levels in the membrane fraction of oxLDL-treated cells than in oxLDL-IC- or KLH-IC-treated cells (Fig. 1D), consistent with data from whole cell lysates shown in Fig. 1A. These data suggested that immune complex-stimulated HSP70B′ message was not translated or that the protein immediately degraded or complexed to undetermined molecule(s).
HSP70B′ Is Up-regulated and Released in Response to oxLDL-IC
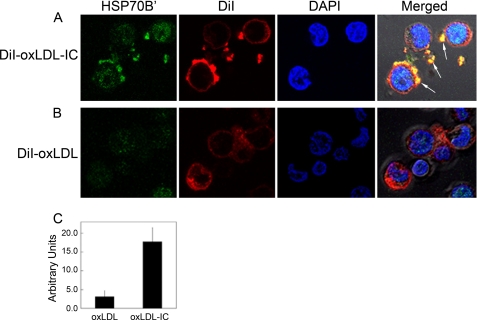
To investigate whether there was an association between HSP70B′ and oxLDL-IC, we used fluorescently labeled oxLDL (DiI-oxLDL) and Alexa Fluor® 488-labeled antibody specific against HSP70B′. Fig. 2 shows that HSP70B′ was expressed at higher levels in response to oxLDL-IC compared with oxLDL alone. Furthermore, HSP70B′ co-localized with extracellular membrane-associated oxLDL-IC. Although both oxLDL and oxLDL-IC induced the release of HSP70B′, as shown in Figs. 1C and 2A, respectively, the membrane-associated HSP70B′ induced by oxLDL-IC was modestly detected using immunoblot analysis (Fig. 1D). This could be explained by poor extractability of membrane-associated HSP70B′, which might have become part of lipid rafts and/or the detergent-resistant membrane fraction.
FIGURE 2.
oxLDL-IC induce up-regulation and release of HSP70B′ in U937 cells. Cells were incubated with DiI-oxLDL-IC (30 μg/ml) (A) or DiI-oxLDL (10 μg/ml) (B) for 5 h and then fixed and permeabilized using the Cytofix/CytopermTM kit. Cells were probed with an Alexa Fluor® 488-labeled monoclonal antibody against HSP70B′ and visualized by confocal microscopy. C, quantification of mean green channel intensity (n = 10; difference between means is significant at p < 0.05). Results are representative of two independent experiments. The arrows point at co-localization of HSP70B′ with membrane-associated and extracellular insoluble oxLDL-IC. Error bars, S.D.
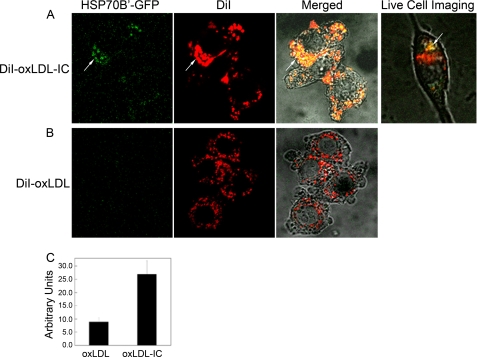
Transfected HSP70B′-GFP Co-localizes with DiI-labeled Lipid Moiety of oxLDL-IC
To verify HSP70B′ co-localization with insoluble oxLDL-IC and to avoid membrane permeabilization required for antibody-based visualization, we cloned HSP70B′ into the pEGFP-N1 expression vector (HSP70B′-GFP) and transfected it in RAW 264.7 cells, an adherent mouse leukemic monocyte macrophage cell line. Fig. 3 shows that HSP70B′-GFP was expressed at higher levels in response to oxLDL-IC compared with oxLDL alone. This is consistent with data shown in Fig. 2. Interestingly, stimulation with oxLDL-IC resulted in co-localization of HSP70B′-GFP with the lipid moiety of oxLDL-IC both in extracellular and intracellular compartments. Fig. 3A shows also live cell imaging of HSP70B′-GFP co-localizing with intracellular DiI-oxLDL-IC between 3 and 4 h post-treatment (see supplemental Video 1).
FIGURE 3.
oxLDL-IC induce co-localization of transfected HSP70B′-GFP with DiI-labeled lipid moiety. A and B, RAW 264.7 cells were transfected with GFP-HSP70B′ and then incubated with DiI-oxLDL-IC (30 μg/ml) (A), or DiI-oxLDL (10 μg/ml) (B) for 4 h, fixed with 3% paraformaldehyde, and visualized by confocal microscopy. Live Cell Imaging panel, HSP70B′ co-localization with DiI-labeled lipid moiety of DiI-oxLDL-IC (see supplemental Video 1). C, quantification of mean green channel intensity (n = 10; difference between means is significant at p < 0.05). Results are representative of three independent experiments. The arrows point at co-localization of HSP70B′-GFP with the lipid moiety of oxLDL-IC. Error bars, S.D.
HSP70-GFP and HSP70B′-GFP Are Up-regulated in Response to oxLDL-IC
HSP70 was also cloned into pEGFP-N1 expression vector (HSP70-GFP) to compare the response of HSP70B′-GFP and HSP70-GFP to oxLDL and oxLDL-IC. Fig. 4 shows that oxLDL-IC but not free oxLDL induced up-regulation of both HSP70-GFP and HSP70B′-GFP. KLH-IC induced also up-regulation of both HSP70-GFP and HSP70B′-GFP similar to oxLDL-IC (data not shown).
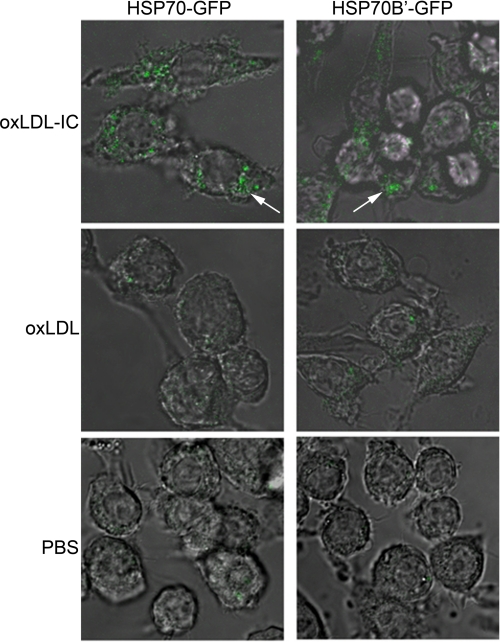
FIGURE 4.
oxLDL-IC induce up-regulation of both HSP70-GFP and HSP70B′-GFP in RAW 264.7 cells. Cells were transfected with HSP70-GFP or HSP70B′-GFP and then treated with oxLDL, oxLDL-IC (150 μg/ml), or PBS vehicle for 6 h, fixed with 4% formaldehyde, and visualized by confocal microscopy. Results are representative scoped fields in three independent experiments.
HSP70B′ Regulates Cell Proliferation and Survival
In an effort to investigate the physiological relevance of HSP70B′ expression, we examined the effect of overexpression of the HSP70B′ in RAW 264.7 cells over 5 days post-transfection with HSP70B′-GFP (Fig. 5A). Interestingly, transfection with HSP70B′ induced decreased cell proliferation compared with cells transfected with the empty GFP control vector. Although both HSP70B′-GFP- and HSP70-GFP-transfected cells showed decreased cell proliferation compared with control cells, HSP70B′-GFP-expressing cells exhibited significantly less proliferation than HSP70-GFP-expressing cells (Fig. 5A), suggesting a similar but possibly selective role for HSP70B′ in macrophage cell growth.
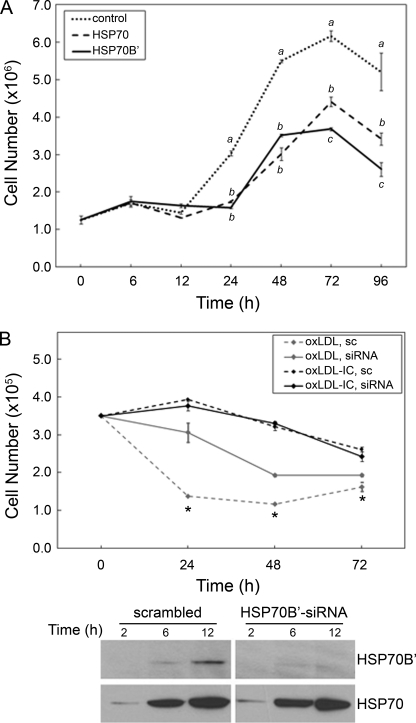
FIGURE 5.
HSP70B′ regulates cell proliferation and survival. A, transfection with HSP70B′-GFP induced decreased cell proliferation compared with HSP70-GFP in RAW 264.7 cells. Cells were transfected with HSP70B′-GFP or HSP70-GFP, maintained in complete media, and then counted at 6, 12, 24, 48, 72, and 96 h post-transfection using a Nexcelom Auto T4 CellometerTM. Plotted values are means ± S.D. (error bars) of triplicate values. Data were analyzed by one-way analysis of variance; different letters denote significant differences among means at each time point (p < 0.05). Results are representative of three independent experiments. B, knockdown of HSP70B′ inhibited oxLDL-induced cell death in U937 cells. Upper panel, cells were transfected with scrambled siRNA (sc) or HSP70B′ siRNA and then treated with oxLDL or oxLDL-IC (150 μg/ml) for 72 h; cell viability was assessed using a CyQUANT DNA assay. Plotted values are means ± S.D. of triplicate values. Differences between means within treatment were evaluated by Student's t test (p < 0.05). *, significant difference between scrambled siRNA-transfected and HSP70B′ siRNA-transfected cells treated with oxLDL-IC but not oxLDL. Results are representative of three independent experiments. Lower panel, cells transfected with scrambled or HSP70B′ siRNA then induced with oxLDL (150 μg/ml) for 2, 6, and 12 h; an equal amount of protein (20 μg) was separated on SDS-PAGE, probed for HSP70B′, and then re-probed for HSP70.
It has been established that free oxLDL elicits cytotoxicity (14–16), whereas oxLDL-IC promote cell survival (17–19). To further elucidate the contribution of HSP70B′ to cell survival, HSP70B′ siRNA was used to selectively knock down HSP70B′ in U937 cells. Fig. 5B shows no significant difference in cell survival in response to oxLDL-IC between cells transfected with HSP70B′ siRNA and scrambled siRNA. However, U937 cells showed decreased mortality following knockdown of HSP70B′ in cells treated with oxLDL. This finding suggests that oxLDL-induced HSP70B′ may serve as a proapoptotic mediator.
HSP70B′ Knockdown Results in Decreased SK1 mRNA and Protein Expression
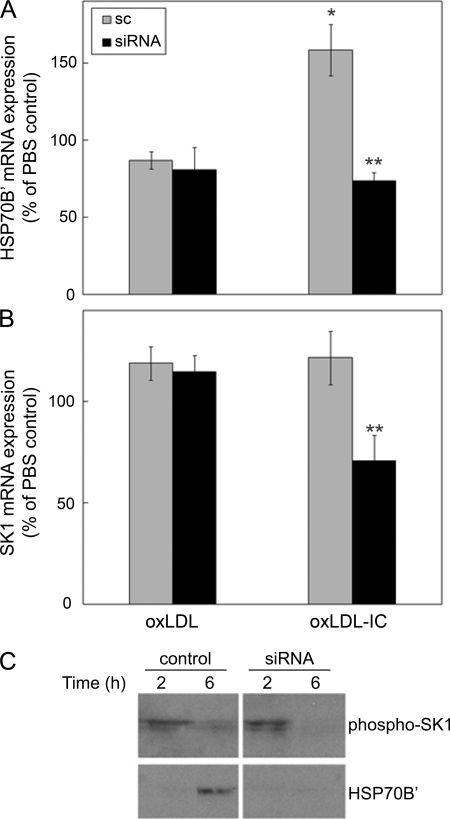
We have previously shown that a prosurvival mechanism associated with oxLDL-IC signaling in U937 cells is mediated by activation of SK1 and that oxLDL-IC, but not oxLDL alone, induce an immediate membrane translocation and release of SK1 (18). To determine a regulatory relationship between HSP70B′, cell survival, and SK1, we examined the effect of HSP70B′ knockdown on SK1 mRNA expression in the presence of oxLDL and oxLDL-IC (Fig. 6). A significant increase in HSP70B′ mRNA expression was induced by oxLDL-IC at 6 h compared with other treatments in cells transfected with control siRNA (Fig. 6A). Interestingly, siRNA knockdown of HSP70B′ inhibited SK1 mRNA expression in response to oxLDL-IC but not oxLDL alone (Fig. 6B), suggesting a regulatory link between HSP70B′ and SK1. Using an antibody raised against phosphorylated SK1, Fig. 6C shows that activated SK1 protein was also decreased in response to knockdown of HSP70B′.
FIGURE 6.
HSP70B′ knockdown results in decreased SK1 mRNA and protein expression in U937 cells. Cells were transfected with scrambled siRNA (sc) or HSP70B′ siRNA, primed with IFN-γ for 18 h, and then incubated in serum-free medium for 2 h prior to treatment with oxLDL, oxLDL-IC (150 μg/ml), or PBS vehicle for 6 h. A, Q-PCR analysis of HSP70B′ mRNA levels; B, Q-PCR analysis of SK1 mRNA levels. Quantification of RNA was performed using the cycle threshold of HSP70B′ and SK1 cDNA relative to that of GAPDH. Data are expressed as means ± S.D. (error bars) of triplicate values. Data were analyzed by two-way analysis of variance with transfection and treatment as variables. Data are representative of two experiments. C, cells transfected with control or HSP70B′ siRNA and then induced with oxLDL (150 μg/ml) for 2 and 6 h; an equal amount of protein (20 μg) was separated on SDS-PAGE, probed for phosphorylated SK1, and then reprobed for HSP70B′. *, significantly different from all other groups (p < 0.05). **, significantly different from scrambled siRNA-transfected oxLDL-IC-treated cells (p < 0.05).
HSP70B′ Knockdown Results in Increased IL-10 Secretion
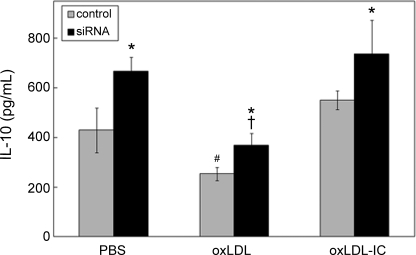
We also examined the effect of HSP70B′ on cell activation and cytokine release in U937 cells induced by oxLDL and oxLDL-IC. Using a multiplex human cytokine detection assay, the secretion of both pro- and anti-inflammatory cytokines known to be involved with macrophage activation was examined. Among the cytokines tested, the anti-inflammatory IL-10 displayed significant differences between control and HSP70B′ siRNA-transfected cells (Fig. 7). In cells treated with oxLDL, whether HSP70B′ was knocked down or not, IL10 levels were significantly decreased compared with both oxLDL-IC and vehicle treatments, with no difference between oxLDL-IC and vehicle treatments. Intriguingly, knockdown of HSP70B′ resulted in an increase in IL10 levels whether cells were treated with oxLDL, oxLDL-IC, or PBS vehicle (Fig. 7).
FIGURE 7.
HSP70B′ knockdown results in increased IL-10 secretion in U937 cells. Cells were transfected with HSP70B′ siRNA, primed with IFN-γ, and treated with oxLDL, oxLDL-IC, or vehicle as described in the legend to Fig. 6. Conditioned medium was collected after 12 h, and IL-10 was quantified using the Bio-Plex human cytokine kit (Bio-Rad). Plotted values are means ± S.D. (error bars) of triplicate values. Differences between means were evaluated by Student's t test (p < 0.05). Results are representative of three independent experiments. *, significantly different from control transfected cells within each treatment; #, significantly different from PBS and oxLDL-IC in control transfected cells; †, significantly different from PBS and oxLDL-IC in HSP70B′ siRNA-transfected cells.
DISCUSSION
In this study, we examined the role of HSP70B′, a poorly characterized member of the heat shock protein 70 family, in foam cell activation and survival induced by oxLDL-IC. HSP70B′ appears to play a unique and complex role in macrophage activation associated with oxLDL-IC signaling. The results showed for the first time that human monocytic cells respond to oxLDL-IC by stimulating and secreting HSP70B′. The results also showed that HSP70B′ appears to become associated with the intracellular as well as membrane-associated oxLDL-IC lipid moiety. Furthermore, the data demonstrated that HSP70B′ is involved in cell survival, and this effect could be mediated by SK1 activation. The regulation of the anti-inflammatory cytokine IL-10 by HSP70B′ provides further evidence of the involvement of the little known member of the HSP70 family, HSP70B′, in foam cell functionality. Investigating the significance of HSPs, not only as classical chaperones and stress proteins, but as complex signaling molecules is of increasing importance in understanding the mechanisms involved in the activity of foam cells, a hallmark of atherosclerotic plaques.
In a recent study, we identified 83 genes as being similarly regulated by oxLDL-IC in the human leukemic monocyte lymphoma U937 cell line (25). Among the up-regulated genes, HSP70B′ showed the highest increase in expression. Despite the substantial increase of HSP70B′ at the gene level in response to oxLDL-IC, the fate of the protein could not be determined using immunoblot analysis (Fig. 1). The lack of HSP70B′ protein suggested aborted translation, improper folding, and/or complexing to an undetermined molecule(s) concealing the antibody binding sites. Due to cross-linking of the Fcγ receptors with immune complexes (oxLDL-IC and KLH-IC), we believe that the HSP70B′ protein and possibly other stimulated proteins, such as SK1 (18, 37), become part of lipid rafts and/or the detergent-resistant membrane fraction. To extract the cell membrane-associated HSP70B′, which is demonstrated clearly in the confocal microscopy images, we used several different detergents and extraction buffers (38) (data not shown). These did not improve the extractability of HSP70B′, from cells induced by immune complexes beyond what is shown in Fig. 1D. Interestingly, we were able to detect more HSP70B′ protein in response to KLH-IC than oxLDL-IC (Fig. 1D), which reflects appropriately the higher gene expression of HSP70B′ in response to KLH-IC compared with oxLDL-IC (25). Apparently, the protein expression of HSP70B′ in response to oxLDL alone is mainly post-translational because it reached a plateau at 6 h post-treatment.
HSP70 has been shown to form complexes with a number of intracellular proteins (39), and more recently HSPs have been shown to bind to intracellular lipids (29, 40, 41). Our data suggest that oxLDL-IC on the surface of the cells become associated with secreted HSP70B′ (Figs. 2 and 3). This association may inhibit the presumed autocrine effect of HSP70B′ on cell signaling.
The differential trafficking of oxLDL and oxLDL-IC probably plays a role in the action of HSP70B′. Differences in trafficking could be due to differences in receptor binding, uptake, and delivery to lysosomes and/or to lysosomal and post-lysosomal processing. Several studies have previously shown that the lipid and protein moieties are metabolized in lysosomes within hours after internalization of oxLDL (42–45). Live cell imaging shown in Fig. 3A demonstrated internalization of oxLDL-IC and co-localization with HSP70B′-GFP at 4 h post-treatment. This novel finding suggests that induced HSP70B′ could bind to cytoplasmic oxLDL-IC but not to oxLDL in the lysosomal compartment.
There have been several reports implicating HSPs in survival of immune cells (46–48), mostly suggesting a protective function against inflammation and other types of stressors. Our data suggest that inducible HSP70B′ may have an inhibitory effect on macrophage cell growth and survival. For example, cells overexpressing HSP70B′ showed significantly decreased cell proliferation and knockdown of HSP70B′-attenuated oxLDL-induced cell death compared with controls.
A mechanism regularly implicated in mediating prosurvival and inflammatory responses to macrophage cell survival is regulation of SK1, an enzyme that is responsible for the generation of the signaling molecule sphingosine 1-phosphate (49). We have recently shown that SK1 mRNA levels increased in response to oxLDL-IC in U937 cells (25), and we also showed that oxLDL-IC prompt the release of SK1 into the medium, suggesting the generation of sphingosine 1-phosphate extracellularly (18). It has recently been reported that overexpression of HSP70 in RAW 264.7 macrophages resulted in both increased SK1 protein and mRNA levels, contributing to the partial reversal of cell death caused by the combination of LPS and SK1 inhibitor (50). In this study, we show that knockdown of HSP70B′ reduced the expression of SK1 by half in response to oxLDL-IC, suggesting that HSP70B′ is an upstream mediator of SK1. Furthermore, phosphorylated SK1 is significantly reduced in U937 cells transfected with HSP70B′ siRNA compared with control cells treated with oxLDL. These data are the first to suggest an interaction between HSP70B′ and SK1 and could significantly influence further research to define SK1 effects mediating macrophage activation and survival.
The anti-inflammatory cytokine, IL-10, has been shown to be cytoprotective in macrophages (51) and induced by the addition of exogenous HSP70 in synovial cells (52, 53), suggesting that HSPs play an active role in suppressing inflammation by the regulation of IL-10. Here we provide evidence that secretion of IL-10 may be regulated by HSP70B′.
Although HSPs are classically viewed as intracellular proteins, we provided evidence that HSP70B′ is induced and secreted in response to cell activation by oxLDL-IC. This is in agreement with previous reports demonstrating that HSPs may be released by viable cells under inflammatory stress (3, 22). Recently, it has been shown that HSP70 can be secreted from cells via exosomes (54, 55). Moreover, it has been shown that in stressed cells, HSPs may be inserted into the plasma membrane before release into the extracellular environment in membrane-associated structures (56). The molecular mechanisms that regulate the mobilization of secretory vesicles and secretion of mediators from inflammatory cells, including macrophages, are still obscure (57, 58).
In conclusion, our current data suggest that HSP70B′ is induced and released by macrophages stimulated by oxLDL-IC and that once released into the extracellular space, HSP70B′ immediately associates with oxLDL-IC. We also provided evidence that a variety of cellular responses, including regulation of SK1 and release of the anti-inflammatory prosurvival cytokine IL-10, could be mediated by HSP70B′. Our findings contribute to the understanding of foam cell survival and activation and may advance efforts to reveal additional therapeutic targets that could mediate the stabilization of vulnerable atherosclerotic plaques.
Supplementary Material
Acknowledgments
We thank Dr. Stuart Pitson for anti-phosphorylated SK1 antibody. We also thank Charlyne Chassereau for assistance with lipoprotein isolation and Dr. Mohammed Al-Gadban and Dr. Mi-Hye Lee for helpful suggestions.
This work was supported, in whole or in part, by National Institutes of Health (NIH) Grant HL079274 and NIH (American Recovery and Investment Act (ARRA)) Grant R01 HL079274–04S1 (to S. M. H.); NIH (National Center for Research Resources) Grant P20 RR17677 (South Carolina Center of Biomedical Research Excellence in Lipidomics and Pathobiology) (to S. M. H.); and NIH Grants P01 HL55782, R01 DK081352, and R01 DK081352–02S1 (ARRA) (to M. F. L.-V.). This work was also supported by funding from the Department of Veterans Affairs (to M. F. L.-V.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Video 1.
- HSP
- heat shock protein
- LDL
- low density lipoprotein(s)
- oxLDL
- oxidized low density lipoprotein(s)
- oxLDL-IC
- oxidized low density lipoprotein immune complexes
- SK1
- sphingosine kinase 1
- IL
- interleukin
- TNF-α
- tumor necrosis factor α
- IFN-γ
- interferon-γ
- KLH-IC
- keyhole limpet hemocyanin immune complexes
- DiI
- dioctadecyl-3,3,3′,3-tetramethylindocarbocyanine perchlorate
- PBS
- phosphate-buffered saline
- Q-PCR
- quantitative PCR
- GFP
- green fluorescent protein
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- siRNA
- small interfering RNA.
REFERENCES
- 1.Morimoto R. I. (1993) Science 259, 1409–1410 [DOI] [PubMed] [Google Scholar]
- 2.Xu Q., Wick G. (1996) Mol. Med. Today 2, 372–379 [DOI] [PubMed] [Google Scholar]
- 3.Pockley A. G. (2002) Circulation 105, 1012–1017 [DOI] [PubMed] [Google Scholar]
- 4.Roma P., Catapano A. L. (1996) Atherosclerosis 127, 147–154 [DOI] [PubMed] [Google Scholar]
- 5.Johnson A. D., Berberian P. A., Tytell M., Bond M. G. (1995) Arterioscler. Thromb. Vasc. Biol. 15, 27–36 [DOI] [PubMed] [Google Scholar]
- 6.Berberian P. A., Myers W., Tytell M., Challa V., Bond M. G. (1990) Am. J. Pathol. 136, 71–80 [PMC free article] [PubMed] [Google Scholar]
- 7.Steinberg D. (1997) J. Biol. Chem. 272, 20963–20966 [DOI] [PubMed] [Google Scholar]
- 8.Virella G., Koskinen S., Krings G., Onorato J. M., Thorpe S. R., Lopes-Virella M. (2000) Clin. Immunol. 95, 135–144 [DOI] [PubMed] [Google Scholar]
- 9.Virella G., Lopes-Virella M. F. (2003) Clin. Diagn. Lab. Immunol. 10, 499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Virella G., Muñoz J. F., Galbraith G. M., Gissinger C., Chassereau C., Lopes-Virella M. F. (1995) Clin. Immunol. Immunopathol. 75, 179–189 [DOI] [PubMed] [Google Scholar]
- 11.Griffith R. L., Virella G. T., Stevenson H. C., Lopes-Virella M. F. (1988) J. Exp. Med. 168, 1041–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang Y., Jaffa A., Koskinen S., Takei A., Lopes-Virella M. F. (1999) Arterioscler. Thromb. Vasc. Biol. 19, 1600–1607 [DOI] [PubMed] [Google Scholar]
- 13.Saad A. F., Virella G., Chassereau C., Boackle R. J., Lopes-Virella M. F. (2006) J. Lipid Res. 47, 1975–1983 [DOI] [PubMed] [Google Scholar]
- 14.Asmis R., Begley J. G. (2003) Circ. Res. 92, e20–29 [DOI] [PubMed] [Google Scholar]
- 15.Martinet W., Kockx M. M. (2001) Curr. Opin. Lipidol. 12, 535–541 [DOI] [PubMed] [Google Scholar]
- 16.Vicca S., Massy Z. A., Hennequin C., Rihane D., Drüeke T. B., Lacour B. (2003) Free Radic. Biol. Med. 35, 603–615 [DOI] [PubMed] [Google Scholar]
- 17.Bianchi G., Montecucco F., Bertolotto M., Dallegri F., Ottonello L. (2007) Ann. N.Y. Acad. Sci. 1095, 209–219 [DOI] [PubMed] [Google Scholar]
- 18.Hammad S. M., Taha T. A., Nareika A., Johnson K. R., Lopes-Virella M. F., Obeid L. M. (2006) Prostaglandins Other Lipid Mediat. 79, 126–140 [DOI] [PubMed] [Google Scholar]
- 19.Oksjoki R., Kovanen P. T., Lindstedt K. A., Jansson B., Pentikäinen M. O. (2006) Arterioscler. Thromb. Vasc. Biol. 26, 576–583 [DOI] [PubMed] [Google Scholar]
- 20.Mehta T. A., Greenman J., Ettelaie C., Venkatasubramaniam A., Chetter I. C., McCollum P. T. (2005) Eur. J. Vasc. Endovasc. Surg. 29, 395–402 [DOI] [PubMed] [Google Scholar]
- 21.Asea A., Rehli M., Kabingu E., Boch J. A., Bare O., Auron P. E., Stevenson M. A., Calderwood S. K. (2002) J. Biol. Chem. 277, 15028–15034 [DOI] [PubMed] [Google Scholar]
- 22.Asea A., Kraeft S. K., Kurt-Jones E. A., Stevenson M. A., Chen L. B., Finberg R. W., Koo G. C., Calderwood S. K. (2000) Nat. Med. 6, 435–442 [DOI] [PubMed] [Google Scholar]
- 23.Multhoff G., Mizzen L., Winchester C. C., Milner C. M., Wenk S., Eissner G., Kampinga H. H., Laumbacher B., Johnson J. (1999) Exp. Hematol. 27, 1627–1636 [DOI] [PubMed] [Google Scholar]
- 24.Svensson P. A., Asea A., Englund M. C., Bausero M. A., Jernås M., Wiklund O., Ohlsson B. G., Carlsson L. M., Carlsson B. (2006) Atherosclerosis 185, 32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammad S. M., Twal W. O., Barth J. L., Smith K. J., Saad A. F., Virella G., Argraves W. S., Lopes-Virella M. F. (2009) Atherosclerosis 202, 394–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parsian A. J., Sheren J. E., Tao T. Y., Goswami P. C., Malyapa R., Van Rheeden R., Watson M. S., Hunt C. R. (2000) Biochim. Biophys. Acta 1494, 201–205 [DOI] [PubMed] [Google Scholar]
- 27.Noonan E. J., Place R. F., Giardina C., Hightower L. E. (2007) Cell Stress Chaperones 12, 393–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung T. K., Rajendran M. Y., Monfries C., Hall C., Lim L. (1990) Biochem. J. 267, 125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noonan E., Giardina C., Hightower L. (2008) Exp. Cell Res. 314, 2468–2476 [DOI] [PubMed] [Google Scholar]
- 30.Sundström C., Nilsson K. (1976) Int. J. Cancer 17, 565–577 [DOI] [PubMed] [Google Scholar]
- 31.Virella G., Thorpe S. R., Alderson N. L., Derrick M. B., Chassereau C., Rhett J. M., Lopes-Virella M. F. (2004) J. Lipid Res. 45, 1859–1867 [DOI] [PubMed] [Google Scholar]
- 32.Lopes-Virella M. F., Koskinen S., Mironova M., Horne D., Klein R., Chassereau C., Enockson C., Virella G. (2000) Atherosclerosis 152, 107–115 [DOI] [PubMed] [Google Scholar]
- 33.Atchley D. H., Lopes-Virella M. F., Zheng D., Kenny D., Virella G. (2002) Diabetologia 45, 1562–1571 [DOI] [PubMed] [Google Scholar]
- 34.Pitas R. E., Innerarity T. L., Weinstein J. N., Mahley R. W. (1981) Arteriosclerosis 1, 177–185 [DOI] [PubMed] [Google Scholar]
- 35.Kirkeby S., Thomsen C. E. (2005) J. Immunol. Methods 301, 102–113 [DOI] [PubMed] [Google Scholar]
- 36.Jones L. J., Gray M., Yue S. T., Haugland R. P., Singer V. L. (2001) J. Immunol. Methods 254, 85–98 [DOI] [PubMed] [Google Scholar]
- 37.Hengst J. A., Guilford J. M., Fox T. E., Wang X., Conroy E. J., Yun J. K. (2009) Arch. Biochem. Biophys. 492, 62–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shogomori H., Brown D. A. (2003) Biol. Chem. 384, 1259–1263 [DOI] [PubMed] [Google Scholar]
- 39.Davidoff A. M., Iglehart J. D., Marks J. R. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 3439–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osterloh A., Kalinke U., Weiss S., Fleischer B., Breloer M. (2007) J. Biol. Chem. 282, 4669–4680 [DOI] [PubMed] [Google Scholar]
- 41.Tsan M. F., Gao B. (2004) Cell. Mol. Immunol. 1, 274–279 [PubMed] [Google Scholar]
- 42.Li W., Yuan X. M., Brunk U. T. (1998) Free Radic. Res. 29, 389–398 [DOI] [PubMed] [Google Scholar]
- 43.Mander E. L., Dean R. T., Stanley K. K., Jessup W. (1994) Biochim. Biophys. Acta 1212, 80–92 [DOI] [PubMed] [Google Scholar]
- 44.Yancey P. G., Jerome W. G. (1998) J. Lipid Res. 39, 1349–1361 [PubMed] [Google Scholar]
- 45.Brown A. J., Mander E. L., Gelissen I. C., Kritharides L., Dean R. T., Jessup W. (2000) J. Lipid Res. 41, 226–237 [PubMed] [Google Scholar]
- 46.Kusher D. I., Ware C. F., Gooding L. R. (1990) J. Immunol. 145, 2925–2931 [PubMed] [Google Scholar]
- 47.Williams R. S., Thomas J. A., Fina M., German Z., Benjamin I. J. (1993) J. Clin. Investig. 92, 503–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirvonen M. R., Brüne B., Lapetina E. G. (1996) Biochem. J. 315, 845–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taha T. A., Hannun Y. A., Obeid L. M. (2006) J. Biochem. Mol. Biol. 39, 113–131 [DOI] [PubMed] [Google Scholar]
- 50.Ding X. Z., Feng X. R., Borschel R. H., Nikolich M. P., Feng J., Li Y. S., Hoover D. L. (2010) Prostaglandins Other Lipid Mediat, in press [DOI] [PubMed] [Google Scholar]
- 51.Arai T., Hiromatsu K., Nishimura H., Kimura Y., Kobayashi N., Ishida H., Nimura Y., Yoshikai Y. (1995) Biochem. Biophys. Res. Commun. 213, 600–607 [DOI] [PubMed] [Google Scholar]
- 52.Detanico T., Rodrigues L., Sabritto A. C., Keisermann M., Bauer M. E., Zwickey H., Bonorino C. (2004) Clin. Exp. Immunol. 135, 336–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo X., Zuo X., Zhang B., Song L., Wei X., Zhou Y., Xiao X. (2008) Cell Stress Chaperones 13, 365–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clayton A., Turkes A., Navabi H., Mason M. D., Tabi Z. (2005) J. Cell Sci. 118, 3631–3638 [DOI] [PubMed] [Google Scholar]
- 55.Lancaster G. I., Febbraio M. A. (2005) J. Biol. Chem. 280, 23349–23355 [DOI] [PubMed] [Google Scholar]
- 56.Vega V. L., Rodríguez-Silva M., Frey T., Gehrmann M., Diaz J. C., Steinem C., Multhoff G., Arispe N., De Maio A. (2008) J. Immunol. 180, 4299–4307 [DOI] [PubMed] [Google Scholar]
- 57.Medzhitov R. (2008) Nature 454, 428–435 [DOI] [PubMed] [Google Scholar]
- 58.Logan M. R., Odemuyiwa S. O., Moqbel R. (2003) J. Allergy Clin. Immunol. 111, 923–932 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.