Abstract
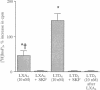
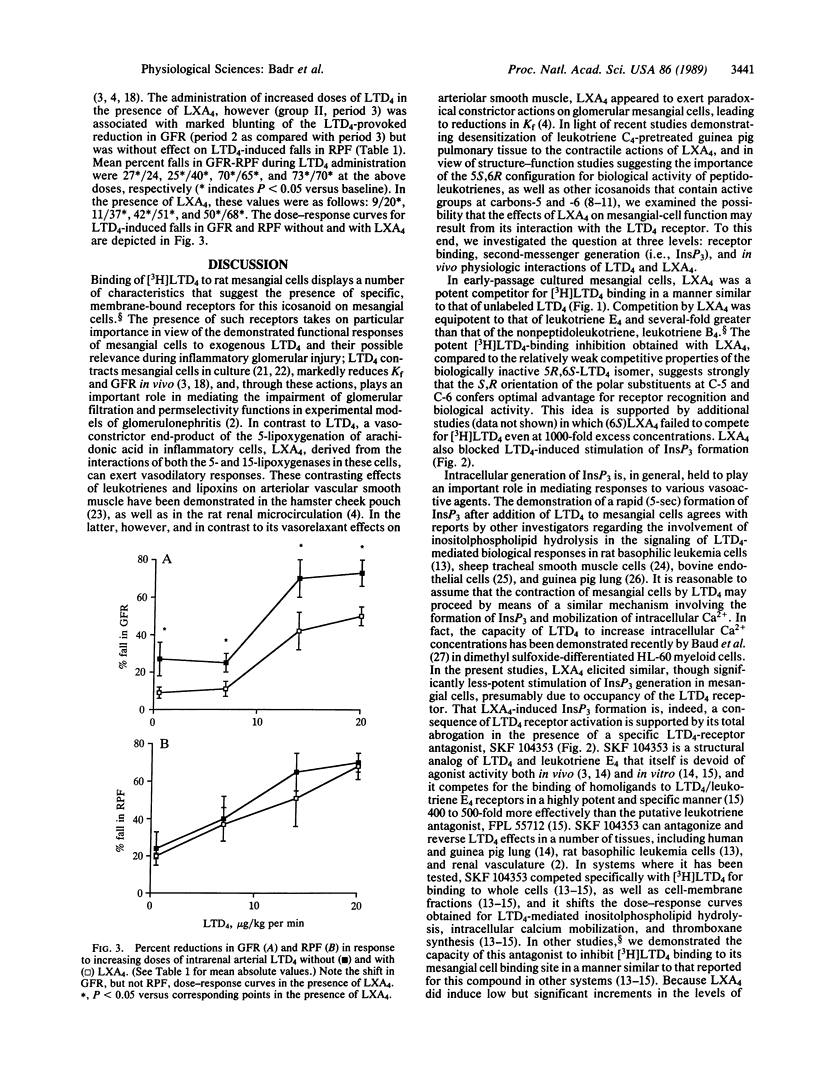
Lipoxin A4 (LXA4) was competitive with [3H]leukotriene D4 (LTD4) for specific binding to cultured rat glomerular mesangial cells. Half-maximal inhibition was obtained with 100 nM LXA4, compared with 10 nM for unlabeled LTD4. At 10 and 50 nM LXA4 induced low, but significant, increases in mesangial-cell inositol trisphosphate generation: 48% and 44% increases as compared to vehicle controls, respectively (compared with 146% and 106% increments obtained for equimolar LTD4), which were abolished in the presence of 100-fold concentrations of the LTD4 receptor antagonist, SKF 104353. In addition, exposure to 100 nM LXA4 prevented mesangial cell inositol trisphosphate generation induced by 10 nM LTD4. To test the in vivo relevance of these results, we established a dose-response curve for the reducing effects of intrarenal arterial LTD4 on glomerular filtration rate and renal plasma flow in anesthetized rats (LTD4 doses were 0.5, 7.0, 14.0, and 20.0 micrograms/kg per min) without or with LXA4 at 1 microgram/kg per min. Mean percent decreases in glomerular filtration rate/renal plasma flow during LTD4 administration were 27*/24, 25*/40*, 70*/65*, and 73*/70* at the above doses, respectively (*P less than 0.05 versus baseline). With LXA4, these values were as follows: 9/20*, 11/37*, 42*/51*, and 50*/68*, the latter value representing a shift in the LTD4/glomerular filtration rate dose-response curve. Thus, LXA4 competes for [3H]LTD4 binding to mesangial cells, its presence prevents LTD4-induced inositol trisphosphate generation, and its own stimulation of mesangial-cell inositol trisphosphate is blocked by an LTD4 receptor antagonist. In vivo, LXA4 antagonizes LTD4-induced falls in glomerular filtration rate but not renal plasma flow, implying prevention of LTD4-mediated reductions in the glomerular ultrafiltration coefficient, a consequence of mesangial-cell contraction. These results suggest that LTD4 and LXA4 interact at a common site on rat mesangial cells at which LXA4 provokes partial agonist responses and competitively antagonizes both the cellular and physiological actions of LTD4. Moreover, these results provide evidence for a potential counterregulatory interaction between leukotrienes and lipoxins that may be relevant during glomerular inflammation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badr K. F., Baylis C., Pfeffer J. M., Pfeffer M. A., Soberman R. J., Lewis R. A., Austen K. F., Corey E. J., Brenner B. M. Renal and systemic hemodynamic responses to intravenous infusion of leukotriene C4 in the rat. Circ Res. 1984 May;54(5):492–499. doi: 10.1161/01.res.54.5.492. [DOI] [PubMed] [Google Scholar]
- Badr K. F., Brenner B. M., Ichikawa I. Effects of leukotriene D4 on glomerular dynamics in the rat. Am J Physiol. 1987 Aug;253(2 Pt 2):F239–F243. doi: 10.1152/ajprenal.1987.253.2.F239. [DOI] [PubMed] [Google Scholar]
- Badr K. F., Schreiner G. F., Wasserman M., Ichikawa I. Preservation of the glomerular capillary ultrafiltration coefficient during rat nephrotoxic serum nephritis by a specific leukotriene D4 receptor antagonist. J Clin Invest. 1988 Jun;81(6):1702–1709. doi: 10.1172/JCI113509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr K. F., Serhan C. N., Nicolaou K. C., Samuelsson B. The action of lipoxin-A on glomerular microcirculatory dynamics in the rat. Biochem Biophys Res Commun. 1987 May 29;145(1):408–414. doi: 10.1016/0006-291x(87)91337-4. [DOI] [PubMed] [Google Scholar]
- Barnett R., Goldwasser P., Scharschmidt L. A., Schlondorff D. Effects of leukotrienes on isolated rat glomeruli and cultured mesangial cells. Am J Physiol. 1986 May;250(5 Pt 2):F838–F844. doi: 10.1152/ajprenal.1986.250.5.F838. [DOI] [PubMed] [Google Scholar]
- Baud L., Goetzl E. J., Koo C. H. Stimulation by leukotriene D4 of increases in the cytosolic concentration of calcium in dimethylsulfoxide-differentiated HL-60 cells. J Clin Invest. 1987 Oct;80(4):983–991. doi: 10.1172/JCI113192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem J. 1984 Jun 1;220(2):345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. A., Conway T. M., Bennett C. F., Crooke S. T., Stadel J. M. Islet-activating protein inhibits leukotriene D4- and leukotriene C4- but not bradykinin- or calcium ionophore-induced prostacyclin synthesis in bovine endothelial cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7320–7324. doi: 10.1073/pnas.83.19.7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha-Melo J. R., Dean N. M., Moyer J. D., Maeyama K., Beaven M. A. The kinetics of phosphoinositide hydrolysis in rat basophilic leukemia (RBL-2H3) cells varies with the type of IgE receptor cross-linking agent used. J Biol Chem. 1987 Aug 25;262(24):11455–11463. [PubMed] [Google Scholar]
- Dahlén S. E., Raud J., Serhan C. N., Björk J., Samuelsson B. Biological activities of lipoxin A include lung strip contraction and dilation of arterioles in vivo. Acta Physiol Scand. 1987 Aug;130(4):643–647. doi: 10.1111/j.1748-1716.1987.tb08187.x. [DOI] [PubMed] [Google Scholar]
- FUHR J., KACZMARCZYK J., KRUTTGEN C. D. Eine einfache colorimetrische Methode zur Inulinbestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr. 1955 Aug 1;33(29-30):729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- Gleason J. G., Hall R. F., Perchonock C. D., Erhard K. F., Frazee J. S., Ku T. W., Kondrad K., McCarthy M. E., Mong S., Crooke S. T. High-affinity leukotriene receptor antagonists. Synthesis and pharmacological characterization of 2-hydroxy-3-[(2-carboxyethyl)thio]-3-[2-(8-phenyloctyl)phenyl] propanoic acid. J Med Chem. 1987 Jun;30(6):959–961. doi: 10.1021/jm00389a001. [DOI] [PubMed] [Google Scholar]
- Hansson A., Serhan C. N., Haeggström J., Ingelman-Sundberg M., Samuelsson B. Activation of protein kinase C by lipoxin A and other eicosanoids. Intracellular action of oxygenation products of arachidonic acid. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1215–1222. doi: 10.1016/0006-291x(86)90380-3. [DOI] [PubMed] [Google Scholar]
- Harris R. C., Hoover R. L., Jacobson H. R., Badr K. F. Evidence for glomerular actions of epidermal growth factor in the rat. J Clin Invest. 1988 Sep;82(3):1028–1039. doi: 10.1172/JCI113659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M. A., Mong S., Vessella R. L., Crooke S. T. Identification and characterization of leukotriene D4 receptors in adult and fetal human lung. Biochem Pharmacol. 1985 Dec 15;34(24):4311–4317. doi: 10.1016/0006-2952(85)90290-4. [DOI] [PubMed] [Google Scholar]
- Mong S., Hoffman K., Wu H. L., Crooke S. T. Leukotriene-induced hydrolysis of inositol lipids in guinea pig lung: mechanism of signal transduction for leukotriene-D4 receptors. Mol Pharmacol. 1987 Jan;31(1):35–41. [PubMed] [Google Scholar]
- Mong S., Miller J., Wu H. L., Crooke S. T. Leukotriene D4 receptor-mediated hydrolysis of phosphoinositide and mobilization of calcium in sheep tracheal smooth muscle cells. J Pharmacol Exp Ther. 1988 Feb;244(2):508–515. [PubMed] [Google Scholar]
- Mong S., Wu H. L., Miller J., Hall R. F., Gleason J. G., Crooke S. T. SKF 104353, a high affinity antagonist for human and guinea pig lung leukotriene D4 receptor, blocked phosphatidylinositol metabolism and thromboxane synthesis induced by leukotriene D4. Mol Pharmacol. 1987 Aug;32(1):223–229. [PubMed] [Google Scholar]
- Samuelsson B., Dahlén S. E., Lindgren J. A., Rouzer C. A., Serhan C. N. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987 Sep 4;237(4819):1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Sarau H. M., Mong S., Foley J. J., Wu H. L., Crooke S. T. Identification and characterization of leukotriene D4 receptors and signal transduction processes in rat basophilic leukemia cells. J Biol Chem. 1987 Mar 25;262(9):4034–4041. [PubMed] [Google Scholar]
- Schlondorff D. The glomerular mesangial cell: an expanding role for a specialized pericyte. FASEB J. 1987 Oct;1(4):272–281. doi: 10.1096/fasebj.1.4.3308611. [DOI] [PubMed] [Google Scholar]
- Serhan C. N., Hamberg M., Samuelsson B. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5335–5339. doi: 10.1073/pnas.81.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C. N., Nicolaou K. C., Webber S. E., Veale C. A., Dahlén S. E., Puustinen T. J., Samuelsson B. Lipoxin A. Stereochemistry and biosynthesis. J Biol Chem. 1986 Dec 15;261(35):16340–16345. [PubMed] [Google Scholar]
- Simonson M. S., Dunn M. J. Leukotriene C4 and D4 contract rat glomerular mesangial cells. Kidney Int. 1986 Oct;30(4):524–531. doi: 10.1038/ki.1986.217. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Finkelstein N., Aliminosa L., Crawford B., Graber M. THE RENAL CLEARANCES OF SUBSTITUTED HIPPURIC ACID DERIVATIVES AND OTHER AROMATIC ACIDS IN DOG AND MAN. J Clin Invest. 1945 May;24(3):388–404. doi: 10.1172/JCI101618. [DOI] [PMC free article] [PubMed] [Google Scholar]