Abstract
Inactivation of the retinoblastoma protein (Rb) through phosphorylation is an important step in promoting cell cycle progression, and hyperphosphorylated Rb is commonly found in tumors. Rb phosphorylation prevents its association with the E2F transcription factor; however, the molecular basis for complex inhibition has not been established. We identify here the key phosphorylation events and conformational changes that occur in Rb to inhibit the specific association between the E2F transactivation domain (E2FTD) and the Rb pocket domain. Calorimetry assays demonstrate that phosphorylation of Rb reduces the affinity of E2FTD binding ∼250-fold and that phosphorylation at Ser608/Ser612 and Thr356/Thr373 is necessary and sufficient for this effect. An NMR assay identifies phosphorylation-driven conformational changes in Rb that directly inhibit E2FTD binding. We find that phosphorylation at Ser608/Ser612 promotes an intramolecular association between a conserved sequence in the flexible pocket linker and the pocket domain of Rb that occludes the E2FTD binding site. We also find that phosphorylation of Thr356/Thr373 inhibits E2FTD binding in a manner that requires the Rb N-terminal domain. Taken together, our results suggest two distinct mechanisms for how phosphorylation of Rb modulates association between E2FTD and the Rb pocket and describe for the first time a function for the structured N-terminal domain in Rb inactivation.
Keywords: Cdk (Cyclin-dependent Kinase), Cell Cycle, E2F Transcription Factor, Protein Phosphorylation, Retinoblastoma (Rb)
Introduction
The retinoblastoma tumor suppressor protein (Rb)2 is a key negative regulator of cell proliferation, and Rb pathway deregulation is ubiquitous in cancer (1, 2). Rb is inactivated by cyclin-dependent kinases (Cdk) in response to positive growth signals, which results in cell cycle progression (3–6). Rb function as a growth inhibitor in part depends on its ability to repress the transcription activity of E2F (7–10). E2F expression or Rb inactivation induces S phase entry, whereas Rb expression arrests cells in G1; these observations directly implicate the Rb-E2F pathway as an essential control mechanism of the G1-S transition and a critical link between growth factor signaling and cell cycle progression (1, 2). In quiescent cells and early G1, Rb is hypophosphorylated and bound to E2F in a manner that inhibits transactivation. Phosphorylation of Rb by both Cdk4/6-cyclin D and Cdk2-cyclin E occurs in late G1 and results in the dissociation of Rb-E2F complexes and E2F activation (4, 5, 11–15). The importance of phosphorylation in Rb inactivation and cellular proliferation is emphasized by the fact that tumor cells often have alterations to upstream regulators that result in Rb hyperphosphorylation (1, 2). However, the molecular basis for how phosphorylation inhibits E2F binding has not been established.
The Rb protein consists of a structured N-terminal domain (RbN) that associates with a structured central domain called the “pocket” (Fig. 1A) (16). The C-terminal domain (RbC) is intrinsically disordered (17). Two additional unstructured sequences exist; one is between RbN and the pocket, which we term the interdomain linker (RbIDL), and the other is a linker within the pocket domain (RbPL) that connects the two structured pocket subdomains (18). The Rb-E2F complex is stabilized by two distinct interactions, both of which have been shown to be necessary for growth suppression and inhibition of E2F transcription activity (19, 20). The Rb pocket domain binds the E2F transactivation domain (E2FTD) (21, 22), whereas RbC associates with the so-called marked box domains of E2F and its heterodimerization partner DP (Fig. 1B) (17).
FIGURE 1.
Domain structure of Rb and interactions with E2F-DP. A, Rb consists of a structured N-terminal domain (RbN) and central pocket domain. Its C-terminal domain (RbC) is disordered except for a short sequence that adopts a structure upon E2F binding. Two other unstructured sequences, the interdomain linker (RbIDL) and pocket linker (RbPL), are indicated. Structured regions are colored, and the conserved consensus Cdk phosphorylation sites are marked. B, Rb makes two distinct contacts with E2F. The pocket domain binds the E2F transactivation domain (E2FTD), and RbC binds the E2F-DP marked box domains.
Human Rb contains 16 Cdk consensus phosphorylation sites, although only a subset of these sites have been found phosphorylated in cells (14). The serine/threonine phosphoacceptor sites are distributed throughout the protein and, with a few exceptions, are in regions of the protein that lack intrinsic structure (Fig. 1A). Cell-based assays to uncover the particular phosphorylation events that result in Rb-E2F dissociation and reversal of Rb growth suppression have shown that phosphorylation at many different sites is capable of inactivating Rb (11–13, 23, 24). Insights into the distinct molecular effects of these phosphorylations is therefore critical to understanding the significance of multiple, seemingly redundant pathways toward Rb-E2F dissociation. One possibility is that different phosphorylation events control the two separate Rb-E2F interactions. Indeed, we previously found that phosphorylation of sites in RbC induces an intramolecular interaction between RbC and the pocket domain that specifically blocks the RbC-marked box association (17). It has also been shown that phosphorylation of sites in the pocket domain is capable of reducing E2FTD binding (22).
Here, we identify unambiguously the key phosphorylation events and characterize the domain rearrangements in Rb that result in inhibition of the E2FTD-pocket domain association. Phosphorylation of Thr356/Thr373 in RbIDL and Ser608/Ser612 in RbPL are each sufficient for partial inhibition of E2FTD binding, but both are necessary for complete inhibition. We show that phosphorylation stimulates an intramolecular interaction between RbPL and the pocket domain that overlaps with the E2FTD binding site. Our data confirm a role for RbPL, RbIDL, and the structured RbN in Rb inactivation and provide the first molecular insights into how phosphorylation disrupts a key cell cycle and growth regulatory complex.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
Human Rb constructs containing a single domain (e.g. RbN or pocket domain) could be expressed with high yields in Escherichia coli. Constructs containing multiple domains required expression in Sf9 cells to obtain quantities suitable for biophysical assays. Thus, Rb55–928, Rb55–787 (wild type and mutants), and Rb380–928 were all expressed in Sf9 cells as His6 fusion proteins. Cells were infected at a density of ∼2 × 106/ml with baculovirus containing the desired gene and incubated for 2–3 days. Proteins were purified by Ni2+-nitrilotriacetic acid affinity purification and heparin sulfate chromatography. Rb352–787, Rb380–787 (wild type and mutants), and RbPΔPL (Rb380–787, with 578–642 deleted) were expressed in E. coli as glutathione S-transferase fusion proteins. Cells were induced overnight at room temperature. The proteins were purified with glutathione affinity chromatography, the glutathione S-transferase tag was cut off, and the Rb domain was isolated by heparin sulfate chromatography. RbPL592–624, Rb338–379, Rb55–379, and E2FTD (E2F1, residues 372–437) were expressed as His6 fusion proteins in E. coli. Cells were induced for 2–4 h at 37 °C, and proteins were purified by Ni2+-nitrilotriacetic acid affinity and anion exchange chromatography. Isotopically labeled RbPΔPL and RbPL592–624 were prepared for NMR as described, except that upon induction, E. coli were switched to M9 minimal medium including [15N]ammonium chloride, [13C]glucose, and D2O as necessary. PP1 catalytic domain (α isoform) was expressed in E. coli and purified with anion exchange and heparin sulfate chromatography. Recombinant Cdk6-CycK (herpesvirus cyclin) and Cdk2-CycA were expressed and purified as described previously (25, 26).
Enzymatic Modifications
Rb protein constructs were concentrated to ∼1–5 mg/ml following purification and then phosphorylated in a reaction containing 10 mm MgCl2, 10 mm ATP, 250 mm NaCl, 25 mm Tris (pH 8.0), and 2% Cdk6-CycK or 10% Cdk2-CycA (percentage of mass of the total substrate in the reaction). Reactions were incubated at room temperature for 1 h. Use of either kinase resulted in similar phosphate incorporation in the reaction described in supplemental Fig. 1. Kinase-treated Rb55–928 was digested with either trypsin or chymotrypsin and analyzed for phosphate incorporation using a Thermo Finnigan liquid chromatography/MS/MS (LTQ) linear ion trap. All MS/MS spectra were processed using Bioworks 3.3. Peptide identifications with better than 0.01 peptide probability were accepted and manually inspected.
Phosphatase reactions were carried out with 10% PP1 (percentage of mass of substrate) in the presence of 1 mm MnCl2, 250 mm NaCl, 25 mm Tris (pH 8.0) at room temperature for 1 h. We have found by mass spectrometry and radioisotope labeling assays that these conditions lead to nearly quantitative dephosphorylation (data not shown). Initially, proteins were purified following the enzymatic treatment and prior to isothermal titration calorimetry (ITC) or NMR with size exclusion chromatography; subsequently it was found this step was not necessary because results were unaffected by the purification step.
ITC
ITC experiments were conducted with a MicroCal VP-ITC calorimeter. Typically, ∼0.5–1 mm E2FTD and 25–50 μm Rb were used in each experiment. Proteins were dialyzed overnight prior to the assay in a buffer containing 100 mm NaCl, 1 mm dithiothreitol, and 25 mm Tris (pH 8.0). Data were analyzed with the Origin calorimetry software package assuming a one-site binding model. n values, reflecting the stoichiometry of the Rb-E2FTD complex, were between 0.8 and 1.2. Experiments were repeated for each Rb construct 2–4 times, and the reported error is the S.D. of each set of measurements.
NMR Spectroscopy
Purified Rb protein constructs were dialyzed into an NMR buffer containing 50 mm sodium phosphate, 5 mm dithiothreitol, and 10% D2O (pH 6.1). For binding experiments involving labeled RbPL592–624, HSQC spectra were recorded at 25 °C on a Varian INOVA 600-MHz spectrometer equipped with an HCN 5-mm cryoprobe (27). Experiments observing labeled RbPΔPL and experiments to assign RbPL592–624 were conducted with an Avance II 900-MHz spectrometer (Bruker-Biospin, Boston, MA) at the Central California 900-MHz NMR facility (Berkeley, CA). The amide resonances were assigned via 1H-15N to side chain correlations observed in two-dimensional 1H-15N HSQC-TOCSY (100-ms mixing time) and two-dimensional 1H-15N HSQC-NOESY (350-ms mixing time) experiments and via 13Ca (i, i − 1) and 13Cb (i, i − 1) linkages observed in a three-dimensional HNCACB experiment (28–30). NMR spectra were processed with NMRPipe and analyzed with NMRViewJ (31, 32).
RESULTS
RbN Is Required for Phosphorylation-induced Inhibition of E2FTD Binding
To determine the precise sequences and phosphorylation sites within Rb required for inhibition of E2FTD binding, we applied an ITC assay to quantitatively measure affinities with purified proteins. We first expressed in Sf9 insect cells an Rb construct containing amino acids 55–928 (Rb55–928). Rb55–928 contains all three domains of Rb and all 15 conserved Cdk consensus sites. Rb55–928 is phosphorylated by endogenous Sf9 kinases (33), so Rb55–928 was dephosphorylated with the Rb phosphatase PP1. As seen in Fig. 2A, the affinity of E2FTD for the PP1-treated protein (dephosRb55–928) is Kd = 0.04 ± 0.02 μm. This value is comparable with the affinity of E2FTD for unphosphorylated pocket domain purified from bacteria (Rb380–787; Kd = 0.045 ± 0.007 μm), which is the Rb domain necessary and sufficient for E2FTD binding (21, 22).
FIGURE 2.
Rb domain requirements for inhibition of E2FTD. A, ITC titration curves show that E2FTD binds to enzymatically dephosphorylated Rb55–928 (Rb55–928; Kd = 0.04 ± 0.02 μm) with a similar affinity as to the unphosphorylated Rb pocket domain (dephosRb380–787; Kd = 0.045 ± 0.007 μm). Phosphorylation of Rb55–928 results in a weaker affinity (phosRb55–928; Kd = 11 ± 3 μm). B, E2FTD dissociation constants were measured by ITC for binding to truncation mutants of Rb. The data demonstrate that RbN is required for phosphorylation-induced inhibition of E2FTD.
We next phosphorylated Rb55–928 with recombinant Cdk-cyclin using reaction conditions that result in quantitative phosphorylation of accessible Cdk consensus sites (supplemental Fig. 1). Phosphorylation was detected at 13 of 15 Cdk consensus sites using phosphopeptide mapping with liquid chromatography/MS/MS (Table 1). Notably, we did not detect phosphorylation at Ser230 or Ser567 despite the large quantity of purified kinase used in the reaction. Both sites are buried in structured domains and have not been observed to be phosphorylated in vivo (14, 21, 22, 34). Calorimetric assays show that E2FTD binds to phosRb55–928 with Kd = 11 ± 3 μm (Fig. 2A), which is ∼250-fold weaker than its association with dephosRb55–928 or unphosphorylated Rb380–787. This result supports a large body of experiments demonstrating loss of E2F binding to Rb upon Cdk phosphorylation (4, 5, 11–15).
TABLE 1.
Identification of Rb55–928 phosphorylation sites following the recombinant kinase reaction
| Residue | Phosphorylateda | Peptide sequence |
|---|---|---|
| 230b | N | F↓IKLSPPML↓L |
| 249, 252 | Y, Y | K↓TAVIPINGS*PRT*PR↓Rc |
| 356 | Y | R↓LFLDHDKTLQTDSIDSFETQRT*PR↓K |
| 373 | Y | R↓KSNLDEEVNVIPPHT*PVR↓T |
| 567 | N | R↓IMESLAWLSDSPLFDLIK↓Q |
| 608, 612b | Y, Y | L↓NLPLQNNHTAADMYLS*PVRS*PK↓K |
| 780 | Y | K↓TNILQYASTRPPTLS*PIPHIPR↓S |
| 788b | Y | L↓SPIPHIPRS*PY↓K |
| 795b | Y | Y↓KFPSS*PLR↓I |
| 807, 811 | Y, Y | R↓IPGGNIYIS*PLKS*PYK↓I |
| 821 | Y | K↓ISEGLPT*PTK↓M |
| 826 | Y | K↓ISEGLPTPTKMT*PR↓S |
a N, unphosphorylated; Y, phosphorylated; Y, Y, phosphorylated at two sites.
b Recovered with chymotrypsin plus trypsin.
c Asterisks represent phosphorylation sites identified by MS/MS.
To identify which Rb domains are required for inhibiting E2FTD association, we carried out a series of ITC experiments using Rb truncation mutants that were phosphorylated in our recombinant Cdk reaction. The results of these assays are summarized in Fig. 2B, and sample ITC data are shown in supplemental Fig. 2. E2FTD binds an RbC truncation mutant (phosRb55–787) with Kd = 13 ± 3 μm, indicating that deletion of RbC has no effect on the phosphorylation-induced change in E2FTD affinity. Therefore, association of phosRbC with the pocket domain is not necessary for inhibition of E2FTD binding, although it remains possible that it has a redundant effect. Part of RbC binds the E2F-DP marked box domains, and previous data demonstrate that RbC phosphorylation specifically inhibits that association (17).
Next, three different N-terminal domain truncation mutants (phosRb380–928, phosRb352–787, and phosRb380–787) were used in binding assays. E2FTD binds significantly tighter to each of these phosphorylated constructs than to phosRb constructs containing the N-terminal domain (Fig. 2B). These differences in affinity demonstrate that RbN is required for the full inhibition of E2FTD binding that occurs upon Rb phosphorylation.
Phosphorylation of Thr356/Thr373 and Ser608/Ser612 Weakens E2FTD Binding to Rb55–787
To define further which phosphorylation events are necessary for inhibition of E2FTD-pocket binding, we purified Rb55–787 constructs containing alanine mutations such that only specific sites were phosphorylated by kinase. E2FTD binds to wild-type dephosRb55–787 with Kd = 0.3 ± 0.2 μm and phosRb55–787 with Kd = 13 ± 3 μm (Table 2). Our mass spectrometry data indicate that within residues 55–787, Rb is phosphorylated at Ser780 and three pairs of other Cdk consensus sites: Ser249/Thr252 in RbN, Thr356/Thr373 in RbIDL, and Ser608/Ser612 in RbPL (Table 1). We purified and phosphorylated three mutant Rb55–787 constructs, each with Ser780 and one of the three pairs of phosphoacceptor sites left intact (Table 2). The affinity of E2FTD for the phosRb55–787 protein with the Thr356/Thr373 and Ser608/Ser612 sites mutated (Kd = 0.33 ± 0.02 μm) is equivalent to its affinity for wild-type dephosRb55–787. This measurement indicates that Cdk phosphorylation of Ser249, Thr252, and Ser780 has no effect on E2FTD binding. The affinities of E2FTD for phosRb55–787 with only either Ser608/Ser612/Ser780 or Thr356/Thr373/Ser780 intact were similar (Kd = 2.7 ± 0.6 μm and Kd = 2.9 ± 0.1 μm, respectively), and both are weaker than wild-type dephosRb55–787 but stronger than wild-type phosRb55–787. Together these data demonstrate that phosphorylation of Thr356/Thr373 and Ser608/Ser612 can both partially inhibit E2FTD binding, but neither pair of phosphoacceptor sites is sufficient alone to reproduce the full inhibition observed upon phosphorylation of wild-type protein. To confirm that phosphorylation of both pairs of sites is together sufficient for inhibition, we generated phosRb55–787Δ249/252 (only Ser249/Thr252 mutated) and found that the affinity of E2FTD for this construct (Kd = 25 ± 1 μm) is similar to wild-type phosRb55–787 (Kd = 13 ± 3 μm).
TABLE 2.
ITC measurements of E2FTD binding to mutants of phosRb55–787
| Construct | Mutations | Accessible phosphorylation sites | E2FTDKd |
|---|---|---|---|
| μm | |||
| dephosRb55–787 | None | NAa | 0.3 ± 0.2 |
| phosRb55–787 | None | Ser249, Thr252, Thr356, Thr373, Ser608, Ser612, Ser780 | 13 ± 3 |
| phosRb55–787Δ356/373/608/612 | T356A, T373A, S608A, S612A | Ser249, Thr252, Ser780 | 0.33 ± 0.02 |
| phosRb55–787Δ249/252/356/373 | S249A, T252A, T356A, T373A | Ser608, Ser612, Ser780 | 2.7 ± 0.6 |
| phosRb55–787Δ249/252/608/612 | S249A, T252A, S608A, S612A | Thr356, Thr373, Ser780 | 2.9 ± 0.1 |
| phosRb55–787Δ249/252 | S249A, T252A | Thr356, Thr373, Ser608, Ser612, Ser780 | 25 ± 1 |
a Not applicable.
It is noteworthy that the E2FTD affinity for phosRb55–787Δ249/252 is ∼25-fold weaker than its affinity for phosRb352–787. Both constructs contain the necessary four phosphorylation sites (Thr356, Thr373, Ser608, and Ser612); however, full inhibition of E2FTD binding was only observed for phosRb55–787Δ249/252, which contains RbN in addition to the required phosphorylation sites. This result suggests further the requirement of RbN for phosphorylation-induced inhibition of E2F, despite the fact that there is no requirement for phosphorylation of the RbN sites. We conclude that the structured RbN must be critical for the mechanism of inhibition.
It is also significant that phosphorylation of Ser608/Ser612 causes similar inhibition in the absence of RbN (∼15-fold; compare in Fig. 2 Kd values for Rb380–787 and phosRb380–787) and in the presence of RbN (∼9 fold; compare in Table 2 Kd values for dephosRb55–787 and phosRb55–787Δ249/252/356/373). Therefore, the effect of Ser608/Ser612 phosphorylation does not require RbN. In sum, our data demonstrate two distinct and independent mechanisms for E2FTD inhibition, each relying on phosphorylation of a specific pair of sites (Thr356/Thr373 or Ser608/Ser612).
Phosphorylation Mediates Binding of RbPL to the Rb Pocket Domain
Having identified the required phosphorylation events for E2FTD inhibition, we next explored the conformational changes in Rb that cause inhibition. The calorimetry data indicate that the partial inhibition induced by phosphorylation of Ser608/Ser612 does not require RbN. Ser608 and Ser612 are located in RbPL, which comprises a disordered stretch of amino acids linking the two pocket subdomains (Fig. 1A). We hypothesized that phosphorylation induces an intramolecular association between the phosphorylated sequence in the linker and the pocket in a manner analogous to that previously observed for phosRbC (17).
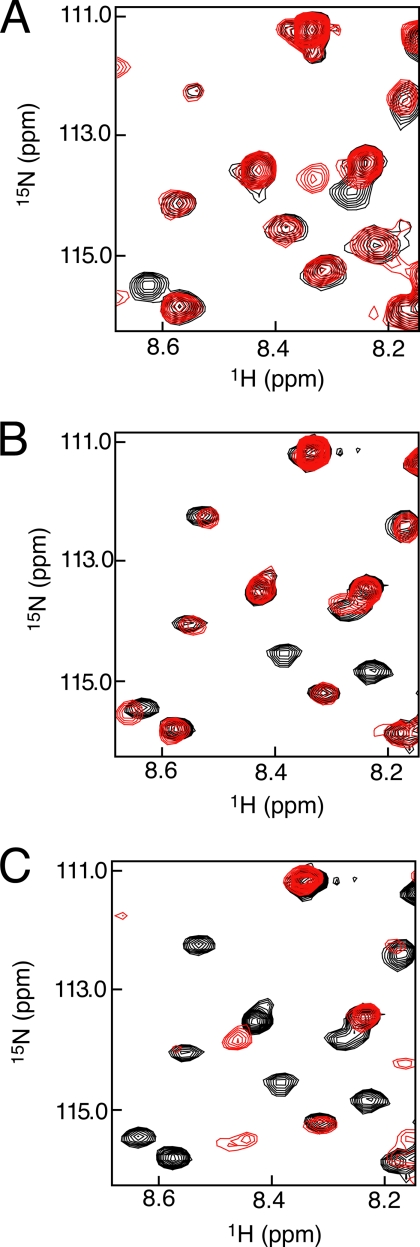
To test this model, we attempted to detect a weak interaction between isolated, phosphorylated RbPL and the pocket domain in trans. ITC experiments titrating phosRbPL into the Rb pocket domain did not yield any significant heat signal (data not shown). Alternatively, we applied an NMR assay that is more sensitive in detecting the anticipated weak intermolecular association. We first generated a uniformly 15N-labeled phosRbPL592–624 construct that contains the phosphorylation sites Ser608 and Ser612 as well as the only sequence of conserved amino acids within RbPL (Fig. 3A). The 1H-15N HSQC spectrum of phosRbPL592–624 shows little chemical shift dispersion in the 1H dimension; this observation is consistent with a lack of structure in the pocket linker (Fig. 3B). We next purified a pocket domain construct (RbPΔPL), previously used in crystallization experiments (21, 22), in which the entire RbPL is deleted. Superposition of the HSQC spectrum of phosRbPL592–624 alone (Fig. 3B, black) and a spectrum of phosRbPL592–624 in the presence of unlabeled RbPΔPL (Fig. 3B, red) shows that chemical shift changes and peak broadening occur in the presence of Rb pocket. This observation is consistent with binding on the fast to intermediate exchange time scale and the increased correlation time of forming a complex. When the HSQC experiment is repeated using unphosphorylated RbPL592–624, no spectral changes are observed in the presence of RbPΔPL, indicating that the association is phosphorylation-dependent (Fig. 3C).
FIGURE 3.
phosRbPL associates with the Rb pocket domain and competes with E2FTD binding. A, alignment of RbPL sequences from human (hs), mouse (mm), chicken (gg), frog (xl), and zebrafish (dr) shows that residues 595–611 (human) are highly conserved (yellow). B, HSQC spectra of 100 μm 15N-labeled phosRbPL592–624 alone (black) and in the presence of 500 μm unlabeled RbPΔPL (red). Broadening of amide resonances occurs selectively for residues 601–610, indicating a binding interaction between the phosphorylated pocket linker and pocket domain in trans. C, spectra of 100 μm 15N-labeled unphosphorylated RbPL alone (black) and in the presence of 400 μm unlabeled RbPΔPL (red). No resonance peak broadening is observed for unphosphorylated RbPL in the presence of the Rb pocket, demonstrating that binding is mediated by phosphorylation of RbPL. D, spectra of 100 μm 15N-labeled phosRbPL alone (black) and with 500 μm unlabeled RbPΔPL and 2 mm unlabeled E2FTD (red). In the presence of excess E2FTD, resonance peaks at chemical shifts corresponding to unbound phosRbPL reappear, indicating that E2FTD competes with phosRbPL for binding to the Rb pocket.
phosRbPL Associates with the Pocket Domain at the E2FTD Binding Site
We next tested whether phosRbPL592–624 and E2FTD directly compete for binding to the Rb pocket. Excess E2FTD was added to the sample containing both 15N-labeled phosRbPL592–624 and unlabeled RbPΔPL pocket. The resulting spectrum (Fig. 3D, red) shows reduced peak broadening compared with the spectrum taken without E2FTD (Fig. 3B, red). This observation is consistent with excess E2FTD displacing phosRbPL592–624 from the pocket domain such that the spectrum in the presence of E2FTD resembles that of free phosRbPL592–624 (Fig. 3D, black). phosRbPL592–624 and E2FTD thus do not bind simultaneously to the pocket domain. The NMR and calorimetry data together demonstrate that RbPL phosphorylation induces a phosRbPL-pocket association that inhibits E2FTD binding.
Upon assigning the HSQC peaks to specific amino acids in RbPL592–624, comparison of peak broadening reveals that the effect is most dramatic for Thr601–Val610 (Fig. 3B). Residues Asp604–Tyr606 are well conserved in orthologs (Fig. 3A) and have some sequence similarity to residues Asp425–Phe427 of the C terminus of E2F1TD. The DLF sequence in E2F1TD makes critical binding contacts to Phe482 and Arg467 in the pocket domain of Rb (Fig. 4A) (21). We supposed that phosRbPL also binds at this site in the pocket. To test this idea, we compared peak broadening in HSQC spectra of 15N-labeled phosRbPL in the presence of unlabeled RbPΔPL, RbPΔPL/F482A, and RbPΔPL/R467A (Fig. 4). The RbPΔPL mutants do not induce the significant peak broadening that is observed with the wild type Rb pocket, suggesting that the affinity of the mutant constructs for phosRbPL is weaker. These data demonstrate that Phe482 and Arg467 are important for mediating binding between phosRbPL and the pocket and accordingly that the phosRbPL and E2FTD binding sites in the pocket domain overlap.
FIGURE 4.
phosRbPL binds the pocket domain at the E2FTD binding site. A, structure of E2FTD bound to the pocket domain. Critical contacts between Asp424 and Phe426 of E2F and Arg467 and Phe482 of Rb are shown. This figure was generated using Protein Data Bank entry 1N4M. B and C, HSQC spectra of 100 μm 15N-labeled phosRbPL592–624 alone (black) and in the presence of 500 μm unlabeled RbPΔPL-R467A (red) and RbPΔPL-F482A (blue), respectively. D, resonance peak intensity ratios of phosRbPL in the presence of wild type RbPΔPL (black) and mutants R467A (red) and F482A (blue). The ratio I/I0 is defined as the peak intensity of phosRbPL in the presence of RbPΔPL (I) divided by the peak intensity of phosRbPL alone (I0). These data demonstrate that Arg467 and Phe482 in the pocket domain are critical for binding phosRbPL as well as E2FTD.
To confirm an important role for residues Asp604–Leu607 in the inhibition of E2FTD binding, we tested the affinity of E2FTD for Rb380–787 mutants in which these residues were mutated to alanine individually or in combination (Table 3). E2FTD binds phosphorylated wild-type Rb380–787 (Kd = 0.7 ± 0.4 μm) with approximately 15-fold less affinity than unphosphorylated protein (Kd = 0.045 ± 0.007 μm). E2FTD binds all of the unphosphorylated mutant proteins in Table 3 with similar affinity as wild type (data not shown). We found that mutation of Met605 had little effect on the affinity of E2FTD for phosphorylated Rb pocket, whereas mutation of Asp604, Tyr606, and Leu607 each had a modest effect. Mutation of Asp604–Tyr606 in combination produced an Rb pocket construct in which phosphorylation does not inhibit E2FTD binding significantly. We conclude that Asp604, Tyr606, and Leu607 all probably contribute to the mechanism of E2FTD inhibition due to Rb phosphorylation at Ser608/Ser612.
TABLE 3.
ITC measurements of E2FTD binding to mutants of phosRb380–787
| Construct | Mutations | Accessible phosphorylation sites | E2FTDKd |
|---|---|---|---|
| unphosRb380–787 | None | NAa | 0.045 ± 0.007 μm |
| phosRb380–787 | None | Ser608, Ser612, Ser780 | 0.7 ± 0.4 μm |
| phosRb380–787Δ604 | D604A | Ser608, Ser612, Ser780 | 0.4 ± 0.2 μm |
| phosRb380–787Δ605 | M605A | Ser608, Ser612, Ser780 | 0.9 ± 0.2 μm |
| phosRb380–787Δ606 | Y606A | Ser608, Ser612, Ser780 | 0.22 ± 0.09 μm |
| phosRb380–787Δ604/605/606 | D604A, M605A, Y606A | Ser608, Ser612, Ser780 | 0.11 ± 0.05 μm |
| phosRb380–787Δ607 | L607A | Ser608, Ser612, Ser780 | 0.3 ± 0.2 μm |
| phosRb380–787Δ608 | S608A | Ser612, Ser780 | 0.15 ± 0.01 μm |
| phosRb380–787Δ612 | S612A | Ser608, Ser780 | 0.7 ± 0.1 μm |
| phosRb380–787Δ608/612 | S608A, S612A | Ser780 | 0.06 ± 0.04 μm |
a Not applicable.
Finally, we asked whether phosphorylation at Ser608 and Ser612 are both required for inhibiting binding between E2FTD and the Rb pocket. We constructed serine to alanine mutants for Ser608 and Ser612 separately and used ITC to quantify changes in E2FTD-phosRb380–787 binding (Table 3). The phosphorylated S612A mutant has a similar affinity for E2FTD (Kd = 0.7 ± 0.1 μm) as wild type phosRb380–787 (Kd = 0.7 ± 0.4 μm), whereas the phosphorylated S608A mutant does not bind E2FTD as weakly (Kd = 0.15 ± 0.01 μm). These data indicate that Ser608 phosphorylation is sufficient for E2FTD inhibition, whereas Ser612 phosphorylation has only a modest effect.
The phosRbPL and phosRbN-RbIDL Binding Sites in the Pocket Domain Each Partially Overlap with the E2FTD Binding Site
Our ITC data demonstrate that RbN and phosphorylation at the RbIDL sites are together capable of inhibiting E2FTD binding. We examined whether phosRbN-RbIDL (phosRb55–379) associates with the pocket by conducting NMR experiments that monitor signals from the pocket domain. A uniformly labeled 2D-15N sample of RbPΔPL results in a well resolved 1H-15N TROSY spectrum (supplemental Fig. 3) (35). We compared the effects of the addition of unlabeled phosRbN-RbIDL (Fig. 5A), phosRbPL (Fig. 5B), or E2FTD (Fig. 5C) on the spectrum (full spectra in supplemental Fig. 3). Titration of unlabeled phosRb55–379 or phosRbPL results in peak broadening indicative of binding with an intermediate exchange time scale. The subset of peaks that broaden in each experiment is different, suggesting that the manner in which phosRbPL and the pocket domain associate is distinct from how phosRb55–379 associates with the pocket. Interestingly, the peaks that broaden upon the addition of both phosRbN-RbIDL and phosRbPL also undergo chemical shift changes upon the addition of E2FTD (Fig. 5C). This observation suggests that the phosRbN-RbIDL and phosRbPL binding sites in the pocket domain partially overlap with the E2FTD binding site. Binding of both has an independent and additive effect toward E2FTD inhibition, as observed in the calorimetry experiments.
FIGURE 5.
Detail of 1H-15N TROSY spectra of 300 μm2H-15N-labeled RbPΔPL alone (black) and in the presence (red) of unlabeled phosRbN-RbIDL (400 μm) (A), phosRbPL (1.5 mm) (B), and E2FTD (2 mm) (C). The observed spectral changes suggest that the binding sites for phosRbN-RbIDL and phosRbPL in the Rb pocket domain each partially overlap with the E2FTD binding site. Full spectra are shown in supplemental Fig. 3.
DISCUSSION
We have applied a calorimetry assay with purified proteins to identify unequivocally which phosphorylation events in Rb are capable of inhibiting E2FTD binding, and our data reveal two distinct mechanisms for this inhibition. In the first mechanism, Ser608/Ser612 phosphorylation induces an intramolecular association between RbPL and the pocket domain. This association occludes the E2FTD binding site in the pocket such that both phosRbPL and E2FTD cannot bind simultaneously. These results mark a novel role for RbPL, which has previously been poorly characterized. Interestingly, both the Rb paralogs p107 and p130 contain linkers in their pocket domains that have a phosphorylation site within a similar sequence context. This homology suggests that a similar phosphorylation-induced structural change may be conserved in the pocket protein family.
The second mechanism for E2FTD inhibition requires both RbN and phosphorylation at sites in RbIDL. Previously, it has been reported that RbN and the Rb pocket domain associate in a manner that is phosphorylation-independent (16). Our data here suggest that RbN and phosRbIDL together bind to the pocket domain in a manner that partially overlaps the E2FTD binding site. Further structural studies are required to examine in detail how phosphorylation changes the interactions between RbN-RbIDL and the pocket.
A critical advantage of our analysis with recombinant proteins is complete control of the sites and extent of phosphorylation. Previous investigations aimed at identifying the critical phosphorylation events that regulate Rb-E2F binding had mixed results (11–13, 23, 24). These studies generally relied on transient transfections of mutagenized proteins in cancer cell lines. Thus, specific conclusions may have been influenced by substoichiometric degrees of phosphorylation at the acceptor sites that varied depending on the mutant and kinase. In an in vitro reaction with large quantities of recombinant kinase, we have achieved nearly quantitative phosphate incorporation, allowing unambiguous interpretation of the molecular effects of phosphorylation. Nevertheless, our results agree with and further explain several key observations from these previous cellular assays. Importantly, in transfection experiments assaying E2F binding, repression of E2F transcription, and growth suppression, cumulative mutation of multiple phosphoacceptor sites was required to abolish the effect of Cdk phosphorylation (11, 13, 23, 24). With previous results, our data reveal that multiple phosphorylation events (Thr356/Thr373, Ser608/Ser612, Ser788/Ser795, and Thr821/Thr826) are capable of inhibiting one of the two interfaces stabilizing the overall complex (Fig. 1), and thus many different combinations of Cdk phosphorylation must all be sufficient to inactivate Rb by disrupting the complex.
Previous studies, which analyzed the effects of Rb phosphorylation at specific sites in cancer cell models, support our molecular characterization of Rb-E2F inhibition in several ways. First, phosphorylation reverses Rb repression of E2F-dependent transcription even if all of the RbC sites have been mutated (13, 24). This observation points to a role for phosphorylation outside of RbC in specifically regulating the E2F transactivation domain and is consistent with our calorimetry data. Second, mutation of Ser608/Ser612 in addition to all of the RbC sites results in an Rb construct that cannot be regulated by phosphorylation, directly demonstrating the importance of Ser608/Ser612 phosphorylation in Rb inactivation (13). Third, Cdk phosphorylation cannot regulate Rb when RbN is deleted and RbC phosphorylation sites are mutated (13). Our data confirm that RbN is required for full phosphorylation-induced E2FTD inhibition. Interestingly, our calorimetry and NMR data suggest that RbN is not required for the partial E2FTD inhibition induced specifically by Ser608/Ser612 phosphorylation. One possible explanation for this discrepancy is that the quantitative calorimetry assay used here is more sensitive than the cellular assays in detecting partial inhibition of E2FTD binding.
The implications of multiple possible phosphorylation pathways to E2F dissociation are intriguing. Different phosphorylation sites are probably preferentially phosphorylated by different Cdk-cyclins (34, 36), and thus diverse upstream regulators can affect Rb-E2F stability. On the other hand, although the sites have seemingly redundant roles in combining to inhibit E2F binding, each phosphorylation event is unique in the structural change it induces in Rb. The resulting conformations can differentially influence interactions of Rb with other proteins. For example, the intramolecular association of phosRbC with the pocket domain upon Thr821/Thr826 phosphorylation competes with the binding of LXCXE-containing proteins in addition to inhibiting the RbC-E2F marked box interface (12, 17, 37). Inhibition of the E2FTD-pocket association through Ser608/Ser612 phosphorylation, however, would still permit LXCXE protein interactions. Thus, in generating distinct phosphorylated Rb structures, the different E2F inhibition mechanisms resulting from various phosphorylation pathways allow for multiple signaling outputs.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants CA132685 (to S. M. R.) and GM68933 (to support the Central California 900-MHz facility), and SS10-RR20939 (to support the University of California Santa Cruz mass spectrometry facility).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- Rb
- retinoblastoma protein
- phosRb
- phosphorylated Rb
- dephosRb
- dephosphorylated Rb
- Cdk
- cyclin-dependent kinase(s)
- MS/MS
- tandem mass spectrometry
- ITC
- isothermal titration calorimetry.
REFERENCES
- 1.Sherr C. J. (1996) Science 274, 1672–1677 [DOI] [PubMed] [Google Scholar]
- 2.Weinberg R. A. (1995) Cell 81, 323–330 [DOI] [PubMed] [Google Scholar]
- 3.Adams P. D. (2001) Biochim. Biophys. Acta 1471, M123–133 [DOI] [PubMed] [Google Scholar]
- 4.Buchkovich K., Duffy L. A., Harlow E. (1989) Cell 58, 1097–1105 [DOI] [PubMed] [Google Scholar]
- 5.Chen P. L., Scully P., Shew J. Y., Wang J. Y., Lee W. H. (1989) Cell 58, 1193–1198 [DOI] [PubMed] [Google Scholar]
- 6.Hinds P. W., Mittnacht S., Dulic V., Arnold A., Reed S. I., Weinberg R. A. (1992) Cell 70, 993–1006 [DOI] [PubMed] [Google Scholar]
- 7.Chellappan S. P., Hiebert S., Mudryj M., Horowitz J. M., Nevins J. R. (1991) Cell 65, 1053–1061 [DOI] [PubMed] [Google Scholar]
- 8.Fattaey A., Helin K., Harlow E. (1993) Philos Trans. R. Soc. Lond. B Biol. Sci. 340, 333–336 [DOI] [PubMed] [Google Scholar]
- 9.Flemington E. K., Speck S. H., Kaelin W. G., Jr. (1993) Proc. Natl. Acad. Sci. U.S.A. 90, 6914–6918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiebert S. W., Chellappan S. P., Horowitz J. M., Nevins J. R. (1992) Genes Dev. 6, 177–185 [DOI] [PubMed] [Google Scholar]
- 11.Brown V. D., Phillips R. A., Gallie B. L. (1999) Mol. Cell. Biol. 19, 3246–3256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harbour J. W., Luo R. X., Dei Santi A., Postigo A. A., Dean D. C. (1999) Cell 98, 859–869 [DOI] [PubMed] [Google Scholar]
- 13.Knudsen E. S., Wang J. Y. (1997) Mol. Cell. Biol. 17, 5771–5783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lees J. A., Buchkovich K. J., Marshak D. R., Anderson C. W., Harlow E. (1991) EMBO J. 10, 4279–4290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundberg A. S., Weinberg R. A. (1998) Mol. Cell. Biol. 18, 753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassler M., Singh S., Yue W. W., Luczynski M., Lakbir R., Sanchez-Sanchez F., Bader T., Pearl L. H., Mittnacht S. (2007) Mol. Cell 28, 371–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rubin S. M., Gall A. L., Zheng N., Pavletich N. P. (2005) Cell 123, 1093–1106 [DOI] [PubMed] [Google Scholar]
- 18.Lee J. O., Russo A. A., Pavletich N. P. (1998) Nature 391, 859–865 [DOI] [PubMed] [Google Scholar]
- 19.Hiebert S. W. (1993) Mol. Cell. Biol. 13, 3384–3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin X. Q., Chittenden T., Livingston D. M., Kaelin W. G., Jr. (1992) Genes Dev. 6, 953–964 [DOI] [PubMed] [Google Scholar]
- 21.Lee C., Chang J. H., Lee H. S., Cho Y. (2002) Genes Dev. 16, 3199–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao B., Spencer J., Clements A., Ali-Khan N., Mittnacht S., Broceño C., Burghammer M., Perrakis A., Marmorstein R., Gamblin S. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 2363–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lents N. H., Gorges L. L., Baldassare J. J. (2006) Cell Cycle 5, 1699–1707 [DOI] [PubMed] [Google Scholar]
- 24.Chew Y. P., Ellis M., Wilkie S., Mittnacht S. (1998) Oncogene 17, 2177–2186 [DOI] [PubMed] [Google Scholar]
- 25.Jeffrey P. D., Tong L., Pavletich N. P. (2000) Genes Dev. 14, 3115–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Russo A. A. (1997) Methods Enzymol. 283, 3–12 [DOI] [PubMed] [Google Scholar]
- 27.Mori S., Abeygunawardana C., Johnson M. O., van Zijl P. C. (1995) J. Magn. Reson. B 108, 94–98 [DOI] [PubMed] [Google Scholar]
- 28.Norwood T. J., Boyd J., Heritage J. E., Soffe N., Campbell I. D. (1990) J. Magn. Reson. 87, 488–501 [Google Scholar]
- 29.Wittekind M., Mueller L. (1993) J. Magn. Reson. B 101, 201–205 [Google Scholar]
- 30.Muhandiram D. R., Kay L. E. (1994) J. Magn. Reson. B 103, 203–216 [Google Scholar]
- 31.Johnson B. A., Blevins R. A. (1994) J. Biomol. NMR 4, 603–614 [DOI] [PubMed] [Google Scholar]
- 32.Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 33.Lin B. T., Gruenwald S., Morla A. O., Lee W. H., Wang J. Y. (1991) EMBO J. 10, 857–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zarkowska T., Mittnacht S. (1997) J. Biol. Chem. 272, 12738–12746 [DOI] [PubMed] [Google Scholar]
- 35.Singh M., Krajewski M., Mikolajka A., Holak T. A. (2005) J. Biol. Chem. 280, 37868–37876 [DOI] [PubMed] [Google Scholar]
- 36.Kitagawa M., Higashi H., Jung H. K., Suzuki-Takahashi I., Ikeda M., Tamai K., Kato J., Segawa K., Yoshida E., Nishimura S., Taya Y. (1996) EMBO J. 15, 7060–7069 [PMC free article] [PubMed] [Google Scholar]
- 37.Knudsen E. S., Wang J. Y. (1996) J. Biol. Chem. 271, 8313–8320 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.