Abstract
Glutamate cysteine ligase (GCL) catalyzes the rate-limiting step in the formation of the cellular antioxidant glutathione (GSH). The GCL holoenzyme consists of two separately coded proteins, a catalytic subunit (GCLC) and a modifier subunit (GCLM). Both GCLC and GLCM are controlled transcriptionally by a variety of cellular stimuli, including oxidative stress. This study addresses post-translational control of GCL activity, which increased rapidly in human lymphocytes following oxidative stress. Activation of GCL occurred within minutes of treatment and without any change in GCL protein levels and coincided with an increase in the proportion of GCLC in the holoenzyme form. Likewise, GCLM shifted from the monomeric form to holoenzyme and higher molecular weight species. Normal rat tissues also showed a distribution of monomeric and higher molecular weight forms. Neither GCL activation, nor the formation of holoenzyme, required a covalent intermolecular disulfide bridge between GCLC and GCLM. However, in immunoprecipitation studies, a neutralizing epitope associated with enzymatic activity was protected following cellular oxidative stress. Thus, the N-terminal portion of GCLC may undergo a change that stabilizes the GCL holoenzyme. Our results suggest that a dynamic equilibrium exists between low and high activity forms of GCL and is altered by transient oxidative stress. This provides a mechanism for the rapid post-translational activation of GCL and maintenance of cellular GSH homeostasis.
Keywords: Antioxidant, Enzyme Kinetics, Glutathione, Oxidative Stress, Protein Assembly, Glutamate Cysteine Ligase
Introduction
The tripeptide glutathione (GSH) is the major low molecular weight cellular thiol. Reduced GSH is present in most cell types at millimolar concentrations, whereas the oxidized forms glutathione disulfide (GSSG)2 and mixed disulfides are ∼100-fold less abundant in the cytosol. GSH plays a role in myriad cellular functions, including maintenance of reduced protein thiols, detoxification of hydrogen peroxide (H2O2) and lipid peroxides, secondary metabolism, and non-enzymatic scavenging of free radicals (1). GSH also protects against apoptotic cell death following exposure to antineoplastic agents, radiation, and receptor-based death signals (2–6).
The initial step in GSH synthesis is catalyzed by glutamate cysteine ligase (GCL), a heterodimeric enzyme with a 73-kDa catalytic subunit (GCLC) and a 31-kDa modifier subunit (GCLM) (7, 8). GCL generates the dipeptide γ-glutamyl cysteine in a reaction requiring ATP; the second, non-rate-limiting step in GSH formation is the addition of glycine by GSH synthetase. GCLC contains the glutamate, cysteine, and ATP binding sites and has catalytic activity as a monomer. However, compared with the GCLC subunit alone, the GCL holoenzyme has enhanced Vmax, increased glutamate and ATP binding affinity, and reduced feedback inhibition by GSH, which competes at the Glu binding site (7, 9). Thus, the GCL holoenzyme has efficient activity over a larger range of cellular conditions, with kinetics that favor activity under physiological glutamate and ATP concentrations.
A model widely cited in the GCL literature holds that GCL holoenzyme exists predominantly as an inactive heterodimer that is oxidized to its activated state by formation of an intermolecular disulfide bond (10). This labile disulfide bridge between GCLC and GLCM could serve as a “redox switch” by sensing cellular oxidation status and altering the efficiency of the GCL holoenzyme to achieve redox homeostasis (11). In this study, we found that GCL activation was not associated with formation of an intermolecular disulfide bond between GCLC and GCLM. We present evidence for an alternative model, in which the activation status of the cellular GCL pool depends on a dynamic equilibrium between monomeric and holoenzyme forms of the enzyme. Increased formation of high activity heterodimeric complexes results in a shift to more efficient GSH production following intracellular oxidative stress.
EXPERIMENTAL PROCEDURES
Animal Use and Care
All animal procedures were approved by the University of Washington Institutional Animal Care and Use Committee. Mouse liver, kidney, and brain and rat kidney and liver samples (rat tissues gift of Dr. James Woods, University of Washington) were obtained immediately after sacrifice.
Chemicals and Reagents
Common laboratory reagents were purchased from Sigma. l-glutamate and l-serine were from ICN Biomedicals (Aurora, OH). Nonidet P-40 was from Calbiochem. Phorone was from Aldrich (Milwaukee, WI). Protein gel mix and ammonium persulfate were from Bio-Rad. Protease inhibitor mix containing 100 mg/ml Pefabloc, 10 mg/ml Nα-tosyl-l-lysine chloromethyl ketone, 1 mg/ml pepstatin A, 1 mg/ml aprotinin, and 1 mg/ml leupeptin (all from Boehringer-Mannheim, Indianapolis, IN) in distilled H2O was used in all cell lysis and tissue homogenization buffers at a 1:1000 dilution.
Cell Culture and Treatments
Jurkat cells (clone E6.1) were cultured in RPMI 1640 medium supplemented with l-glutamine, sodium pyruvate, penicillin/streptomycin (Invitrogen), and 10% fetal bovine serum (HyClone, Logan, UT), at an initial density of 0.5 × 106 cells/ml. Phorone was used as a model GSH-depleting agent, which depletes cellular GSH stores by glutathione S-transferase-mediated conjugation reactions. For phorone treatment, cells were pulse-treated with 500 μm phorone for 90 min in non-permeable flasks, followed by a 60-min recovery phase in fresh medium except where indicated. H2O2 was used as a model oxidant, because it converts GSH to its oxidized disulfide GSSG by GSH peroxidases. H2O2 can also oxidize cysteine groups on proteins, which are normally reduced by thioredoxin. Regeneration of thioredoxin, by thioredoxin reductase, consumes NAD(P)H. Catalase and peroxiredoxins (which are reduced in turn by thioredoxin reductase or sulfiredoxin) can also reduce H2O2. For H2O2 treatment, cells were re-suspended in fresh medium, treated for 10 min with 10 mm H2O2, and harvested by centrifugation. Titration of H2O2 in phosphate-buffered saline or RPMI medium supplemented with 10% fetal bovine serum was performed to assess the impact of catalase and other potential antioxidants in the medium. Based on quantitative analysis of phosphotyrosine in cell extracts, we demonstrated that a 10 mm dose in medium was equivalent to a treatment of 1.1 mm H2O2 in phosphate-buffered saline without serum.
GCL Activity Assays
GCL activity was assayed by a modification of the high-performance liquid chromatography-based method of Hamel et al. (12, 13). Protein concentrations were assayed by the Bradford method (Bio-Rad), or for Nonidet P-40 extracts, by the bicinchoninic acid method (Pierce). Baseline GSH concentrations were determined in duplicate, and GCL activities were assayed in triplicate. GCL enzyme kinetics was analyzed using a modification of the NADH recycling assay of Seelig and Meister (14). Briefly, recombinant mouse GCLC or GCL holoenzyme was eluted from the His-bind resin (Novagen) with 400 mm imidazole buffer, and 0.5–1.0 μg of protein was added to 96-well plates containing varying concentrations of cysteine and glutamate, or GSH. After a 5-min preincubation at 37 °C, the reaction was started by addition of 4× assay buffer (400 mm Tris, pH 8.0, 600 mm KCl, 20 mm ATP, 8 mm phosphoenolpyruvate, 8 mm EDTA, 80 mm MgCl2). Absorbance at 340 nm was monitored for 5 min, and initial rates of reaction were calculated from linear portions of the curves.
GSH Assays
GSH was assayed by a modification of the high-performance liquid chromatography-based method of Hamel et al. (12, 13), or in some cases by using the Tietze method adapted for use in 96-well microtiter plates (15).
In-gel Activity Assays
Immobilized His-tagged recombinant mouse GCLC protein (16, 17) was incubated with bacterial extract containing untagged GCLM for 0–60 min at 4 °C. The complexes were washed repeatedly with low imidazole buffer (20 mm Tris, 500 mm NaCl, 40 mm imidazole, pH 7.9) and eluted from the His-bind resin as described above. Western blot analysis was performed to assess the degree of association of GCLM with His-GCLC. Eluted GCL complexes were resolved by native PAGE on 4–15% gradient gels. The gels were equilibrated for 5 min at 37 °C in GCL assay buffer (100 mm Tris, pH 7.4, 10 mm MgCl2, 10 mm ATP, 0.5 mm EDTA, 15 mm glutamate). Reactions were initiated with fresh assay buffer containing 15 mm glutamate, 5 mm cysteine, and 3 mm cerium chloride (CeCl3), and the gels were incubated at 37 °C for 30–60 min with shaking. The in-gel activity assay exploits the deposition of CePO4 crystals following ATP hydrolysis (18, 19). CePO4 crystals were visualized on a Gel-doc video capture system (Bio-Rad) using an MVI Darklite trans-illuminating light source (Meridian, Kent, WA) adapted to orthogonally illuminate the gel.
PAGE and Western Blotting
Jurkat cells were lysed in Nonidet P-40 lysis buffer (50 mm Tris, pH 8.0, 1% Nonidet P-40, 150 mm NaCl) containing protease inhibitors, and the supernatants were mixed with an equal volume of 2× native gel sample buffer (120 mm Tris, pH 6.8, 20% glycerol, 0.001% bromphenol blue), and resolved on 4–15% pre-cast gradient gels (Bio-Rad) for native PAGE, or boiled for 3 min in 2× Laemmli buffer containing 10% β-mercaptoethanol and resolved on 12.5% SDS-polyacrylamide gels for denaturing PAGE. All gels were run with Tris/Gly buffer on ice for 1 h at 150 V, and proteins were transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA), blocked with phosphate-buffered saline/0.5% Tween 20 containing 5% milk, and probed with polyclonal rabbit antisera (1:20,000) reactive against murine GCLC or GCLM (20) followed by a goat-anti-rabbit-horseradish peroxidase secondary antibody (1:5,000, Boehringer Manheim, Indianapolis, IN). Western blots were developed (ECL, Amersham Biosciences), and protein levels were visualized and quantified using the Gel-Doc system (Bio-Rad).
Size-exclusion Chromatography
Rat kidney and liver cytosol were prepared by homogenization in TES/SB buffer (20 mm Tris, pH 7.4, 1 mm EDTA, 250 mm sucrose, 20 mm serine, and 1 mm boric acid) in the absence or presence of 1 mm DTT. Clarified extracts (2 mg per run) were separated on a Waters high-performance liquid chromatograph equipped with a 300W size-exclusion chromatography column (Waters, Milford, MA), and fractions were collected for immunoblot analysis. For Jurkat cell experiments, a 250-mm inner diameter × 45-cm column was prepared with Sepharose S-200 HR size-exclusion chromatography medium (Amersham Biosciences) and characterized using Bio-Rad gel filtration chromatography standards under established flow conditions. Clarified extracts from control and treated cells (total protein between 0.5 and 1.5 mg) were injected (t = 0), and fractions were collected at 1.5- or 2.0-min intervals starting at t = 60 min and ending at t = 96 min or, in one experiment, t = 120 min, on a Frac-100 automated fraction collector (Amersham Biosciences). Fractions were analyzed for GCLC and GCLM expression by immunoblotting, and densitometry was performed as described above.
Immunoprecipitation of GCLC and GCLM
Polyclonal antibodies were generated in rabbits and have been described elsewhere (20). The peptide antigens were selected on the basis of homology between human, rat, and mouse amino acid sequences, presence of hydrophilic residues, and expert judgment of the peptide synthesis team. Rabbits responding with high titers were repeatedly immunized with the peptide antigen to promote affinity maturation of the polyclonal antisera; individual bleeds were characterized by Western blot of serial dilutions. For native immunoprecipitation, Nonidet P-40 lysates of Jurkat cells were incubated on ice with a 1:200 dilution of anti-GCLC or anti-GCLM serum for the indicated times. Immune complexes were recovered with Protein-A-Sepharose beads (Repligen, Cambridge, MA), washed extensively, boiled in 2× Laemmli loading buffer containing 10% β-mercaptoethanol, and analyzed by immunoblotting and densitometry as described above. For immunoprecipitation from denatured cell extracts, Jurkat cell Nonidet P-40 lysates (50 μl) were denatured by addition of 10 μl of 10% SDS and incubation at 100 °C for 3 min. Samples were diluted with 1.2 ml of modified radioimmune precipitation assay buffer (150 mm NaCl, 50 mm Tris, 1% Nonidet P-40, 0.5% deoxycholate, pH 8.0) and incubated on ice for 15 min prior to addition of antisera and immunoprecipitation as described above.
Statistical Analysis
Data management and descriptive statistics, and Student's t test were performed using MS Excel (Microsoft Corp., Redmond, WA). A Wilcoxon signed rank test was also used (Systat, Inc., Evanston, IL). Statistical analysis was performed on raw data in all cases, whereas data were sometimes presented as percentage of control in the figures shown.
RESULTS
Enzyme Kinetics of Murine Monomeric GCLC and GCL Holoenzyme
The kinetics of recombinant mouse GCLC and GCL holoenzyme were determined using a modification of the NADH recycling assay of Seelig and Meister. Heterodimeric GCL holoenzyme was prepared by mixing His-tagged GCLC with an excess of GCLM to obtain quantitative GCL complex formation and optimal GCL activity. Similar to rat and human GCL (10, 11, 21), mouse GCL holoenzyme exhibited a higher Vmax, lower Km for Glu, and higher Ki for GSH than monomeric GCLC (Table 1). These results are consistent with previously reported values for murine GCLC and GCL holoenzyme (9, 22, 23).
TABLE 1.
Kinetic constants for recombinant mouse GCLC and GCL holoenzyme
Activity assays were performed on eluted recombinant proteins, using a modification of the NADH recycling assay (14). Kinetic parameters were estimated using Lineweaver-Burk fits for the initial rate of NADH consumption at varying concentrations of glutamate. The average (±S.D.) is reported for three to six experiments. Determinations of GSH inhibition represent the mean of two experiments.
| Construct | Vmaxa | Km Glu | Ki GSH |
|---|---|---|---|
| mm | mm | ||
| GCLC | 3.1 (0.38) | 2.2 (0.75) | 0.23 |
| Holoenzyme | 7.5 (1.1) | 0.86 (0.23) | 1.3 |
a Micromoles of γ-GC produced per milligram of GCLC per minute.
Formation of Heterodimeric GCL Complex Is Associated with Increased Activity
Compared with the GCLC subunit alone, GCL holoenzyme has increased specific activity and kinetics that favor increased activity at lower Glu and ATP concentrations, and decreased feedback inhibition by GSH (9, 10). An in-gel GCL activity assay was developed to allow comparison of GCLC monomer activity (lower band) with that of GCL holoenzyme (upper band) simultaneously under optimal substrate conditions (Fig. 1). This assay shows the contribution of the separate forms of GCLC to product formation, which is not possible in a “test tube” reaction. Briefly, recombinant mouse GCL complexes were resolved by either denaturing SDS-PAGE or native PAGE. Staining for GCLC (top panel) and GCLM (second panel) showed that equivalent amounts of GCLC were eluted in complex with varying amounts of GCLM. Heterodimerization of GCLM with GCLC was detected by a complete shift to a lower mobility form in native PAGE (third panel, Coomassie stain). For the in-gel activity assay, samples were separated by native PAGE, and the gel was incubated in the presence of GCL substrates at concentrations that support maximal activity of the GCLC monomer. Deposition of CePO4 was used as a semi-quantitative readout for GCL activity (bottom panel). This assay shows that the ability of GCLM to enhance GCLC specific activity requires the formation of a heterodimeric GCL complex and results in increased specific activity under conditions permissive of maximal activity in the GCLC monomer.
FIGURE 1.
Heterodimeric GCL holoenzyme has increased activity relative to monomeric GCLC. Immobilized recombinant mouse GCLC was incubated with excess GCLM for up to 60 min to form GCL holoenzyme. Proteins were eluted and resolved by SDS-PAGE or native PAGE. Top panels show GCLC and GCLM immunoblots. Bottom panels show native PAGE gels: Coomassie stain of GCLC and holoenzyme, and relative activity of GCLC and holoenzyme forms, measured by CePO4 deposition.
Rapid Activation of GCL following Oxidative Stress
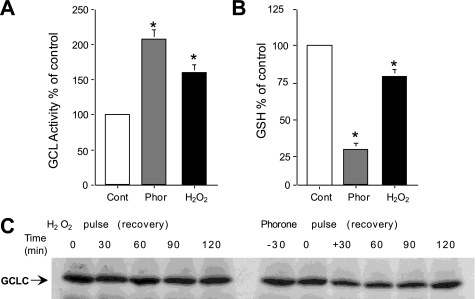
To examine the effects of oxidative stress and GSH depletion on cellular GCL activity, Jurkat cells were treated with phorone (a glutathione S-transferase substrate that depletes intracellular GSH), or H2O2. Both phorone and H2O2 increased GCL activity ∼2-fold (Fig. 2A). Cellular GSH concentrations were decreased following both phorone and H2O2 treatments (Fig. 2B). Activity of GCL was inversely proportional to the relative levels of GSH in these studies. The rapid kinetics of GCL activation in response to phorone (90 min) and H2O2 (10 min), suggest a post-translational modification; immunoblot analysis showed that neither treatment had affected GCLC protein levels over the time course examined (Fig. 2C and Fig. 3C). Treatment of Jurkat cells with other model oxidants, including diethyl maleate, diamide, ionizing radiation, and anti-Fas stimulation, also acutely activated GCL without affecting GCLC or GCLM protein levels (data not shown).
FIGURE 2.
Rapid activation of GCL following oxidative stress. Jurkat cells were treated with phorone for (90 min) or H2O2 (10 min) and GCL activity (A) and cellular GSH levels (B) were measured. (Mean percentage of control activity ± S.E. are shown; *, statistically different from control by Wilcoxon signed rank test (p < 0.05)). C, immunoblot analysis of GCLC protein in extracts of Jurkat cells treated with H2O2 (left lanes) or phorone (right lanes).
FIGURE 3.
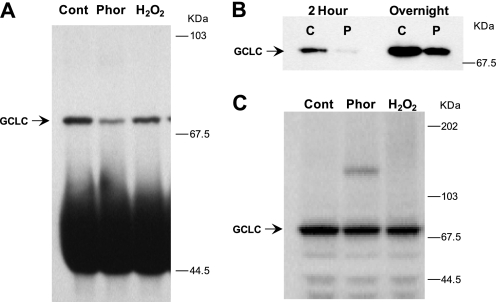
Oxidative stress increases protection of an N-terminal epitope in GCLC. A, Jurkat cells treated with phorone or H2O2 were subjected to immunoprecipitation (IP) and immunoblotting with an antisera directed against peptide Glu76–Thr91 of GCLC. B, time-dependent GCLC recovery from native IP of control and phorone-treated Jurkat cell extracts for 2 h or overnight. C, GCLC expression levels in control and treated Jurkat cells. Whole cell extracts were resolved by SDS-PAGE and immunoblotted for GCLC.
Epitope Protection of GCLC following Oxidative Stress
Post-translational regulation of GCL activity may involve direct modifications to the GCL subunits. Topological changes associated with holoenzyme formation may also occur during GCL activation. We developed a high affinity polyclonal antibody directed against the N-terminal peptide sequence Glu76–Thr91 in GCLC (20) for use in immunoprecipitation (IP) studies from human lymphocytes, which have relatively low levels of GCL. It was observed that, despite the increased GCL activity in the treated Jurkat cell extracts, recovery of GCLC by IP was reduced, whereas there was no difference in the protein amounts in immunoblots of whole cell extracts (Fig. 3). The same antibody was used for immunoprecipitation and detection of GCLC. In whole cell extracts, and in IP reactions of extracts that were denatured, and then partially re-natured to allow epitope recognition by the antisera, the same amount of GCLC was recovered from control and treated cells (Fig. 2C, Fig. 3C, and supplemental Fig. 1). By comparison, the non-denaturing IP recovered much less GCLC than the denaturing IP strategy did from the same cell extracts (supplemental Fig. 1). Increasing the length of the IP incubation from 2 h to overnight improved GCLC recovery from native samples, yet lower recovery was still observed in extracts from phorone-treated cells (Fig. 3B and supplemental Fig. 2). Thus, a small proportion of the GCLC pool in human lymphocytes exists in a state where the Glu76–Thr91 epitope is exposed, and oxidative treatments further decrease the epitope availability. These data also suggest that the change in epitope availability is not due to covalent modification to the epitope itself, since antibody binding was not blocked in the denatured protein.
The reduced recovery of GCLC from native extracts of cells exposed to oxidative stress was observed with polyclonal sera from three different rabbits immunized against the Glu76–Thr91 epitope (data not shown). Immune complex GCL activity assays were not possible, because GCL activity was not detectable in immunoprecipitates using the antibodies directed against the Glu76–Thr91 epitope (data not shown). The Glu76–Thr91 epitope is relatively close to Lys38, which has been reported to be important for glutamate binding and GCLC activity, and Glu103, mutation of which abrogates GCL activity without affecting heterodimerization (24, 39).
An increase in recovery of GCLC from longer IP reactions is consistent with a dynamic process by which the Glu76–Thr91 becomes available over time. Serial immunoprecipitations from the same cell extract demonstrated that the GCLC epitope becomes available at a fairly constant rate in reactions performed at 4 °C (supplemental Fig. 2). To demonstrate that the IP reaction was not limited by the concentration or affinity of the antibody, an immunoaffinity resin was used. Recovery of GCLC was saturated more quickly in extracts from phorone-treated than from control cells (supplemental Fig. 2). Thus, the IP experiments show evidence of a process by which a portion of the GCLC pool shifts between a lower activity state with a more accessible Glu76–Thr91 and an activated state in which the epitope is more protected.
Absence of an Intermolecular Disulfide Bond in Activated GCL
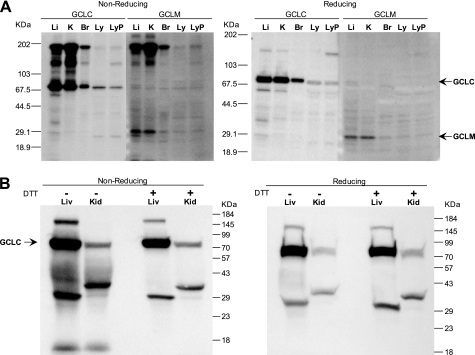
Thiol oxidation of the GCL holoenzyme can result in the formation of a disulfide bridge between the two subunits (10); indeed, rat GCLM was originally identified as a disulfide-linked component of GCL preparations (25). We have noted high molecular mass forms of GCL on non-reducing gels, including bands at 100, 160–170, and 200 kDa, which are mostly absent in gels run under standard reducing conditions (Fig. 4). In our experience, these high molecular mass species are detected in tissue or cell extracts after extended storage and are likely the result of nonspecific oxidation of protein sulfhydryl groups. The high molecular mass complexes contain GCLM, which is only visible at ∼30 kDa in the reducing gels (Fig. 4A, right panel). By contrast, in fresh extracts made from rat kidney or liver, high molecular weight complexes account for a very small fraction of the GCLC detected. Fig. 4B shows GCLC from liver and kidney extracts prepared with or without 5 mm DTT in the homogenization buffer. Both reducing and non-reducing gels show the main band staining for GCLC at 70 kDa. Minor bands between 30 and 40 kDa are present in both tissues. On the non-reducing gel (left panel), a high molecular mass band (∼150 kDa) in kidney is less prominent in the DTT extracts, suggesting that formation of this complex was limited by the presence of the thiol reductant. In reducing gels, a small amount of this high molecular mass band is still visible (right panel).
FIGURE 4.
Disulfide bond formation and high molecular weight forms of GCL. A, extensive formation of high molecular weight complexes in frozen extracts from mouse liver (Li), kidney (K), and brain (B), but not in control (Ly) and phorone-treated Jurkat cells (LyP). Samples were resolved by SDS-PAGE under non-reducing or reducing conditions and stained for GCLC and GCLM. B, in fresh rat kidney and liver, prepared with or without 5 mm DTT, high molecular weight form is a minor component of GCLC. Samples were resolved by SDS-PAGE under non-reducing or reducing conditions and stained for GCLC.
Treatment of Jurkat cells with phorone to activate GCL did not result in the formation of a disulfide bond in GCLC (Fig. 4A and supplemental Fig. 1). Notably, phorone treatment generates a high molecular mass band (∼160 kDa) that is immunoreactive for both GCLC and GCLM. This band is present in SDS gels run under reducing conditions and thus is unlikely to result from a simple disulfide bridge. Phorone is an α,β-unsaturated ketone, which can undergo Michael addition to nucleophiles. It may be that at the dose used (500 μm) some of the phorone was able to cross-link GCLC and GCLM protein, and perhaps some other protein(s) resulting in this higher molecular mass form (supplemental Fig. 1). Experiments using glutaraldehyde or ruthenium to cross-link cell extracts for identification of potential GCLC and GCLM binding partners were unsuccessful. However, we have noted cross-linking of the subunits when GCL holoenzyme is treated in vitro with α,β-unsaturated aldehydes, such as acrolein or 4-hydroxy-2-nonenol.3
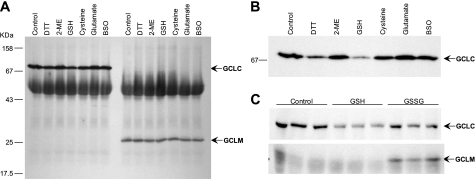
Another line of evidence that counters the hypothesis that GCL holoenzyme normally exists as a disulfide-linked heterodimer is the absence of co-precipitation of the complementary subunit by antibodies directed against GCLC or GCLM. In our experiments, co-precipitation of either subunit was not observed (Fig. 5A and data not shown). To consider alternative mechanisms by which changes in intracellular oxidation status might affect the heterodimeric state of GCL, we tried adding GSH, other thiol-reactive substances, and the GCL substrates glutamate, cysteine, and buthionine sulfoximine (a cysteine analog and GCL inhibitor) to the IP reaction. Although enzyme substrates, buthionine sulfoximine, and β-mercaptoethanol did not alter the recovery of GCLC by IP, addition of GSH or DTT (5 mm) resulted in decreased amounts of GCLC (Fig. 5B). There was no difference in recovery of GCLM with any of the additives. However, addition of 5 mm GSSG to the GCLC IP reaction resulted in co-precipitation of GCLM (Fig. 5C). This result suggests that formation of an intermolecular disulfide cross-link per se does not prevent access to the Glu76–Thr91 epitope of GCLC. Taken together, the co-precipitation studies and the lack of a 100-kDa form of GCL on non-reducing gels demonstrate that the GCL subunits are not linked by an intermolecular disulfide bond in normal or activated tissues.
FIGURE 5.
Effects of thiols and GCL substrates on immunoprecipitation. A, no co-precipitation of GCLC or GCLM was observed in IP reactions containing DTT, β-mercaptoethanol, GSH, cysteine, or glutamate at 5 mm, or buthionine sulfoximine at 1 mm. GCLC (left lanes) and GCLM (right lanes) were immunoprecipitated from Jurkat cell extracts, resolved by SDS-PAGE, and stained for both GCLC and GCLM. B, lower exposure image of GCLC, showing decreased recovery from IP reactions containing GSH and DTT. C, co-precipitation of GCLM with GCLC, in the presence of GSSG. Jurkat cell extracts were subjected to native IP reactions with 5 mm GSH or 5 mm GSSG; triplicate reactions were run. The top panel shows GCLC; the bottom panel shows co-precipitation of GCLM with GCLC in the same reactions.
High Molecular Weight Forms of GCL in Rat Kidney and Liver
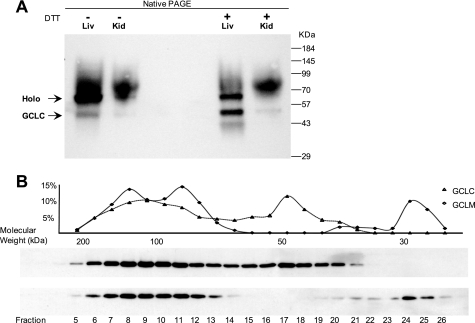
Our IP studies suggest that GCL may normally be present in multiple molecular weight forms within the cell. Fresh rat kidney and liver extracts resolved by native PAGE contained two major GCLC bands, which were only partially dissociated in extracts prepared with 5 mm DTT (Fig. 6A). Because separation of proteins by native PAGE does not result in good estimates of molecular weight, we used size-exclusion chromatography (SEC) to estimate the size of protein complexes containing GCLC and GCLM in freshly prepared rat kidney extracts. Molecular mass fractions containing GCLC ranged from 60 to 200 kDa, whereas GCLM was present in two separate fractions at ∼30 kDa and from 100 to 200 kDa (Fig. 6B). This analysis showed that ∼75% of the GCLM in the kidney was eluted with GCLC, whereas ∼40% of the GCLC was present in monomeric form (Fig. 6C). No difference in the size distribution of GCL subunits was observed between extracts prepared in the presence or absence of 5 mm DTT (data not shown). The results demonstrate that GCL subunits are present in both monomeric and heterodimeric forms in normal (untreated) tissue and that higher molecular mass forms (>100 kDa) represent a fraction of the GCL pool.
FIGURE 6.
Multiple high molecular weight forms of GCL in tissues. A, freshly prepared extracts from rat kidney or liver were resolved by native PAGE and stained for GCLC. Extracts were prepared with or without 5 mm DTT as indicated. B, rat kidney extracts were separated by SEC; the fractions were eluted, resolved by SDS-PAGE, and stained for GCLC and GCLM. The percentage of total recovered GCLC and GCLM in each fraction was determined by densitometry analysis of the immunoblots. Mobility of molecular size standards in the SEC column, shown under the graph, also corresponds to the size of elution fractions collected in this series of experiments.
Oxidative Stress Shifts GCLC and GCLM into Heterodimeric Complexes
Native analysis of GCLC and GCLM was used to determine the effects of oxidative stress on GCL composition in extracts of Jurkat cells treated with phorone or H2O2. Immunoblotting for GCLC revealed two distinct immunoreactive bands with differing electrophoretic mobilities in untreated Jurkat cells (Fig. 7A, left panel). The lower mobility band was also immunoreactive with GCLM antisera (Fig. 7A, right panel), indicating that this band is GCL holoenzyme, and the higher mobility bands are monomeric GCLC (left panel) and GCLM (right panel). Treatment of Jurkat cells with phorone or H2O2 quantitatively shifted GCLC from its monomeric form into the GCL holoenzyme complex (Fig. 7A). Other high molecular weight bands were also immunoreactive for GCLC and/or GCLM, suggesting higher order enzyme complexes are increased in the treated samples (Fig. 7A). Densitometry analysis revealed that, in both control and treated cells, less than half the total GCLC pool existed as GCLC monomer (Fig. 7B). Interestingly, phorone treatment caused a shift predominantly to the 100-kDa form, whereas H2O2 treatment also promoted formation of higher molecular mass forms immunoreactive with GCLC antisera. These results provide evidence that oxidative activation of GCL is mediated by the formation of highly active heterodimeric GCL holoenzyme from subunits that are available as monomers in the cell. Oxidizing conditions also promote the oligomerization of a portion of the GCL pool into higher order complexes.
FIGURE 7.
GCL high molecular weight forms increase following oxidative stress. A, extracts of control and phorone-treated Jurkat cells were resolved by native PAGE and stained for GCLC (left panel) and GCLM (right panel). Duplicate lanes were run with 100 μg and 50 μg of cytosolic protein. Markers indicate position of BSA run as a standard on the gel. B, densitometry analysis of the GCLC monomer, holoenzyme, and high molecular weight forms detected following native PAGE of Jurkat cells treated with phorone or H2O2 (n = 3–6 experiments). The percentages of total GCLC in each form, average ± S.D., are shown. *, statistically different from control by Wilcoxon rank sum test (p < 0.05). C, SEC analysis of control and phorone-treated Jurkat cells. SEC fractions were eluted, resolved by SDS-PAGE, and stained for GCLC. An elution profile of GCLC for a single experiment is shown. D, cumulative percentage of GCLC eluted from three SEC experiments; 2-ml elution fractions are presented as cumulative flow volume.
To further examine the effects of oxidative stress on GCL size, SEC was performed on extracts from control and phorone-treated Jurkat cells. The SEC fractions were collected and resolved by SDS-PAGE, and the presence of GCLC was assessed by immunoblotting. A higher percentage of GCLC eluted in early (higher molecular mass) fractions in phorone-treated cell extracts compared with controls (Fig. 7C). The percentage of total GCLC eluted was increased in higher molecular weight fractions and cumulative recovery of GCLC was shifted to earlier elutions in phorone-treated cells (Fig. 7D). There was overlap in the molecular weight ranges containing GCLC from control and treated cell extracts, consistent with results from the native PAGE and SEC analyses of rat kidney extracts. These results support a model of cellular GCL as a pool of both monomeric GCLC and heterodimeric forms, which upon intracellular oxidative stress converts to a higher activity form as the ∼100 kDa holoenzyme and higher molecular mass forms of the GCL heterodimer complex.
DISCUSSION
Although transcriptional up-regulation of GCLC and GCLM has been well documented, rapid oxidative activation of GCL has been reported to occur in the absence of de novo protein synthesis, suggesting the involvement of post-translational control mechanisms (26–28). Few studies have investigated post-translational regulation of GCL activity. Phosphorylation of GCLC was reported to suppress GCL activity (29, 30). In our hands, 32P labeling studies showed no phosphorylation of GCLC or GCLM in control Jurkat cells or cells treated with phorone or H2O2, although treatment with pharmacological activators of PKA and PKC rapidly increased GCL activity (data not shown). Although GCLC is subject to caspase-3-dependent cleavage during apoptosis, functional effects of GCLC cleavage are not known (31–33).
In this study we examined changes associated with increased activity of GCL following transient intracellular oxidative stress. Our data show that oxidative stress, GSH depletion or GSSG formation, rapidly activates the cellular GCLC pool and that high enzyme activity is attributable to the GCL heterodimer (Figs. 1 and 2). Immunoaffinity studies suggest a redox-sensitive dynamic equilibrium exists between monomeric GCLC and GCLM subunits and the higher activity GCL holoenzyme. The shift of GCLC to the high activity pool involved a change in GCLC, such that an N-terminal GCLC epitope associated with enzyme activity was protected in extracts with high GCL activity (Fig. 3 and supplemental Fig. 1). This topological change in GCLC was also associated with the formation of high molecular weight GCL complexes, observed as altered electrophoretic mobility of GCLC and GCLM on native PAGE gels and earlier elution of the GCLC subunit in SEC studies. The detection of high molecular mass GCL complexes (160–200 kDa, Figs. 6 and 7) by native immunoblot analysis in human lymphocytes, rat kidney, and rat liver, opens the possibility that other binding partners may contribute to the activation of GCL in high molecular mass GCL enzyme complexes.
As expected, GCL holoenzyme had an increased Vmax and decreased apparent Km for Glu and Cys, compared with monomeric GCLC (Table 1). Using recombinant mouse GCL, we demonstrated that GCL holoenzyme has higher specific activity under substrate conditions permissive for optimal GCLC activity (Fig. 1). This is similar to reported effects of GCLM on GCLC activity in the mouse and other species (22, 23). The enhancement of GCLC enzymatic activity by GCLM suggests a mechanism for the dynamic regulation of GSH biosynthesis within the cell, based on rapid shift in the balance between low activity (monomeric) and high activity forms of GCL in response to intracellular oxidation status. This may provide a highly efficient response mechanism for maintaining intracellular GSH homeostasis.
A model widely cited in the GCL literature states that GCL exists predominantly as an inactive heterodimer that is oxidized to an activated state by formation of an intermolecular disulfide bond (10). Our evidence does not support this model (Fig. 4 and supplemental Fig. 1). The present results suggest that the reversible disulfide linkage between GCLC and GCLM, observed in the isolated rat enzyme, was a result of protein oxidation during the lengthy purification process. In addition, we show that part of the GCLC and GCLM subunits exist as monomers in untreated cells (Figs. 4, 6, and 7) and shift to higher molecular weight forms following treatment (Fig. 7). Altered redox balance appears to favor formation of heterodimeric complexes and/or to decrease the dissociation rate of the binding partners in the GCL holoenzyme.
Our immunoaffinity results show that concurrently with the activation of GCL, the Glu76–Thr91 epitope of GCLC was protected. Tu et al. reported that an N terminus His tag on GCLC, decreased activity by ∼40%, and deletion of residues 1–25 abrogated activity (21). Deletion of N-terminal residues also prevented interaction with GLCM (9). Critical active site residues have been identified at Lys38 and Glu103, relatively close to the GCLC epitope described here (24, 39). The absence of GCL activity in immune complex activity assays is likely due to the proximity of the Glu76–Thr91 epitope to the catalytic pocket of GCLC. Homology/interaction modeling suggests that changes in GCLC structure due to holoenzyme formation may alter access to the Glu76–Thr91 epitope, protecting the enzyme from immunoprecipitation while in the holoenzyme form.4
This study focused on the GCLC antisera, which has higher affinity and greater specificity than the GCLM-directed reagent. Antisera directed against GCLC did not co-precipitate GCLM, except in the presence of exogenous GSSG, and antisera directed against GCLM did not co-precipitate GCLC (Fig. 5). Our native PAGE and SEC results demonstrate the presence of holoenzyme in untreated extracts of normal tissues; thus the lack of co-precipitation also suggests that heterodimerization hinders access to epitopes recognized by these antibodies.
Several alternative mechanisms would also be consistent with our findings, because large and/or sequestered enzyme complexes are known to exist in a number of pathways. Nonetheless, equivalent recovery was observed from denatured extracts of control and treated cells (Fig. 3 and supplemental Fig. 1). Therefore, differential recovery by IP from native cell extracts appears to be due to a bona fide change in epitope protection, rather than a reduction in the extractable GCLC content due to association with insoluble complexes, or to epitope loss due to a covalent modification. Interestingly, GCL purified from Drosophila appears to form a trimeric enzyme complex containing one GCLC and two GCLM subunits (34). We found that a portion of GCLC was detected at a molecular mass exceeding the expected ∼100 kDa for the GCL heterodimer (Figs. 6 and 7). It is possible that similar trimeric GCL complexes exist in mammalian cells, although our data suggest that higher order complexes could also occur naturally.
The lack of evidence for an intermolecular disulfide bridge does not rule out the involvement of cysteine groups as redox sensors on GCL, via the formation of mixed disulfides with low molecular weight thiols, or even via intramolecular disulfide bonds. Kinetic studies comparing GSH and its non-thiol analog ophthalmic acid demonstrated feedback inhibition of GCL holoenzyme by GSH due to binding at the glutamate site and a thiol interaction at a non-specified site (35). Cys553 of GCLC is required for formation of fully functional GCL holoenzyme, because heterodimeric GCL made with mutant GCLC (C553G) exhibited a Vmax similar to that of wild-type monomeric GCLC. Interestingly, the inability of GCLM to enhance GCLC(C553G) activity was not due to an effect on GCL holoenzyme formation, but rather a defect inherent to the mutant GCLC(C553G) (35). The Glu76–Thr91 epitope does not contain a cysteine residue. However, because IP reactions with 5 mm DTT or GSH yielded less GCLC (Fig. 5), it appears that thiol agents can alter the topology of GCLC indirectly, protecting the Glu76–Thr91 epitope.
Although this study focused on GCL activation following treatment of Jurkat cells with H2O2 and phorone as model oxidative treatments, similar activation of GCL was observed after phorone treatment in other cell models, including mouse and monkey cell lines, primary human T cells and lymphoblasts, and human pulmonary fibroblasts. Rapid activation of GCL without increased subunit expression has also been observed in Jurkat cells following treatments with anti-Fas antibodies, ionizing radiation, diamide, diethyl maleate, dibutyryl cAMP, phorbol 12-myristate 13-acetate, and even buthionine sulfoximine at early time points.5 This suggests that rapid post-translational activation of GCL after perturbation of the intracellular redox state is a general phenomenon. As a mechanism to maintain intracellular GSH, the activation of GCL complements the well documented transcriptional up-regulation of GCLC and GCLM following oxidative stress.
Furthermore, differential control of GCLC and GCLM transcription may be understood in light of a dynamic equilibrium between monomeric and heterodimeric pools of GCL. In this model, increased GCLM levels would favor a larger fraction of enzyme in the high activity state, or facilitate a shift to the active state following oxidative stress in certain tissues. There are striking differences in GCL subunit expression in different tissues, in both the absolute quantities and the ratiometric expression of GCLC and GCLM (22, 36). If formation of GCL holoenzyme complexes governs a tissue's ability to activate GCL, the relative expression of the GCL subunits may affect the sensitivity of a tissue to oxidative injury. In vivo studies show that mice deficient in GCLM are significantly more susceptible to liver damage following administration of acetaminophen (paracetamol) than wild-type mice (37) but could be made resistant to acetaminophen by conditionally restoring GCLM expression (38). Compensatory GSH biosynthesis is likely to temper the activation of redox-sensitive transcription factors and may play an important role in modulating the transcriptional regulation of stress-responsive genes, including GCLC and GCLM. In summary, the rapid activation of GCL could represent a major homeostatic mechanism for intracellular redox balance.
Supplementary Material
Acknowledgments
We thank Dr. Jim Blake of Bristol-Myers Squibb for synthesis of the GCLC and GCLM peptides to develop the polyclonal antisera, and Lorelyn Mackie for competent veterinary care of the rabbits used for this purpose. Dr. Steve Nadler of Bristol-Myers Squibb and Dennis Sloane of University of Washington provided guidance for the SEC studies. We thank Dr. Ken Lewis of ZymoGenetics, Inc., for careful reading of the manuscript and valuable feedback and Dr. Henry Jay Forman for helpful discussion.
This work was supported, in whole or in part, by National Institutes of Health Grants R01ES10849 (to T. J. K.), P01AG01751 (to T. J. K.), P42ES04696 (to T. J. K.), P30ES07033 (to T. J. K.), T32ES07032 (to C. M. K.), and R01CA90473 (to C. C. F.). This work was also supported by the Bristol-Myers Squibb Pharmaceutical Research Institute.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
C. C. Franklin and D. S. Backos, manuscript in preparation.
D. S. Backos, K. S. Fritz, J. R. Roede, D. R. Petersen, and C. C. Franklin, manuscript in preparation.
C. Krejsa, unpublished observations.
- GSSG
- oxidized glutathione disulfide
- GCL
- glutamate cysteine ligase
- GCLC
- glutamate cysteine ligase catalytic subunit
- GCLM
- glutamate cysteine ligase modifier subunit
- IP
- immunoprecipitation
- DTT
- dithiothreitol
- SEC
- size-exclusion chromatography
- TES
- 2-{[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]amino}ethanesulfonic acid.
REFERENCES
- 1.Meister A., Anderson M. E. (1983) Annu. Rev. Biochem. 52, 711–760 [DOI] [PubMed] [Google Scholar]
- 2.Forman H. J., Liu R., Shi M. M. (1995) in Biothiols in Health and Disease (Packer L., Cadenas E. eds) 1st Ed., pp. 189–212, Marcel Dekker, New York [Google Scholar]
- 3.Hall A. G. (1999) Eur J. Clin. Invest. 29, 238–245 [DOI] [PubMed] [Google Scholar]
- 4.Meister A. (1991) Pharmacol. Ther. 51, 155–194 [DOI] [PubMed] [Google Scholar]
- 5.Manna S. K., Kuo M. T., Aggarwal B. B. (1999) Oncogene 18, 4371–4382 [DOI] [PubMed] [Google Scholar]
- 6.Botta D., Franklin C. C., White C. C., Krejsa C. M., Dabrowski M. J., Pierce R. H., Fausto N., Kavanagh T. J. (2004) Free Radic Biol. Med. 37, 632–642 [DOI] [PubMed] [Google Scholar]
- 7.Griffith O. W., Mulcahy R. T. (1999) Adv. Enzymol. Relat. Areas Mol. Biol. 73, 209–267, xii [DOI] [PubMed] [Google Scholar]
- 8.Seelig G. F., Meister A. (1984) J. Biol. Chem. 259, 3534–3538 [PubMed] [Google Scholar]
- 9.Yang Y., Chen Y., Johansson E., Schneider S. N., Shertzer H. G., Nebert D. W., Dalton T. P. (2007) Biochem. Pharmacol. 74, 372–381 [DOI] [PubMed] [Google Scholar]
- 10.Huang C. S., Chang L. S., Anderson M. E., Meister A. (1993) J. Biol. Chem. 268, 19675–19680 [PubMed] [Google Scholar]
- 11.Huang C. S., Anderson M. E., Meister A. (1993) J. Biol. Chem. 268, 20578–20583 [PubMed] [Google Scholar]
- 12.Hamel D. M., White C., Eaton D. L. (1991) Toxicol. Mech. Methods 1, 273–288 [Google Scholar]
- 13.White C. C., Krejsa C. M., Eaton D. L., Kavanagh T. J. (1999) in Current Protocols in Toxicology (Maines M. D., Costa L. G., Reed D. J., Sassa S., Sipes I. G. eds) pp. 6.5.1–6.5.14, John Wiley & Sons, Inc., New York [Google Scholar]
- 14.Seelig G. F., Meister A. (1985) Methods Enzymol. 113, 379–390 [DOI] [PubMed] [Google Scholar]
- 15.Baker M. A., Cerniglia G. J., Zaman A. (1990) Anal. Biochem. 190, 360–365 [DOI] [PubMed] [Google Scholar]
- 16.Franklin C. C., Backos D. S., Mohar I., White C. C., Forman H. J., Kavanagh T. J. (2009) Mol. Aspects Med. 30, 86–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krzywanski D. M., Dickinson D. A., Iles K. E., Wigley A. F., Franklin C. C., Liu R. M., Kavanagh T. J., Forman H. J. (2004) Arch. Biochem. Biophys. 423, 116–125 [DOI] [PubMed] [Google Scholar]
- 18.Seitz J., Keppler C., Fahimi H. D., Völkl A. (1991) Electrophoresis 12, 1051–1055 [DOI] [PubMed] [Google Scholar]
- 19.Song J., Bosch K. S., Tigchelaar W., Van Den Munckhof R. J., Schellens J. P., Van Noorden C. J., Frederiks W. M. (1995) Histochem. J. 27, 914–922 [PubMed] [Google Scholar]
- 20.Thompson S. A., White C. C., Krejsa C. M., Diaz D., Woods J. S., Eaton D. L., Kavanagh T. J. (1999) Toxicol. Lett. 110, 1–9 [DOI] [PubMed] [Google Scholar]
- 21.Tu Z., Anders M. W. (1998) Arch. Biochem. Biophys. 354, 247–254 [DOI] [PubMed] [Google Scholar]
- 22.Chen Y., Shertzer H. G., Schneider S. N., Nebert D. W., Dalton T. P. (2005) J. Biol. Chem. 280, 33766–33774 [DOI] [PubMed] [Google Scholar]
- 23.Yang Y., Dieter M. Z., Chen Y., Shertzer H. G., Nebert D. W., Dalton T. P. (2002) J. Biol. Chem. 277, 49446–49452 [DOI] [PubMed] [Google Scholar]
- 24.Chang L. S. (1996) J. Protein Chem. 15, 321–326 [DOI] [PubMed] [Google Scholar]
- 25.Seelig G. F., Simondsen R. P., Meister A. (1984) J. Biol. Chem. 259, 9345–9347 [PubMed] [Google Scholar]
- 26.Ochi T. (1995) Archives of Toxicology 70, 96–103 [DOI] [PubMed] [Google Scholar]
- 27.Ochi T. (1996) Toxicology 112, 45–55 [DOI] [PubMed] [Google Scholar]
- 28.Jez J. M., Cahoon R. E., Chen S. (2004) J. Biol. Chem. 279, 33463–33470 [DOI] [PubMed] [Google Scholar]
- 29.Sun W. M., Huang Z. Z., Lu S. C. (1996) Biochem. J. 320, 321–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toroser D., Yarian C. S., Orr W. C., Sohal R. S. (2006) Biochim. Biophys. Acta 1760, 233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siitonen T., Alaruikka P., Mäntymaa P., Savolainen E. R., Kavanagh T. J., Krejsa C. M., Franklin C. C., Kinnula V., Koistinen P. (1999) Ann. Oncol. 10, 1361–1367 [DOI] [PubMed] [Google Scholar]
- 32.Franklin C. C., Krejsa C. M., Pierce R. H., White C. C., Fausto N., Kavanagh T. J. (2002) Am. J. Pathol. 160, 1887–1894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franklin C. C., Rosenfeld-Franklin M. E., White C., Kavanagh T. J., Fausto N. (2003) FASEB J. 17, 1535–1537 [DOI] [PubMed] [Google Scholar]
- 34.Fraser J. A., Saunders R. D., McLellan L. I. (2002) J. Biol. Chem. 277, 1158–1165 [DOI] [PubMed] [Google Scholar]
- 35.Tu Z., Anders M. W. (1998) Biochem. J. 336, 675–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gipp J. J., Bailey H. H., Mulcahy R. T. (1995) Biochem. Biophys. Res. Commun. 206, 584–589 [DOI] [PubMed] [Google Scholar]
- 37.McConnachie L. A., Mohar I., Hudson F. N., Ware C. B., Ladiges W. C., Fernandez C., Chatterton-Kirchmeier S., White C. C., Pierce R. H., Kavanagh T. J. (2007) Toxicol. Sci. 99, 628–636 [DOI] [PubMed] [Google Scholar]
- 38.Botta D., White C. C., Vliet-Gregg P., Mohar I., Shi S., McGrath M. B., McConnachie L. A., Kavanagh T. J. (2008) Drug Metab. Rev. 40, 465–477 [DOI] [PubMed] [Google Scholar]
- 39.Backos D. S., Brocker C. N., Franklin C. C. (2010) Toxicol. Appl. Pharmacol. 243, 35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.