Abstract
GRASP proteins share an N-terminal GRASP domain and mediate homotypic tethering of Golgi cisternae to form extended Golgi ribbons. The golgin GM130 is thought to bind the C-terminal side of the GRASP domain to recruit GRASP65 onto the Golgi whereas stable membrane association appears to also depend on anchoring of the N terminus by myristoylation. Here, we examine the nature of the GM130/GRASP65 interaction and test whether the dual membrane contacts of the GRASP domain have a role in tethering beyond membrane recruitment. GM130 was found to contain a C-terminal PDZ ligand that binds the putative groove of the second PDZ-like domain in GRASP65. To test tethering activity independent of targeting, we took advantage of a tethering assay carried out on the mitochondrial membrane in which the GRASP membrane attachment points were individually or simultaneously substituted with mitochondrially targeted transmembrane sequences. N-terminally anchored constructs tethered only if the C terminus was also anchored; and likewise, C-terminally anchored constructs tethered only if the N terminus was anchored. One explanation for the role of this dual anchoring is that it orients the GRASP domain to prevent cis interactions within the same membrane thereby favoring trans interactions between adjacent membranes. Indeed, singly anchored GRASP constructs, although nonfunctional in tethering, interacted with one another and also bound and inhibited dually anchored constructs. This work thus elucidates the GM130/GRASP65 interaction and supports a novel orientation-based model of membrane tether regulation in which dual membrane contact orients the tethering interaction interface to favor trans over cis interactions.
Keywords: Golgi, Membrane Fusion, Membrane Proteins, Membrane Trafficking, Mitochondria
Introduction
The tethering of membranes prior to SNARE2-mediated fusion is accomplished by factors present in each membrane that interact to form a bridge thereby increasing the fidelity and efficiency of membrane fusion (1). The interaction can be heterotypic, such as vesicle fusion with a target organelle, or homotypic, as is the case when identical membranes fuse. In heterotypic tethering, each membrane contributes a distinct partner to form a heteromeric-tethering complex. After fusion, the complex is presumably disassembled, and one partner, if still membrane-associated, is sorted from the other in the plane of the membrane and packaged into vesicles for recycling. Disassembly and sorting are needed because interactions in cis between the tether partners would prevent further rounds of tethering just as cis SNARE interactions block membrane fusion (2). In homotypic tethering, each membrane may contribute distinct partners that bind to one another or identical partners that self-associate but because the membranes are identical each has a full complement of the tethering components, and this state is also maintained after fusion. Thus, for homotypic tethering, mechanisms other than sorting and recycling are needed to prevent cis complex formation.
In higher eukaryotes, the juxtanuclear Golgi membrane network, or Golgi ribbon, is a product of homotypic fusion between analogous cisternae in adjacent mini stacks. This fusion is mediated, in part, by the tethering action of the GRASP family of proteins beginning with GRASP65 on the cis face of the Golgi (3, 4). The GRASP65 interaction is homotypic (5) and is presumed to involve an internal PDZ ligand within a GRASP65 partner on one membrane binding to the first of two PDZ binding pockets in a GRASP65 partner on the opposing membrane (6). Currently, however, it is unknown how GRASP65 interactions in cis are prevented.
Our work has shown that the N-terminal myristoylation site of GRASP65 is required for it to tether membranes (6). As a transmembrane domain substitutes for this function, it suggests that the purpose of myristoylation is to anchor the first PDZ domain, PDZ1, promoting interactions in trans. Interestingly, the C-terminal side of PDZ1 is also anchored. Next to PDZ1 is PDZ2, which appears to mediate GRASP65 interactions with its Golgi-localized receptor GM130 (6, 7). PDZ ligands are typically a short peptide stretch at the C terminus and use the terminal carboxylic moiety to interact with a conserved GLGF motif in the PDZ binding pocket (8, 9). The GM130 C terminus may bind the PDZ2 pocket for dual anchoring of PDZ1, but significant questions remain. Even though the GM130 C terminus was shown to function as a PDZ ligand (10, 11), this is of uncertain physiological relevance as it involved binding to a mitochondrial, rather than Golgi, protein. Further, GM130 binding to GRASP65 was shown to depend on a GLGF-like motif in the second PDZ domain of GRASP65 (7), but there are three GLGF-like motifs in this domain, and it is unclear which of these, if any, is actually part of a PDZ binding groove. Typically, the GLGF motif is present near the N terminus of PDZ domains just prior to the second β-strand, which forms one side of the binding pocket (8), whereas the GLGF motif of GRASP65 mutated in previous work is near the C terminus just after the predicted sixth β-strand. Finally, it is not known whether anchoring the C-terminal side of PDZ1, i.e. establishing a dually anchored PDZ1 domain, plays a functional role in tethering beyond targeting the complex to the membrane.
We thus set out to test whether membrane recruitment of GRASP65 by GM130 involves a bone fide PDZ ligand interaction that dually anchors the tether to promote trans interactions. Our results indicate that GM130 does, indeed, bind to the second PDZ domain of GRASP65 via a traditional PDZ/C-terminal ligand interaction. We further show that this interaction is sufficient to recruit GRASP65 to membranes and regulates its ability to tether membranes in trans by anchoring both ends of the molecule possibly geometrically restricting it to prevent cis interactions.
EXPERIMENTAL PROCEDURES
Constructs
G65-His, G65-myc, G65-GFP-ActA, T20-G65-GFP-ActA, T20-GFP-GM130Cterm, and GFP-ActA were described (6). Point mutations for PDZ1, PDZ2, and the myristoylation mutant as well as the addition of a stop codon after GFP in the T20-G65-GFP-ActA construct and the addition of arginine to the T20-GFP-GM130Cterm construct were introduced using the QuikChange protocol (Stratagene, La Jolla, CA). A loop-out modification to the QuikChange protocol was used to abut the Tom20 transmembrane domain and mCherry in-frame. GST-GM130Cterm and GST-GM130Cterm+R were made by PCR amplification of residues 592–888 of mouse GM130 (3) and insertion into pGEX-2 (GE Healthcare).
Cell Culture and Immunofluorescence
HeLa cells were grown in minimum Eagle's medium containing 10% fetal bovine serum and maintained at 37 °C in a 5% CO2 incubator. Transient transfection was performed with jetPEI (Genesse Scientific, San Diego, CA) according to manufacturer's specifications, and cells were fixed 16–20 h after transfection with 3% paraformaldehyde at room temperature or methanol at −20 °C for 15 min. Mitochondrial construct transfections were treated with brefeldin A (Sigma) at 10 μg/ml for 30 min prior to fixation. Immunofluorescence (12) and image capture and analysis (6) were described previously.
Antibodies
Rabbit anti-His (Bethyl Labratories, Montgomery, TX) and rabbit anti-GFP (Sigma) were used at 1:2000. Rabbit anti-GRASP65 and rabbit anti-GPP130 were used at 1:1000. Monoclonal mouse anti-myc was used at 1:200. Goat anti-mouse or rabbit Alexa Fluor 488 and 568 secondary antibodies (Invitrogen) were used at 1:500 in immunofluorescence assays, and goat anti-mouse or rabbit horseradish peroxidase (Bio-Rad) were used at 1:2000 for Western blotting.
Protein Purification and Binding Assays
Protein purification was described previously (13) with dialysis of eluted proteins into phosphate-buffered saline containing 14 mm β-mercaptoethanol and 1 mm imidazole (Fisher Scientific). Myristoylated proteins were induced in cells co-transformed with a myristoyltransferase plasmid (kindly provided by Meir Aridor, University of Pittsburgh, Pittsburgh, PA). GST pulldown experiments were done in HKT buffer (10 mm HEPES (pH 7.2), 100 mm KCl, 1% Triton X-100, 50 mm phenylmethylsulfonyl fluoride, and 10 μg/ml leupeptin and pepstatin) with a 2-h incubation of proteins followed by a 2-h incubation with glutathione beads and washed with HKT. Co-immunoprecipitations were carried out with anti-myc antibodies cross-linked to protein A-Sepharose beads (GE Healthcare) with 20 mm dimethylpimelimidate (Sigma) as described (14). Cells were co-transfected with plasmids as described above and lysed in HKT buffer. Lysates were centrifuged to remove insoluble material and incubated with beads for 2 h followed by washing.
RESULTS
GRASP65 PDZ2 Binds a PDZ Ligand in GM130 for Membrane Recruitment
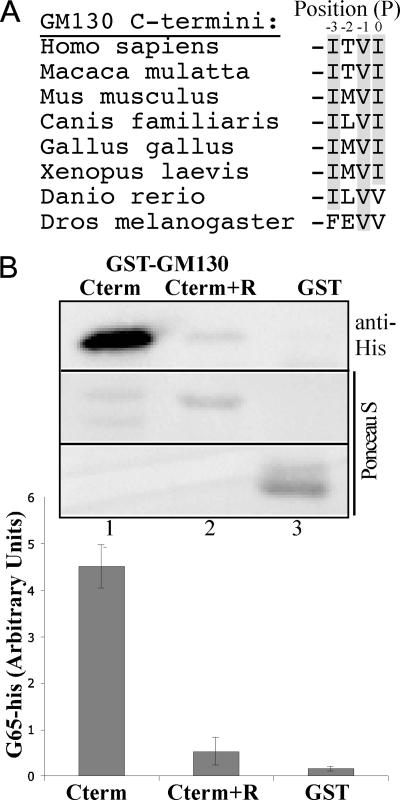
Conventional PDZ ligand sequences have been classified on the basis of residue positioning relative to the C-terminal residue termed P0, with the residue at P−2 considered most important (15). In common with many PDZ ligand sequences the GM130 P0 residue is aliphatic, but the residue in the P−2 position varies from species to species (Fig. 1A). However, GM130 does have a conserved valine at position P−1, giving it a double aliphatic sequence at the terminus. A double hydrophobic motif mediates binding of select p24 proteins (16), CD8a, and Fz4 (17) and may reflect an important binding motif for GRASP PDZ-like domains. Because it does contain a ligand motif, we wanted to confirm that GM130 binds GRASP65 as a ligand of its second PDZ-like domain. To this end, we created a GST fusion construct with the GM130 C terminus, residues 592–888 (GST-GM130Cterm), and a version with an additional arginine residue at the C terminus (GST-GM130Cterm+R). Our reasoning was that the appended arginine should interfere with the ligand binding into the hydrophobic portion of the binding pocket without changing any GM130 sequence. Myristoylated GRASP65 with a C-terminal His6 tag was purified after expression in bacteria expressing myristoyltransferase (6). GRASP65 bound GM130, but the additional arginine blocked the binding, suggesting that the GM130 C terminus binds in a ligand-like fashion (Fig. 1B).
FIGURE 1.
GM130 contains a C-terminal PDZ ligand. A, schematic of the C termini of GM130 through evolution from its first appearance in Drosophila melanogaster to Homo sapiens. B, purified myristoylated G65-His (2 μg) incubated with the GST-tagged GM130 C terminus with or without an appended arginine (2 μg). Recovery on glutathione-agarose beads was determined by immunoblotting (anti-His) and staining with Ponceau S. Binding was quantified (n = 3, ±S.E. (error bars)).
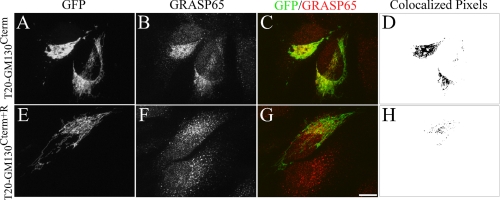
To test the interaction in cells, we made use of a recently developed assay for GM130-dependent GRASP65 recruitment in which the GM130 C terminus is targeted to the mitochondrial outer membrane using an N-terminal transmembrane domain from Tom20 serving as a mitochondrial targeting sequence (6). Consistent with the previous report, the mitochondrial version of GM130 (T20-GM130Cterm) recruited endogenous GRASP65 to mitochondria and the mitochondria became clustered due to GRASP65 tethering activity (Fig. 2, A–D). In contrast, the presence of an arginine at the C terminus (T20-GM130Cterm+R) blocked recruitment of endogenous GRASP65, and the mitochondria remained dispersed (Fig. 2, E–H). Thus, membrane recruitment of GRASP65 depends on the PDZ ligand characteristics of the GM130 C terminus.
FIGURE 2.
GM130 recruits GRASP65 to membranes using a C-terminal ligand. Cells were transfected with mitochondrially targeted GM130 C terminus (T20-GFP-GM130Cterm) without (A–C) or with an additional arginine (E–G). The cells were treated with brefeldin A to disperse the Golgi and visualized to detect GFP fluorescence and endogenous GRASP65 using an anti-GRASP65 antibody. The levels of GRASP65 recruitment are also indicated by representations of the co-localized pixels (D and H). Scale bar, 10 μm.
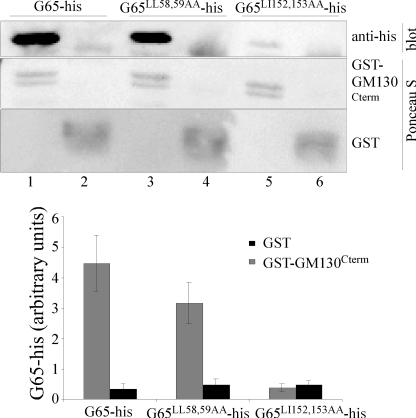
To test whether GM130 binds to PDZ2 of GRASP65, we assayed binding of GST-GM130Cterm to purified GRASP65 in which mutations were introduced into the binding pockets of either PDZ1 or PDZ2. Although the GRASP65 structure has not been solved, PDZ domains have a characteristic array of β-strands and α-helices. In GRASP65, however, the only residues that can be confidently predicted to be in the binding pocket are those in the α2-helices (6). Therefore, we mutated two hydrophobic residues in the putative α2-helices of PDZ1 and PDZ2. Purified GRASP65 with mutations in PDZ1 (G65L58A/L59A-His) bound the GM130 C terminus, but GRASP65 with mutations in PDZ2 (G65L152A/I153A-His) did not (Fig. 3). Quantification verified that the binding was specific and dependent on PDZ2 but not PDZ1.
FIGURE 3.
C terminus of GM130 binds PDZ2 of GRASP65. Purified, myristoylated G65-His (2 μg) as wild type or with mutations in the predicted binding pocket of PDZ1 (LL58,59AA) or PDZ2 (LI152,153AA) was incubated with GST-GM130Cterm or GST (2 μg). Recovery on glutathione-agarose beads was determined by immunoblotting (anti-His) and staining with Ponceau S. Binding was quantified (n = 3, ±S.E. (error bars)).
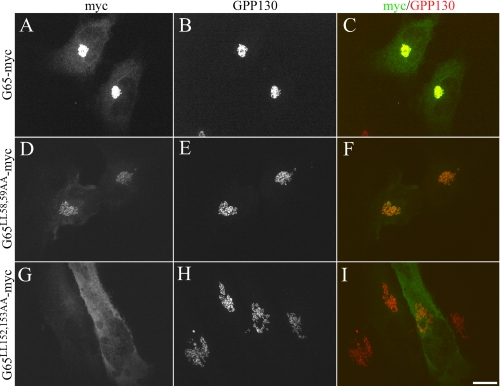
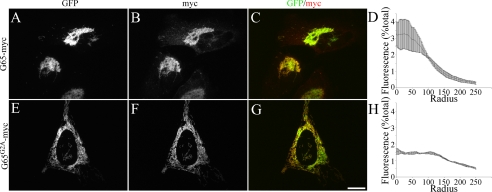
To confirm this result in cells, we assayed targeting of the mutated versions of GRASP65 to the Golgi because GRASP65 targeting depends on its binding to GM130 (3). As expected, a version of GRASP65 with a C-terminal myc tag (G65-myc) was Golgi localized as indicated by colocalization with the Golgi protein GPP130 after expression in HeLa cells (Fig. 4, A–C). Significantly, Golgi targeting was maintained for the version with PDZ1 pocket mutations (G65L58A/L59A-myc), whereas the version with mutations in the PDZ2 pocket (G65L152A/I153A-myc) failed to localize to the Golgi (Fig. 4, D–I). Thus, Golgi localization of GRASP65 depends on an interaction between the putative binding pocket in its second PDZ domain and a C-terminal PDZ ligand in GM130.
FIGURE 4.
GRASP65 is recruited to the Golgi by the PDZ2/GM130 ligand interaction. Cells were transfected with the wild type G65-myc (A–C), the PDZ1 mutant G65L58A/L59A-myc (D–F), or the PDZ2 mutant G65L152A/I153A-myc (G–I) and stained using antibodies against myc and GPP130 (to stain the Golgi). Scale bar, 10 μm.
Dual Anchoring of GRASP65 Promotes trans Pairing
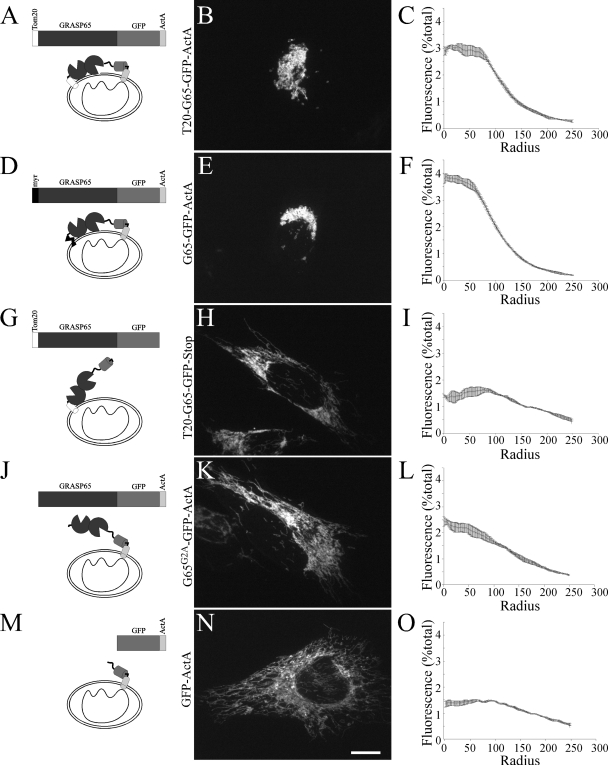
As stated above, our previous work indicated that anchoring of the GRASP65 N terminus is required for its tethering activity suggesting that anchoring might orient the molecule to favor trans interactions over inhibitory cis interactions (6). To test this, we first wanted to know the importance of C-terminal anchoring on the GRASP domain, which, as just shown, normally occurs via binding of PDZ2 to GM130. In other words, will a single N-terminal anchor suffice, or is dual anchoring required? The tethering assay on the mitochondrial outer membrane is advantageous because it is an exogenous site, which uses mitochondrial-targeting sequences that are also membrane anchors to allow decoupling of the roles of GRASP65 sequences in targeting and tethering. Thus, we used the N-terminal Tom20 signal to localize GRASP65 to mitochondrial membranes and anchor the N terminus while the C terminus was anchored with another mitochondrial localization signal, ActA (Fig. 5A). As expected (6), this T20-G65-GFP-ActA construct was localized to mitochondria and induced clustering (Fig. 5B). Clustering was quantified using radial profile analysis, which measures the distribution of fluorescence as a function of distance from a central point (Fig. 5C). Clustering by this construct was indistinguishable from that with the wild type myristoylated N terminus (Fig. 5, D–F). Significantly, when the C-terminal ActA anchor was removed, freeing the C terminus from the membrane, the resulting T20-G65-GFP construct was no longer able to tether mitochondrial membranes (Fig. 5, G–I). As previously reported (6), freeing the N terminus by mutating the myristoylation site and leaving the C terminus anchored also blocked tethering activity (Fig. 5, J–L), and a control construct lacking GRASP65 also failed to induce clustering (Fig. 5, M–O). Thus, dual anchoring appeared necessary for GRASP65-mediated tethering.
FIGURE 5.
C-terminal anchoring of GRASP65 is required for tethering of mitochondria. Cells were transfected with the indicated mitochondrially targeted constructs and visualized using GFP fluorescence followed by quantification of mitochondrial clustering with radial profile analysis (n = 3, 15 cells each trial, ±S.E.). Scale bar, 10 μm.
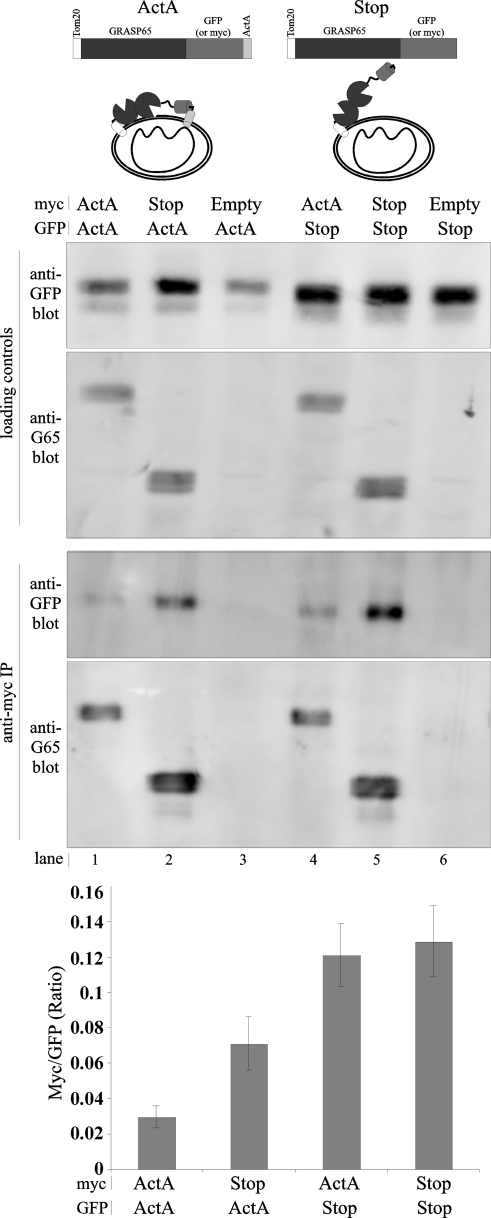
These results suggest that trans interactions only take place when the GRASP domain is dually anchored. However, it could be that dual anchoring activates GRASP65 by altering its conformation to make it binding-competent. In this case, singly anchored constructs could not bind. Alternatively, singly anchored constructs may be binding-competent, but preferentially bind in cis, outcompeting interactions in trans. To distinguish between these possibilities, we assayed binding competence of the singly anchored constructs using co-immunoprecipitation. Cells were co-transfected with GFP- and myc-tagged versions of the mitochondrially targeted constructs (diagrammed in Fig. 6 and termed ActA or STOP for dually and singly anchored, respectively). The myc-tagged proteins were then immunoprecipitated with anti-myc antibodies. Perhaps because of epitope accessibility, the singly anchored forms were recovered about 1.5 times better, so the co-precipitation results were quantified using the apparent ratio of the proteins in the precipitated complexes (Fig. 6, graph). As expected, the dually anchored control co-precipitated a fraction of its dually anchored, GFP-tagged partner (Fig. 6, lane 1), whereas the negative control did not (Fig. 6, lane 3). The low but significant recovery is consistent with the presence of trans pairs that bridge mitochondria and little or no cis pairs. Importantly, the level of co-immunoprecipitation was substantially higher between the singly anchored constructs (Fig. 6, lane 5 and graph). Clearly, the singly anchored versions of GRASP65 possessed binding activity and formed complexes even though they failed to induce clustering, strongly suggesting that cis complexes formed and prevented interactions in trans.
FIGURE 6.
Singly anchored GRASP65 is binding-competent. Cells co-transfected with the indicated myc and GFP-tagged mitochondrial GRASP65 constructs were subjected to immunoprecipitation with anti-myc antibodies followed by immunoblotting with anti GRASP65 (G65, to detect myc-tagged constructs) or anti-GFP antibodies (to detect co-precipitation). Constructs were T20-G65 with either a myc or GFP tag followed by either a C-terminal anchor (ActA) or a stop codon (Stop). Co-transfection with an empty vector was used as a negative control (Empty). Loading control is 10% of total extract incubated with beads. Binding was quantified as the ratio of the amount of co-precipitated GFP-tagged protein to recovered myc-tagged protein (n = 3, ±S.E. (error bars)).
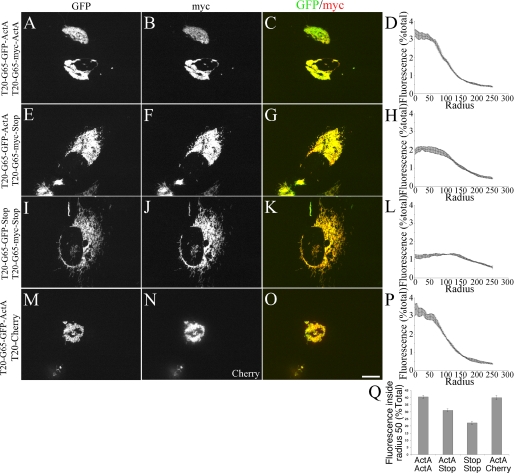
We next tested whether singly anchored constructs would bind and inhibit the tethering activity of the dually anchored constructs. Immunoprecipitation of the singly anchored construct resulted in co-immunoprecipitation of the dually anchored construct (Fig. 6, lane 2) and, likewise, immunoprecipitation of the dually anchored construct yielded recovery of the singly anchored form (Fig. 6, lane 4). Thus, the singly anchored forms not only bound themselves but also bound the dually anchored forms. Note that the quantified ratio shows that the singly anchored version co-precipitated less dually anchored construct than the reciprocal reaction. This may have reflected its self-interaction in cis, leaving fewer molecules available to form complexes with the dually anchored version. In any case, given that the singly anchored construct bound the dually anchored construct, we expected it to inhibit trans interactions competitively. Indeed, compared with co-expression of the dually anchored molecules (Fig. 7, A–D), the level of clustering was reduced when a singly anchored version was co-expressed along with a dually anchored form (Fig. 7, E–H). As expected, co-transfection of singly anchored constructs was even less effective at clustering (Fig. 7, I–L), and co-transfection of a construct lacking GRASP65 had no effect on the clustering induced by the dually anchored form (Fig. 7, M–P). The inhibitory effect of the singly anchored construct on clustering was also evident in a direct comparison of the percent of the total mitochondrial fluorescence encompassed by a single centrally focused circle with a radius of 50 pixels (Fig. 7Q).
FIGURE 7.
Singly anchored GRASP65 inhibits the tethering of mitochondria by doubly anchored GRASP65. HeLa cells co-transfected with the indicated mitochondrially targeted GRASP65 constructs were visualized using GFP fluorescence and myc staining, and clustering was quantified using radial profile analysis (n = 3, 15 cells each trial, ±S.E. (error bars)). Shown are two dually anchored versions (A–D), a dual with a single (E–H), two singly anchored versions (I–L), and a dual with a negative control (M–P). Scale bar, 10 μm. Direct comparison of the fluorescence within radius 50 pixels is also shown (Q).
Because these experiments took advantage of an artificial anchor at the C terminus, we next tested whether N-terminal myristoylation, and hence dual anchoring, would also be required if the physiological PDZ2-GM130 C-terminal anchor were in place. To do so, we asked whether GRASP65 targeted to the membrane by GM130 binding would still require N-terminal myristoylation for its tethering activity. Previous work had indicated that myristoylation is required for targeting (7), but we reasoned that GM130 expression at high levels on mitochondrial membranes might bypass this requirement. Indeed, similar to the case for GRASP65 with an intact myristoylation site (Fig. 8, A–D), GRASP65 lacking its myristoylation site was recruited to mitochondria when co-expressed with mitochondrially targeted GM130 (Fig. 8, E–G). Importantly, however, whereas the wild type GRASP65 induced clustering, the version lacking its myristoylation site failed to tether the membranes. Thus, if present at sufficient levels, GM130 was capable of recruiting GRASP65, but dual anchoring was needed for tethering. As just shown above, singly anchored GRASP65 not only failed to tether but also inhibited dually anchored GRASP65, and this could be attributed to interactions in cis. It is therefore likely that the expressed nonmyristoylated form in these experiments inhibited the endogenous GRASP65 recruited to the mitochondria as well. In sum, these experiments demonstrate the importance of dual anchoring of the GRASP domain and strongly suggest that it orients the molecule to favor interactions in trans.
FIGURE 8.
Nonmyristoylated GRASP65 is recruited to mitochondrial membranes and inhibits tethering by endogenous GRASP65. HeLa cells co-transfected with the mitochondrial GM130 C terminus (T20-GFP-GM130Cterm) and either G65-myc (A–D) or the nonmyristoylated G65G2A-myc (E–H) were visualized using GFP fluorescence and myc staining, and clustering was quantified using radial profile analysis (n = 3, 15 cells each trial, ±S.E.). Scale bar, 10 μm.
DISCUSSION
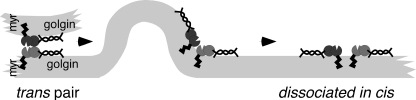
These studies support a model (Fig. 9) in which GRASP65 is recruited onto the Golgi by GM130 whereupon GRASP65 membrane attachment is stabilized by insertion of its N-terminal myristic acid. These two contact points, membrane binding by the myristic acid and GM130 binding, orient the GRASP65 homotypic binding interface in such a way that it can interact in trans but not in cis. GRASP65 self-association in trans is then followed by fusion, presumably mediated by a SNARE complex involving syntaxin-5, which interacts with GM130 (18). The resulting membrane rearrangement might then cause GRASP65 disassembly because it would impart a torque on the complex due to the orientation conferred by the dual anchors; that is, membrane rearrangement would force an unfavorable cis configuration leading to partner dissociation. The relatively weak PDZ interaction (19) lends itself to this novel type of regulation in which protein/protein interaction would be regulated by membrane dynamics. Another noteworthy feature of this model is that localization of the tether and tether activity are linked because both stable localization and activity require dual anchoring. This elegantly ensures that all GRASP65 molecules on the Golgi are dually anchored so that cis interactions do not interfere with trans pairing and membrane tethering.
FIGURE 9.
Model of trans pair disassembly by membrane rearrangement upon fusion. Note that the interaction interface is oriented by the combined myristic acid and golgin attachment of the GRASP domain in such a way that it prevents cis interactions before and after fusion.
Orientation may be a key regulator of many tethering reactions. In the case of tethering of endosome membranes by EEA1, Rab5 and phosphatidylinositol binding to the EEA1 C terminus anchors it to the membrane orienting its coiled-coil domain to project outwards, perpendicular to the membrane (20). This positions an array of binding sites away from interactions that might occur in cis thereby promoting association with other endosomal membranes. In an analogous fashion, coiled-coil tethers containing the GRIP domain are likely oriented perpendicular to the membrane by the GRIP interaction with the Arl1 GTPases that mediate their membrane association on the trans Golgi network (21). Whether these orientation features are required and whether membrane rearrangement upon fusion plays a role in complex disruption remain to be investigated. In these examples, membrane association is regulated by GTPases and is highly dynamic. In the case of GRASP65 tethering, however, it does not appear to be regulated by cycles of membrane recruitment and detachment, so aspects of its disassembly in cis may be unique.
The GRASP65 paralog, GRASP55, is thought to act in a fashion similar to GRASP65 in tethering membranes of the medial and trans Golgi (4). Their GRASP domains share many similarities at the sequence level, with 69% identity and 78% of the changes being of a conservative nature. Similar to GRASP65, the N terminus of GRASP55 is myristoylated (22), and this modification is required for its tethering activity.3 Further, paralleling GRASP65 binding to GM130, GRASP55 binds the C terminus of golgin-45 (23), which has a conserved PDZ ligand motif. Interestingly, GRASP55 appears to be in two separate complexes within the cell, only one of which contains golgin-45 (16). The other contains transiting molecules including secretory cargo with C-terminal PDZ ligand motifs, and at least some of these bind GRASP65 as well (16, 17, 22). The similarity of the ligand motifs to those of GM130 and golgin-45 suggests that they bind the GRASP PDZ2 domain leaving PDZ1 available for tethering. This raises the interesting possibility that cargo functions interchangeably with golgins in anchoring GRASP proteins. GRASP55 is also palmitoylated (22), so, unlike GRASP65, it may be able to remain active during transient absence of either golgin or cargo binding due to the dual myristic and palmitic acid contacts.
A final consideration is whether dual anchoring of the GRASP domain is critical for GRASP-mediated cisternal stacking (24–26) or nonconventional secretion (27, 28). In the case of stacking, this seems likely because the current model invokes homotypic interactions in trans even though the linkage is formed between distinct Golgi subcompartments. Thus, cis interactions need to be prevented, and an orientation of the homotypic binding interface that is dependent on dual anchoring provides a simple solution. One possibility that warrants further investigation is whether GRASP·golgin complexes mediate ribbon formation whereas GRASP·cargo complexes contribute to cisternal stacking due perhaps to localization of the former complexes to Golgi rims and the latter to compact zones. In the case of the participation of the GRASP homologs in Dictyostelium and Drosophila in nonconventional secretion it remains unclear whether the relevant activity depends on PDZ interactions or membrane cross-bridging. Nevertheless, from an evolutionary perspective, the GRASP family of proteins may have developed first as cargo transport factors, then as participants in stacking, and finally, with the emergence of a second GRASP protein, as mediators of ribbon formation. In any case, our study further elucidates the basis of the GM130/GRASP65 interaction and shows its importance, along with myristoylation, in the favoring of trans interactions, which in conjunction with pericentrosomal positioning, allow for the formation of a single copy Golgi organelle with an extended ribbon structure.
Acknowledgments
We thank Tina Lee, Debrup Sengupta, and Smita Yadav for comments on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant GM-56779 (to A. D. L.).
C. Bachert and A. D. Linstedt, unpublished results.
- SNARE
- soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- GFP
- green fluorescent protein.
REFERENCES
- 1.Waters M. G., Pfeffer S. R. (1999) Curr. Opin. Cell Biol. 11, 453–459 [DOI] [PubMed] [Google Scholar]
- 2.Whiteheart S. W., Schraw T., Matveeva E. A. (2001) Int. Rev. Cytol. 207, 71–112 [DOI] [PubMed] [Google Scholar]
- 3.Puthenveedu M. A., Bachert C., Puri S., Lanni F., Linstedt A. D. (2006) Nat. Cell Biol. 8, 238–248 [DOI] [PubMed] [Google Scholar]
- 4.Feinstein T. N., Linstedt A. D. (2008) Mol. Biol. Cell 19, 2696–2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Satoh A., Warren G. (2005) J. Biol. Chem. 280, 4921–4928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sengupta D., Truschel S., Bachert C., Linstedt A. D. (2009) J. Cell Biol. 186, 41–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barr F. A., Nakamura N., Warren G. (1998) EMBO J. 17, 3258–3268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris B. Z., Lim W. A. (2001) J. Cell Sci. 114, 3219–3231 [DOI] [PubMed] [Google Scholar]
- 9.Hung A. Y., Sheng M. (2002) J. Biol. Chem. 277, 5699–5702 [DOI] [PubMed] [Google Scholar]
- 10.Murwantoko, Yano M., Ueta Y., Murasaki A., Kanda H., Oka C., Kawaichi M. (2004) Biochem. J. 381, 895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Runyon S. T., Zhang Y., Appleton B. A., Sazinsky S. L., Wu P., Pan B., Wiesmann C., Skelton N. J., Sidhu S. S. (2007) Protein Sci 16, 2454–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jesch S. A., Linstedt A. D. (1998) Mol. Biol. Cell 9, 623–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Y., Punj V., Sengupta D., Linstedt A. D. (2008) Mol. Biol. Cell 19, 2830–2843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harlow E., Lane D. (1988) Antibodies: A Laboratory Manual, pp. 522–523, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 15.Jeleñ F., Oleksy A., Smietana K., Otlewski J. (2003) Acta Biochim. Pol. 50, 985–1017 [PubMed] [Google Scholar]
- 16.Barr F. A., Preisinger C., Kopajtich R., Körner R. (2001) J. Cell Biol. 155, 885–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Angelo G., Prencipe L., Iodice L., Beznoussenko G., Savarese M., Marra P., Di Tullio G., Martire G., De Matteis M. A., Bonatti S. (2009) J. Biol. Chem. 284, 34849–34860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diao A., Frost L., Morohashi Y., Lowe M. (2008) J. Biol. Chem. 283, 6957–6967 [DOI] [PubMed] [Google Scholar]
- 19.Nourry C., Grant S. G., Borg J. P. (2003) Sci. STKE 2003, RE7. [DOI] [PubMed] [Google Scholar]
- 20.Dumas J. J., Merithew E., Sudharshan E., Rajamani D., Hayes S., Lawe D., Corvera S., Lambright D. G. (2001) Mol. Cell 8, 947–958 [DOI] [PubMed] [Google Scholar]
- 21.Panic B., Perisic O., Veprintsev D. B., Williams R. L., Munro S. (2003) Mol. Cell 12, 863–874 [DOI] [PubMed] [Google Scholar]
- 22.Kuo A., Zhong C., Lane W. S., Derynck R. (2000) EMBO J. 19, 6427–6439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Short B., Preisinger C., Körner R., Kopajtich R., Byron O., Barr F. A. (2001) J. Cell Biol. 155, 877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barr F. A., Puype M., Vandekerckhove J., Warren G. (1997) Cell 91, 253–262 [DOI] [PubMed] [Google Scholar]
- 25.Shorter J., Watson R., Giannakou M. E., Clarke M., Warren G., Barr F. A. (1999) EMBO J. 18, 4949–4960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiang Y., Wang Y. (2010) J. Cell Biol. 188, 237–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinseth M. A., Anjard C., Fuller D., Guizzunti G., Loomis W. F., Malhotra V. (2007) Cell 130, 524–534 [DOI] [PubMed] [Google Scholar]
- 28.Schotman H., Karhinen L., Rabouille C. (2008) Dev. Cell 14, 171–182 [DOI] [PubMed] [Google Scholar]