Abstract
Particulate matter (PM) has been the primary focus of studies aiming to understand the relationship between the chemical properties of ambient aerosols and adverse health effects. Size and chemical composition of PM have been linked to their oxidative capacity which has been postulated to promote or exacerbate pulmonary and cardiovascular diseases. But in the last few years, new studies have suggested that volatile and semivolatile components may also contribute to many adverse health effects. The objectives of this study were: i) assess for the first time the redox and electrophilic potential of vapor-phase components of ambient aerosols, and ii) evaluate the relative contributions of particle- and vapor-fractions to the hazard of a given aerosol. To achieve these objectives vapor- and particle-phase samples collected in Riverside (CA) were subjected to three chemical assays to determine their redox and electrophilic capacities. The results indicate that redox active components are mainly associated with the particle-phase, while electrophilic compounds are found primarily in the vapor-phase. Vapor-phase organic extracts were also capable of inducing the stress responding protein, heme-oxygenase-1 (HO-1), in RAW264.7 murine macrophages. These results demonstrate the importance of volatile components in the overall oxidative and electrophilic capacity of aerosols, and point out the need for inclusion of vapors in future health and risk assessment studies.
Keywords: redox toxicity, Fenton chemistry, electrophilic activity, Heme-oxygenase 1, ambient aerosols
INTRODUCTION
Although exposure to ambient air pollutants includes chemicals present in both the vapor- and particle-phases, most health assessment studies have focused on ambient particulate matter (PM) and diesel exhaust particles (DEP) (Brauer et al. 2002; Gottipolu et al. 2008; Hoek et al. 2002; Klemm et al. 2004; Mauderly and Chow 2008; Naeher et al. 2007). Health effects observed after exposure to these pollutants include among others exacerbation of asthma and cardiovascular diseases (Brauner et al. 2007; Castorena-Torres et al. 2008; De Vizcaya-Ruiz et al. 2006; Pereira et al. 2007; Xia et al. 2004).
Recently, several studies have indicated that volatile (VOCs) and semivolatile (SVOCs) organic compounds may also be involved in diverse health effects (Arif and Shah 2007; Boeglin et al. 2006; Rumchev et al. 2004). For example, ambient levels of VOCs have been correlated with the frequency of hospital visits due to ischemic heart disease and myocardial infarctions (Klemm et al. 2004; Tolbert et al. 2001). In a study evaluating the cardiovascular effects of highway aerosols in rats, a decrease in heart rate was associated with the vapor phase components and not the particles (Elder et al. 2007). A similar study conducted by Lund and collaborators (Lund et al. 2007) showed that the effects of exposure to gasoline emissions on oxidative stress and pro-atherosclerotic tissue changes in aorta of mice were not reduced when PM was removed from the ambient air using high efficiency particulate air (HEPA) filters. In addition, previous laboratory studies demonstrated that the toxic potency of freshly emitted SVOCs was higher than that of the emitted PM (Seagrave et al. 2001; Seagrave et al. 2003).
The ability of aerosols to induce a state of cellular oxidative stress has been suggested as a common pathway leading to these adverse health effects (Balakrishna et al. 2009; Blanchet et al. 2004; Delfino et al. 2005; Donaldson et al. 2003; MacNee and Donaldson 2003). Oxidative stress is typically caused by reactive oxygen species (ROS) such as hydrogen peroxide and hydroxyl radical, and electrophiles such as α,β-unsaturated carbonyls. In this context, the chemical components of both vapors and particles are key players. Transition metals present in PM are capable of generating ROS through the Fenton reaction, and concentrations of iron and copper have been correlated with the ability of ambient PM and DEP to induce a state of oxidative stress (DiStefano et al. 2009; Gottipolu et al. 2008; Ohyama et al. 2007; Shinyashiki et al. 2009). Besides the contribution of transition metals, organic components of PM and ambient air have been shown to induce oxidative stress. The organic content of PM, in particular polycyclic aromatic hydrocarbons and quinones, has also been correlated with the ability of the particles to induce oxidative stress and inflammatory responses by the respiratory system (Chung et al. 2006; Hiyoshi et al. 2005; Inoue K et al. 2007; Inoue KI et al. 2007; Li et al. 2003). More recent studies have also found that ambient PM and DEP contain compounds that can inactivate thiol proteins through covalent bonding (Iwamoto et al. 2007; Rodriguez et al. 2005; Shinyashiki et al. 2008).
The main purpose of this study was to assess, for the first time, the redox and electrophilic potential of vapor-phase components of aerosols, and to evaluate the relative contributions of particle- and vapor-fractions to the overall redox potential and electrophilic content of ambient air. To achieve these goals three different chemical assays were used to: the dithiothreitol (DTT) assay, which measures the ability of the sample to generate ROS; the dihydroxybenzoic acid (DHBA) assay, which assesses the capacity of the sample to catalyze the Fenton reaction; and the glyceraldehyde-3-phosphate dehydrogenase (GADPH) assay, which measures electrophilic activity through thiol protein inactivation. We also conducted an in-vitro assay to measure the ability of the vapor-phase extract to induce the stress protein hemeoxygenase-1 (HO-1) in macrophages.
MATERIALS AND METHODS
Sample Collection
Samples were collected at the Riverside Agricultural Center (CA) using PM2.5 medium-vol samplers (Tisch Environmental Model 1202, Cleves, OH) during April-May and October–December 2007, and June–August 2008 (Table 1). PM2.5 was collected on Teflon coated glass fiber filters (Pall Corp., East Hills, NY) and vapors in XAD-4 resin (Acros, Thermo Fisher Scientific). Two parallel instruments were run at 113 l/min for 6 hours/day over 6-day period. Samples were collected from 7:00 am to 1:00pm parallel to animal exposure studies. Sampling details and matrix cleaning procedures have been previously published (Eiguren-Fernandez et al. 2004). During the last week of October 2007, Southern California had a significant fire, which emissions impacted the Riverside are were samples were been collected.
Table 1.
Sampling information and total sample volume (m3)
| Sample ID | Sampling dates | Total volume (m3) |
|---|---|---|
| RIV041607 | April 16–24, 2007 | 399 |
| RIV042507 | April 25 – May 3, 2007 | 423 |
| RIV050707 | May 7–16, 2007 | 415 |
| RIV102507 | Oct 25 – Nov 8, 2007 | 578 |
| RIV111307 | Nov 11–21, 2007 | 339 |
| RIV112607 | Nov 26 – Dec 18, 2007 | 808 |
| RIV062608 | Jun 26 –Jul 3, 2008 | 339 |
| RIV070709 | Jul 7–15, 2008 | 401 |
| RIV072108 | Jul 21–29, 2008 | 400 |
| RIV811108 | Aug 11–19, 2008 | 402 |
As samples were collected during several days, and although they were kept in the freezer between sample collection periods, it is important to acknowledge that sampling artifacts may have occurred. As teflon coated filters where used for the sample collection no positive artifacts were expected. To minimize losses due to negative artifacts (volatilization of organic compounds associated with the collected PM) a double filter system was used. However, the volatilized organic carbon is not always recaptured by the backup filter; if this was the case volatiles would have been captured by the downstream XAD bed.
Sample extraction
Vapor-phase samples collected in parallel were pooled and extracted by sonication (30 min) with dichloromethane (DCM) (Fisher Scientific, PA). The suspension was filtered through a 0.45um nylon filter (Millipore, Billerica, Massachusetts), volume reduced, and solvent exchanged to dimethyl sulfoxide (DMSO) (Fisher Scientific, PA). Final concentration of the organic extract was ~ 250 m3/mL. Aliquots of the DMSO solution were used for posterior chemical and toxicological analyses. Blank XAD was prepared as described previously and used as control. PM2.5 samples were extracted by two different methods depending on the study: i) by sonicating filter punches in water and obtaining water suspensions of particles; and ii) extracting filter punches with DCM followed by filtration through 0.45 µm nylon filter to obtain an organic extract, which was solvent exchanged to DMSO prior to the assay. The chemical assays were conducted using aliquots of the suspension and organic extract. Highly polar substances associated with the vapor phase would not be included in the analysis as the XAD resin bed as some of these species would not be extracted with DCM.
DTT assay
This method assesses the redox activity of the sample based on its ability to transfer electrons from dithiothreitol (DTT) to oxygen (Cho et al. 2005; Kumagai et al. 2002). In this assay, aliquots of vapor-phase and PM2.5 organic extracts and PM2.5 water suspensions were incubated with DTT (Sigma Chemical Co., MO) for times varying from 10 to 30 minutes. The reaction was quenched at specific times and, after addition of 5,5’-dithiobisbis-(2-dinitro)benzoic acid (DTNB) (Sigma Chemical Co., MO) to complex with the remaining DTT, the absorption at 412 nm measured. Rates are calculated averaging duplicate runs, and are blank corrected.
Since DTT can be oxidized by high concentrations of metal ions (Kachur et al. 1997; Netto and Stadtman 1996), the contribution of metals to the DTT-based redox activity was also determined adding the metal chelator diethylenetriaminepentaacetic acid (DTPA) (10 µM) to one set of the samples
DHBA assay
This analytical procedure was developed to quantify metal-based redox activity by measuring the ability of transition metals associated with the ambient particles to catalyze the Fenton reaction in which hydrogen peroxide is converted to hydroxyl radical (DiStefano et al. 2009). Briefly, aliquots of PM2.5 water suspensions were incubated with ascorbic acid and salicylate (Sigma Chemical Co., MO) for times varying from 15 to 45 minutes. At each time point, the reaction was quenched by the addition of metaphosphoric acid (Sigma Chemical Co., MO). A second set of tubes containing the metal chelator, DTPA was included in each condition to evaluate the participation by metal ions such as iron and copper in the reaction. Concentrations of 2, 3- and 2, 5-DHBA were then determined by high performance liquid chromatography-electrochemical detector. All samples are run in duplicate and blank corrected.
The DHBA assay was performed only on the aqueous suspensions of filters as the DCM extracts would be devoid of metals.
GAPDH assay
This assay measures the content of electrophiles in the sample, based on their ability to inhibit or inactivate the thiolate enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH), through covalent bonding. Inhibition of GAPDH by vapor and PM2.5 samples was determined under anaerobic conditions according to the method described previously (Shinyashiki et al. 2008). In brief, chicken GAPDH (Sigma Chemical Co., MO) or 1 unit of rabbit GAPDH was incubated with aliquots of the organic extracts of vapors and particles or water suspension under argon gas at 25°C for 120 minutes. At this time point the reaction was quenched by adding an equal volume of cold DTT solution, and GAPDH activity, measured as rate of nicotinamide adenine dinucleotide (NADH) formation, was monitored by its absorption at 340 nm. Samples were run in triplicate and values reported as averages.
Although the assay is limited to those structures capable of interacting with the catalytic center of the enzyme, it provides a quantitative measure of the electrophilic content which can be used in comparison studies.
HO-1 induction
RAW264.7 cells (2 × 106/well in 6-well plate) were cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 10% serum overnight and exposed to XAD extracts (0.7 m3/well/2 ml) for 6 and 18 hours. Cells were lysed in lysis buffer consisting of 10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 2 mM EDTA, 8 mM EGTA, 1% Protease Inhibitor III (Calbiochem) and protein concentrations were measured by the DC protein assay kit (Bio-Rad) with bovine serum albumin as a standard.
HO-1 expression was assayed by Western blot analysis according to the method previously described (Shinyashiki et al. 1996). Thus, total lysate protein (25 µg) was separated on SDS-PAGE (12%) and transferred to PVDF membrane. HO-1 was probed with anti-HO-1 polyclonal antibody (Stressgen), followed by anti-rabbit immunoglobulin G (IgG) polyclonal antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology). HO-1 bands were detected with ECL plus kit (GE Healthcare). The membranes were re-probed with anti-β-actin rabbit polyclonal antibody and used for loading controls. The bands were scanned and representative data are shown. The density of HO-1 and β-actin bands was analyzed by image analyzing program, Image J64 (NIH). The average ratio HO-1/β-actin of three independent experiments was plotted as fold-changes versus blank XAD extracts.
RESULTS AND DISCUSSION
Oxidative capacity of PM2.5 and vapors
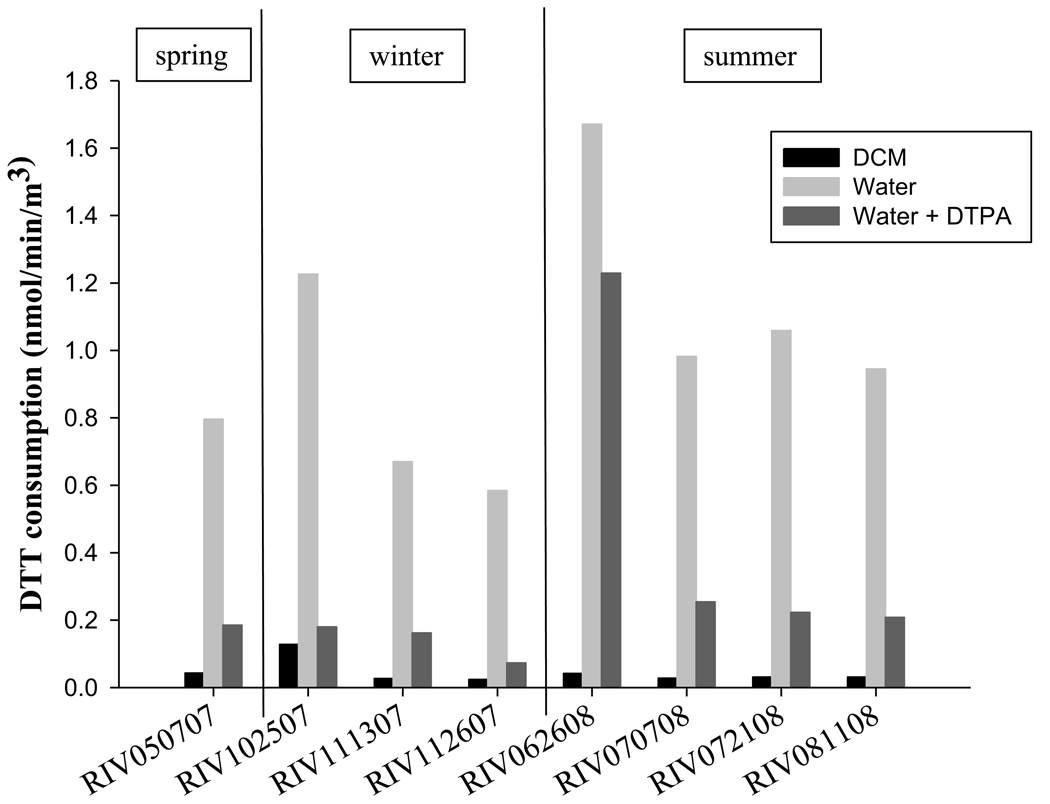
The DTT based redox activity of the PM2.5 samples varied with the different preparations (Figure 1). Aqueous suspensions of the filters were 10 to 40 times more active than the organic extracts, suggesting that redox active organic compounds or metals associated with the particles and not extracted by DCM, have a significant contribution to the overall activity. The metal contribution was assessed by adding DTPA, and even though the DTT activity was significantly decreased by addition of the metal chelator, about 50% remained, indicating that other water soluble or particle bound species also contributed to overall redox activity of the particles.
Figure 1.
Redox activity of PM2.5 measured as DTT consumption rate (nmol/min/m3) for organic extract (DCM), water suspension, and water suspension with metal chelator (DTPA)
Another factor to be considered, and whose contribution has not been assessed, is the possibility of physical interactions occurring between particle surface and DTT. A recent study conducted with DEP samples has shown that the physical presence of particles in the water suspension contribute significantly to the total redox activity of the DEP (Shinyashiki et al. 2009). Although the physical properties of DEP and Riverside PM2.5 may be different, these interactions may be important when assessing the individual contributions of the physical and chemical properties of PM2.5 to the overall toxicity.
A seasonal effect was observed on the redox capacity of the PM2.5; summer samples were in general more active than the winter samples although the differences were dependent of the extraction method. Summer samples were 20% and 50% higher than winter samples for the DCM extracts and water suspensions respectively. In addition, the activity due to non-metals in the water suspension was 75% higher in summer, suggesting that the organic chemicals present in the sample were more active during the summer season. These seasonal differences may reflect contributions from secondary organic aerosols, formed by photochemical reactions, to the redox activity of the PM2.5 samples. A recent study conducted in the Los Angeles Basin has shown that ambient concentrations of phenanthraquinone (PQ), a highly redox active compound, increase as the air parcel moves inland towards Riverside due to photochemical activity during transport (Eiguren-Fernandez et al. 2008). The increase in concentrations of compounds such as PQ may explain the higher activity observed for the summer PM2.5 samples.
Vapor-phase associated redox activity was much lower than that observed for the aqueous suspensions of PM2.5, but was much greater than the DCM extract of PM2.5 (Table 2), indicating that levels of redox active organic compounds are higher in the vapor phase. DTT activity was very similar over the entire sampling interval, which included both summer and winter seasons. The DTT values averaged 0.14 (± 0.02 SEM) nmol/min/ m3, suggesting that ambient levels of chemical components contributing to the activity were very similar. This observation is consistent with previous studies which have shown that vapor-phase levels of quinones, the likely contributors to vapor-phase redox activity, do not significantly vary with season (Eiguren-Fernandez et al. 2007; Eiguren-Fernandez et al. 2004).
Table 2.
DTT activity (nmol/min/m3) (95% CI) for all samples and each extraction method.
| PM activity | Vapor-phase | |
|---|---|---|
| Sample ID | Non-metal activity | DCM extract |
| RIV041607 | n.a. | 0.155 (0.118–0.192) |
| RIV042507 | n.a. | 0.160 (0.114–0.206) |
| RIV050707 | 0.185 (0.137–0.233) | n.a. |
| RIV102507 | 0.180 (0.055–0.305) | 0.159 (0.109–0.209) |
| RIV111307 | 0.162 (0.092–0.231) | 0.147 (0.126–0.168) |
| RIV112607 | 0.073 (0.019–0.127) | 0.106 (0.076–0.136) |
| RIV062608 | 1.229 (1.096–1.362) | 0.125 (0.100–0.150) |
| RIV070708 | 0.254 (0.198–0.310) | 0.140 (0.119–0.161) |
| RIV072108 | 0.223 (0.175–0.271) | 0.153 (0.131–0.175) |
| RIV081108 | 0.208 (0.143–0.273) | 0.116 (0.058–0.175) |
n.a.: sample was not available for analysis
Overall, redox activity was highest in the aqueous suspension of the PM2.5 fraction than the DCM extracts.
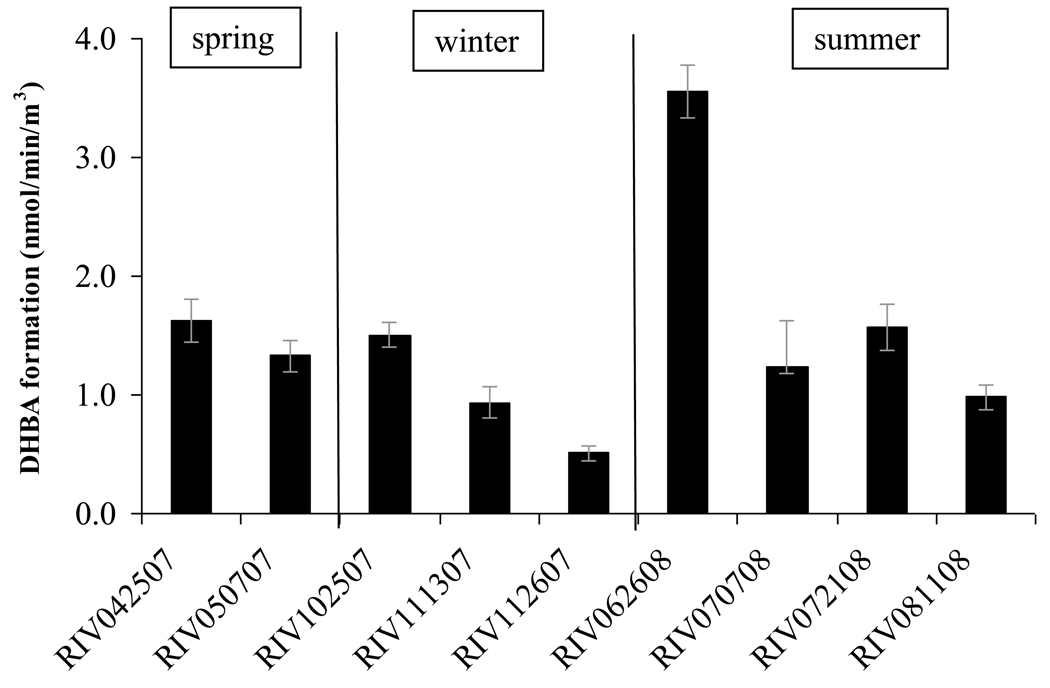
Fenton reaction catalysis
All samples collected in this study exhibited Fenton reaction capabilities with DHBA formation rates varying from 0.51 to 3.56 nmol/min/m3 (Figure 2). However, the sample collected for the period of June 26-July 3, 2008, was unusually high. Previous studies conducted with ambient ultrafine particles and DEPs have shown correlation between copper concentration in PM and the ability of the particles to generate hydroxyl radical (Di Stefano el al., 2009). Other metals such as iron also participate but their relative activities vary considerably (Halliwell and Gutteridge 2007). Regardless of the specific metal, Fenton based redox activity is an important contributor to the ability of PM2.5 to generate oxidative stress.
Figure 2.
Ability of water suspensions of PM2.5 to generate hydroxyl radical measured as DHBA formation rate (± 95% CI) (nmol/min/m3)
Protein inhibition: presence of electrophiles
In addition to the ability of PM to induce cellular stress by generating ROS, aerosols contain several electrophiles which can react with cellular components, initiating a second general pathway for toxicity. In this case, organic species containing an electrophilic center can react with nucleophilic centers in metabolic proteins such as thiols and amino groups to form covalent bonds. This reaction can lead to glutathione depletion and protein inhibition, with their recovery depending on protein synthesis (Guengerich 2005; LoPachin and DeCaprio 2005; Rachakonda et al. 2008; Waidyanatha et al. 2002).
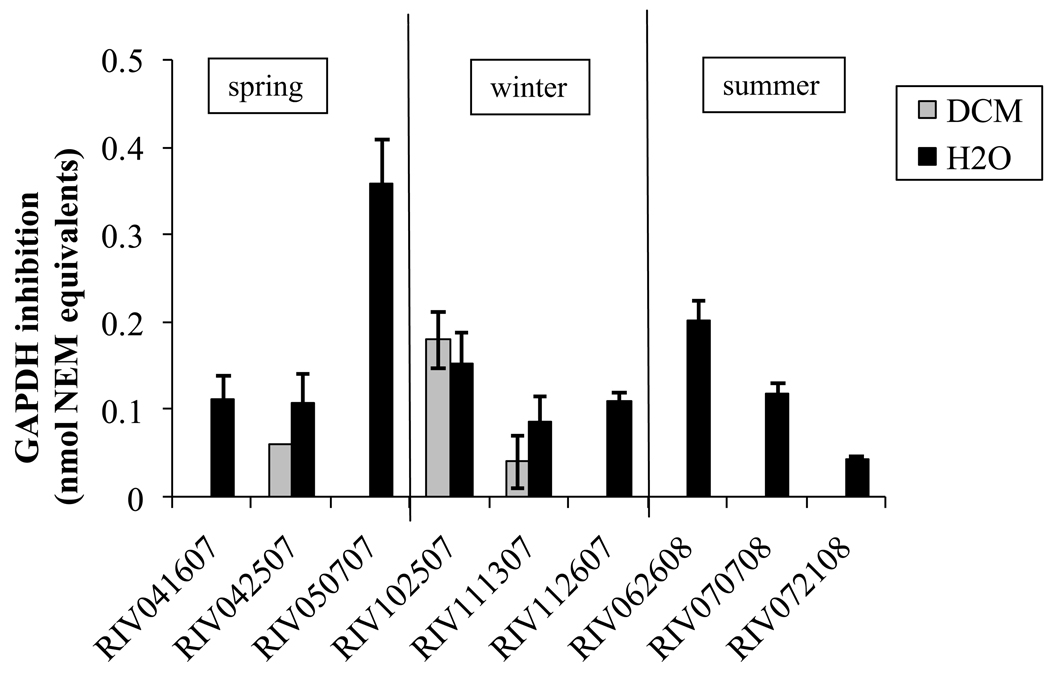
In this study PM2.5 water suspensions showed significantly higher electrophile content in than DCM extracts (Figure 3), indicating that the electrophiles in the particle-phase are more soluble in water than in organic solvents.
Figure 3.
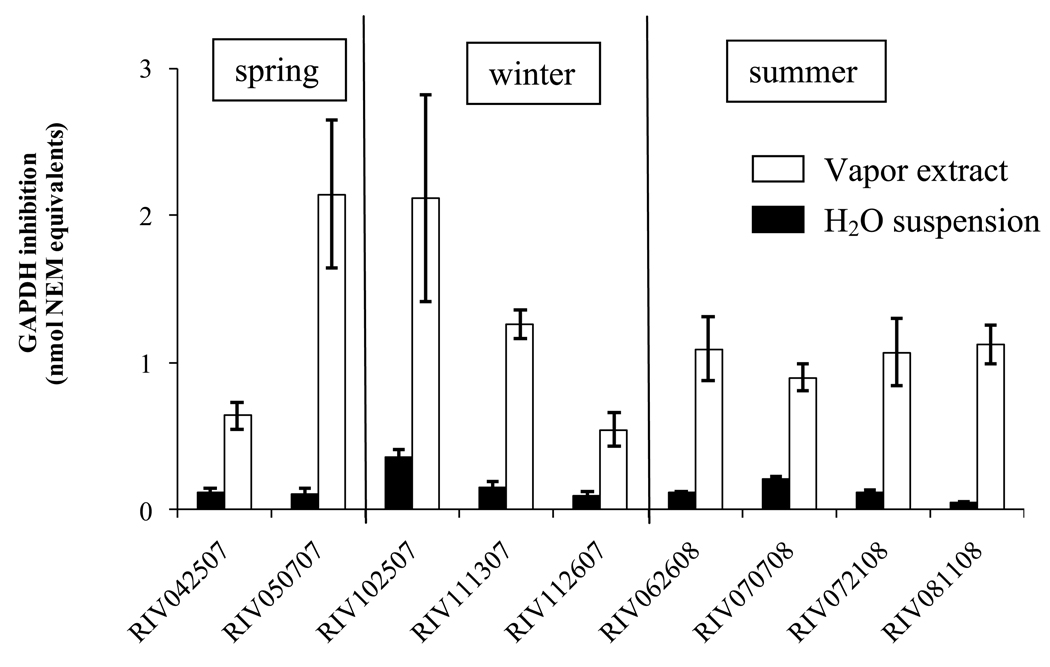
GAPDH inhibition in NEM equivalent (±SE) by electrophilic compounds present in water suspensions and organic extract of PM2.5
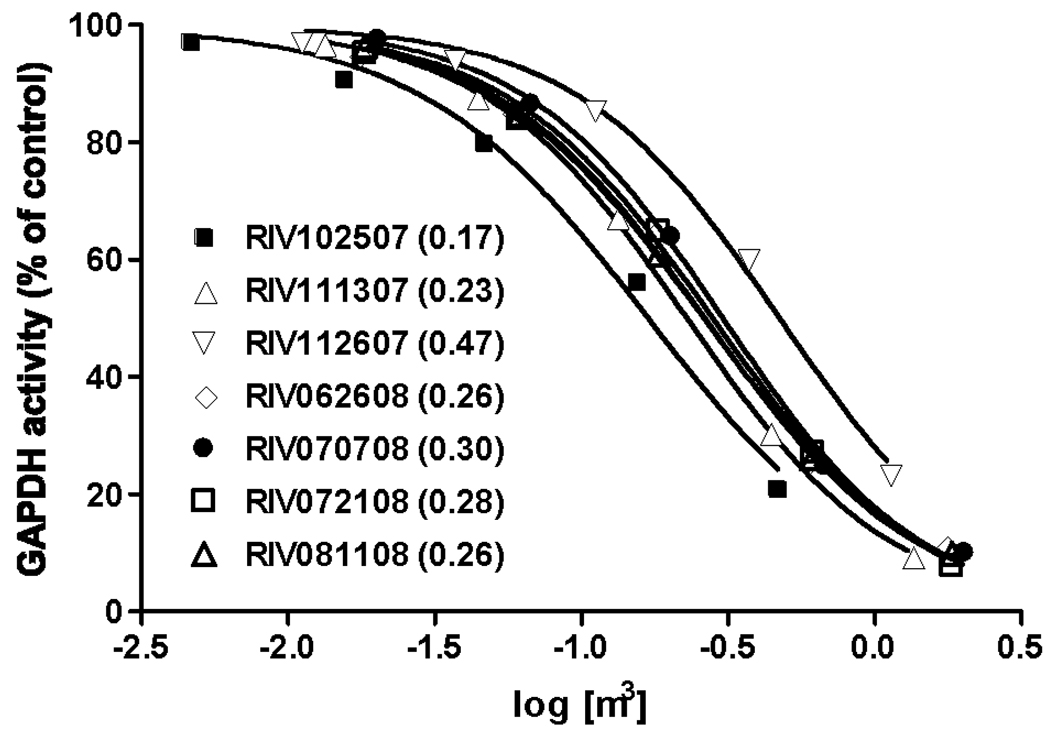
As vapor-phase samples have not been previously studied, the interactions of 7 vapor-phase samples with GAPDH were examined in a concentration-inactivation study. The results indicated that concentrations equivalent to 0.26 m3 of air, on average, were capable of inducing a 50% inhibition of the GAPDH activity under the conditions of the experiment (Figure 4). Results of the application of the general electrophilic assay to all samples are shown in figure 5. Electrophilic species associated with the vapor-phase, had on average, 10 times higher inhibitory capacity than that of the corresponding PM2.5 samples. These results suggest that most of the ambient compounds with electrophilic capacity are associated with the vapor-phase.
Figure 4.
Dose-response curves and EC50 values calculated for organic extract of seven vaporphase samples. EC50 values are shown in parenthesis.
Figure 5.
Comparison between electrophilic activity of vapor-phase components and water suspension of PM2.5 based on their ability to inhibit GAPDH (NEM equivalents)
HO-1 induction
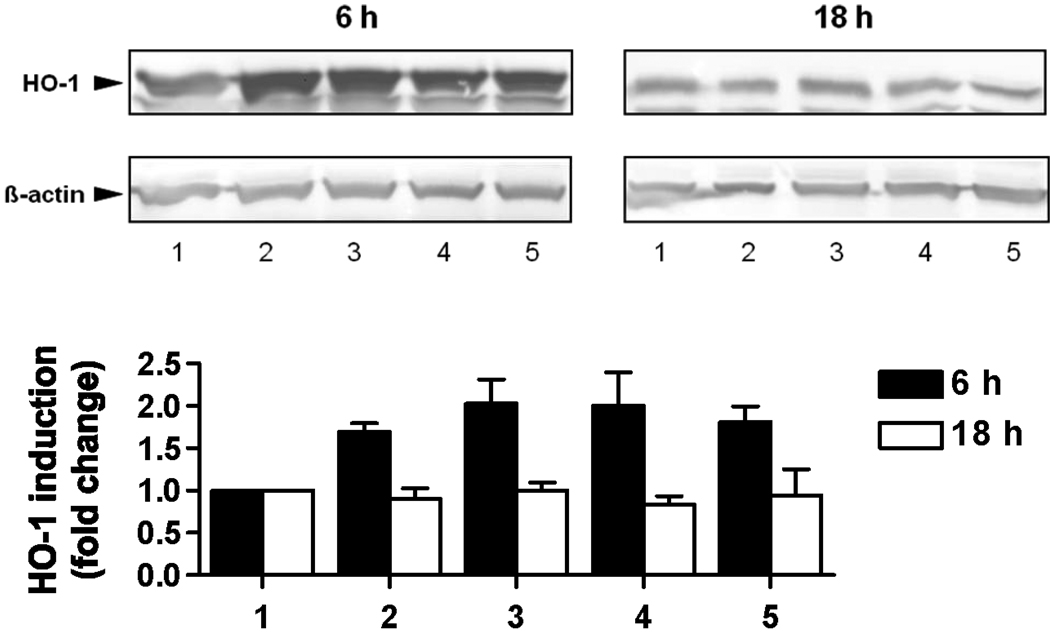
Several studies have linked the induction of the stress protein HO-1 to the chemical content of PM (Chin et al. 2003; Duvall et al. 2006; Roberts et al. 2007), but not to vapor-phase organic compounds. To further characterize the vapor-phase samples collected in this study, a murine macrophage cell line (RAW264.7) was exposed to vapor-phase organic extracts at concentrations equivalent to 0.7 m3 per 2mL DMEM and HO-1 induction assessed after 6 and 18 hrs of incubation. After six hours of exposure a 2-fold increase in HO-1 expression was observed for all samples over the control sample (Figure 6). HO-1 levels returned to control levels at 18 hours, suggesting a time-dependent response of cells to the presence of redox active compounds. Previous studies conducted with different PM fractions reported 16 hrs exposure as the optimum for HO-1 induction (Li et al. 2003) so the time course of the response to vapor-phase components appears to be different.
Figure 6.
HO-1 induction by vapor-phase organic extracts. RAW264.7 cells were incubated with DCM extracts (0.35 m3/mL) for 6 and 18 hours and HO-1 expression assayed by Western blotting. Three independent experiments were performed and a representative blot (A) and average of fold changes in HO-1/β -actin ratio (B) are shown.
1. Blank XAD; 2. RIV062608; 3. RIV070708; 4. RIV072108; 5. RIV081108
CONCLUSIONS
Toxicological properties of ambient PM have been studied extensively over the last decade, and only few studies have reported the effects of chemicals associated with the vapor-phase on the overall toxicity of ambient aerosols. The results of this study, in which a comparison of the chemical properties of particles and vapor components relevant to toxicity were examined, indicated that the overall redox activity was concentrated in the particle-phase, reflecting in part, the presence of metals, whereas the electrophiles, which are organic molecules, appear to be mostly volatile.
As a result, the toxicokinetics of reactive species associated with the two fractions can elicit different responses in spite of having similar chemical reaction capabilities. The differences in distribution of redox activity and electrophiles between the two phases, together with the different toxicokinetics of the two phases suggest that the effects of exposure to particles and vapors can be very different. For this reason assessing the relative activities of each phase is critical for more comprehensive exposure assessment studies. Therefore, future health and risk assessment studies should include both particles and vapors when assessing the ability of ambient aerosols to exert toxicological events.
Acknowledgements
The authors thank Dr. Michael T. Kleinman and Glenn Gookin from UC Riverside for sample collection.
FUNDING SOURCES
This work was supported in part by the U.S. Environmental Protection Agency-funded Southern California Center for Airborne Particulate Matter (Grant RD-83241301), and award number 040623 for Asthma Consortium, and National Institutes of Environmental Health Sciences funded Southern California Environmental Health Science Center (Grant 5P30 ES07048). Although the research described in this article has been funded wholly or in part by the United States Environmental Protection Agency, it has not been subjected to the Agency’s required peer and policy review and therefore does not necessarily reflect the views of the Agency. No official endorsement should be inferred.
Abbreviations
- PM
particulate matter
- DEP
diesel exhaust particles
- VOCs
volatile organic compounds
- SVOCs
semi-volatile organic compounds
- ROS
reactive oxygen species
- PAHs
polycyclic aromatic hydrocarbons
- DCM
dichloromethane
- DMSO
dimethyl sulfoxide
- DTT
dithiothreitol
- DTPA
Diethylenetriaminepentaacetic acid
- DHBA
dihydroxybenzoic acid
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HO-1
heme-oxygenase 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arif AA, Shah SM. Association between personal exposure to volatile organic compounds and asthma among US adult population. International Archives of Occupational and Environmental Health. 2007;80:711–719. doi: 10.1007/s00420-007-0183-2. [DOI] [PubMed] [Google Scholar]
- Balakrishna S, Lomnicki S, McAvey KM, Cole RB, Dellinger B, Cormier SA. Environmentally persistent free radicals amplify ultrafine particle mediated cellular oxidative stress and cytotoxicity. Particle and Fibre Toxicology. 2009:6. doi: 10.1186/1743-8977-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchet S, Ramgolam K, Baulig A, Marano F, Baeza-Squiban A. Fine particulate matter induces amphiregulin secretion by bronchial epithelial cells. American Journal of Respiratory Cell and Molecular Biology. 2004;30:421–427. doi: 10.1165/rcmb.2003-0281RC. [DOI] [PubMed] [Google Scholar]
- Boeglin ML, Wessels D, Henshel D. An investigation of the relationship between air emissions of volatile organic compounds and the incidence of cancer in Indiana counties. Environmental Research. 2006;100:242–254. doi: 10.1016/j.envres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Brauer M, Hoek G, Van Vliet P, Meliefste K, Fischer PH, Wijga A, Koopman LP, Neijens HJ, Gerritsen J, Kerkhof M, Heinrich J, Bellander T, Brunekreef B. Air pollution from traffic and the development of respiratory infections and asthmatic and allergic symptoms in children. American Journal of Respiratory and Critical Care Medicine. 2002;166:1092–1098. doi: 10.1164/rccm.200108-007OC. [DOI] [PubMed] [Google Scholar]
- Brauner EV, Forchhammer L, Moller P, Simonsen J, Glasius M, Wahlin P, Raaschou-Nielsen O, Loft S. Exposure to ultrafine particles from ambient air and oxidative stress-induced DNA damage. Environmental Health Perspectives. 2007;115:1177–1182. doi: 10.1289/ehp.9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castorena-Torres F, de Leon MB, Cisneros B, Zapata-Perez O, Salinas JE, Albores A. Changes in gene expression induced by polycyclic aromatic hydrocarbons in the human cell lines HepG2 and A549. Toxicology in Vitro. 2008;22:411–421. doi: 10.1016/j.tiv.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Chin BY, Trush MA, Choi AMK, Risby TH. Transcriptional regulation of the HO-1 gene in cultured macrophages exposed to model airborne particulate matter. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2003;284:L473–L480. doi: 10.1152/ajplung.00297.2002. [DOI] [PubMed] [Google Scholar]
- Cho AK, Sioutas C, Miguel AH, Kumagai Y, Schmitz DA, Singh M, Eiguren-Fernandez A, Froines JR. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environmental Research. 2005;99:40–47. doi: 10.1016/j.envres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Chung MY, Lazaro RA, Lim D, Jackson J, Lyon J, Rendulic D, Hasson AS. Aerosol-borne quinones and reactive oxygen species generation by particulate matter extracts. Environmental Science & Technology. 2006;40:4880–4886. doi: 10.1021/es0515957. [DOI] [PubMed] [Google Scholar]
- De Vizcaya-Ruiz A, Gutierrez-Castillo ME, Uribe-Ramirez M, Cebrian ME, Mugica-Alvarez V, Sepulveda J, Rosas I, Salinas E, Garcia-Cuellar C, Martinez F, Alfaro-Moreno E, Torres-Flores V, Osornio-Vargas A, Sioutas C, Fine PM, Singh M, Geller MD, Kuhn T, Miguel AH, Eiguren-Fernandez A, Schiestl RH, Reliene R, Froines J. Characterization and in vitro biological effects of concentrated particulate matter from Mexico City. Atmospheric Environment. 2006;40:S583–S592. [Google Scholar]
- Delfino RJ, Sioutas C, Malik S. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environmental Health Perspectives. 2005;113:934–946. doi: 10.1289/ehp.7938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano E, Eiguren-Fernandez A, Delfino RJ, Sioutas C, Froines JR, Cho AK. Determination of metal-based hydroxyl radical generating capacity of ambient and diesel exhaust particles. Inhalation Toxicology. 2009:1–8. doi: 10.1080/08958370802491433. iFirst. [DOI] [PubMed] [Google Scholar]
- Donaldson K, Stone V, Borm PJA, Jimenez LA, Gilmour PS, Schins RPF, Knaapen AM, Rahman I, Faux SP, Brown DM, MacNee W. Oxidative stress and calcium signaling in the adverse effects of environmental particles (PM10) Free Radical Biology and Medicine. 2003;34:1369–1382. doi: 10.1016/s0891-5849(03)00150-3. [DOI] [PubMed] [Google Scholar]
- Duvall R, Norris G, Burke J, McGee J, Gilmour MI, Devlin R. Source apportionment of fine particulate matter in the United States and associations with lung inflammatory markers IL-8, COX-2, and HO-1. Epidemiology. 2006;17:S141–S141. doi: 10.1080/08958370801935117. [DOI] [PubMed] [Google Scholar]
- Eiguren-Fernandez A, Avol EL, Thurairatnam S, Hakami M, Froines JR, Miguel AH. Seasonal influence on vapor- and particle-phase polycyclic aromatic hydrocarbon concentrations in school communities located in Southern California. Aerosol Science and Technology. 2007;41:438–446. [Google Scholar]
- Eiguren-Fernandez A, Miguel AH, Froines JR, Thurairatnam S, Avol EL. Seasonal and spatial variation of polycyclic aromatic hydrocarbons in vapor-phase and PM 2.5 in Southern California urban and rural communities. Aerosol Science and Technology. 2004;38:447–455. [Google Scholar]
- Eiguren-Fernandez A, Miguel AH, Lu R, Purvis K, Grant B, Mayo P, Di Stefano E, Cho AK, Froines J. Atmospheric formation of 9,10-phenanthraquinone in the Los Angeles air basin. Atmospheric Environment. 2008;42:2312–2319. [Google Scholar]
- Elder A, Couderc JP, Gelein R, Eberly S, Cox C, Xia XJ, Zareba W, Hopke P, Watts W, Kittelson D, Frampton M, Utell M, Oberdorster G. Effects of on-road highway aerosol exposures on autonomic responses in aged, spontaneously hypertensive rats. Inhalation Toxicology. 2007;19:1–12. doi: 10.1080/08958370600985735. [DOI] [PubMed] [Google Scholar]
- Gottipolu RR, Landa ER, Schladweiler MC, McGee JK, Ledbetter AD, Richards JH, Wallenborn GJ, Kodavanti UP. Cardiopulmonary responses of intratracheally instilled tire particles and constituent metal components. Inhalation Toxicology. 2008;20:473–484. doi: 10.1080/08958370701858427. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. Gluthione Transferases and Gamma-Glutamyl Transpeptidases. vol 401. 2005. Activation of alkyl halides by glutathione transferases; pp. 342–353. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge J. Free Radicals in Biology and Medicine. Oxford University; 2007. [Google Scholar]
- Hiyoshi K, Takano H, Inoue KI, Ichinose T, Yanagisawa R, Tomura S, Kumagai Y. Effects of phenanthraquinone on allergic airway inflammation in mice. Clinical and Experimental Allergy. 2005;35:1243–1248. doi: 10.1111/j.1365-2222.2005.02297.x. [DOI] [PubMed] [Google Scholar]
- Hoek G, Brunekreef B, Goldbohm S, Fischer P, van den Brandt PA. Association between mortality and indicators of traffic-related air pollution in the Netherlands: a cohort study. Lancet. 2002;360:1203–1209. doi: 10.1016/S0140-6736(02)11280-3. [DOI] [PubMed] [Google Scholar]
- Inoue K, Takano H, Hiyoshi K, Ichinose T, Sadakane K, Yanagisawa R, Tomura S, Kumagai Y. Naphthoquinone enhances antigen-related airway inflammation in mice. European Respiratory Journal. 2007;29:259–267. doi: 10.1183/09031936.00033106. [DOI] [PubMed] [Google Scholar]
- Inoue KI, Takano H, Ichinose T, Tomura S, Yanagisawa R, Sakurai M, Sumi D, Cho AK, Hiyoshi K, Kumagai Y. Effects of naphthoquinone on airway responsiveness in the presence or absence of antigen in mice. Archives of Toxicology. 2007;81:575–581. doi: 10.1007/s00204-007-0186-5. [DOI] [PubMed] [Google Scholar]
- Iwamoto N, Sumi D, Ishii T, Uchida K, Cho AK, Froines JR, Kumagai Y. Chemical knockdown of protein-tyrosine phosphatase 1B by 1,2-naphthoquinone through covalent modification causes persistent transactivation of epidermal growth factor receptor. Journal of Biological Chemistry. 2007;282:33396–33404. doi: 10.1074/jbc.M705224200. [DOI] [PubMed] [Google Scholar]
- Kachur AV, Held KD, Koch CJ, Biaglow JE. Mechanism of production of hydroxyl radicals in the copper-catalyzed oxidation of dithiothreitol. Radiation Research. 1997;147:409–415. [PubMed] [Google Scholar]
- Klemm RJ, Lipfert FW, Wyzga RE, Gust C. Daily mortality and air pollution in Atlanta: Two years of data from ARIES. Inhalation Toxicology. 2004;16:131–141. doi: 10.1080/08958370490443213. [DOI] [PubMed] [Google Scholar]
- Kumagai Y, Koide S, Taguchi K, Endo A, Nakai Y, Yoshikawa T, Shimojo N. Oxidation of proximal protein sulfhydryls by phenanthraquinone, a component of diesel exhaust particles. Chemical Research in Toxicology. 2002;15:483–489. doi: 10.1021/tx0100993. [DOI] [PubMed] [Google Scholar]
- Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang MY, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environmental Health Perspectives. 2003;111:455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoPachin RM, DeCaprio AP. Protein adduct formation as a molecular mechanism in neurotoxicity. Toxicological Sciences. 2005;86:214–225. doi: 10.1093/toxsci/kfi197. [DOI] [PubMed] [Google Scholar]
- Lund AK, Knuckles TL, Lucero J, Seagrave J, McDonald JD, Campen MJ. Exposure to gasoline engine emissions increases vascular reactive oxygen species and activates molecular pathways involved in progression of atherosclerosis. Arteriosclerosis Thrombosis and Vascular Biology. 2007;27:E71–E71. [Google Scholar]
- MacNee W, Donaldson K. Mechanism of lung injury caused by PM10 and ultrafine particles with special referance to COPD. European Respiratory Journal. 2003;21:47S–51S. doi: 10.1183/09031936.03.00403203. [DOI] [PubMed] [Google Scholar]
- Mauderly JL, Chow JC. Health effects of organic aerosols. Inhalation Toxicology. 2008;20:257–288. doi: 10.1080/08958370701866008. [DOI] [PubMed] [Google Scholar]
- Naeher LP, Brauer M, Lipsett M, Zelikoff JT, Simpson CD, Koenig JQ, Smith KR. Woodsmoke health effects: A review. Inhalation Toxicology. 2007;19:67–106. doi: 10.1080/08958370600985875. [DOI] [PubMed] [Google Scholar]
- Netto LES, Stadtman ER. The iron-catalyzed oxidation of dithiothreitol is a biphasic process: Hydrogen peroxide is involved in the initiation of a free radical chain of reactions. Archives of Biochemistry and Biophysics. 1996;333:233–242. doi: 10.1006/abbi.1996.0386. [DOI] [PubMed] [Google Scholar]
- Ohyama M, Otake T, Adachi S, Kobayashi T, Morinaga K. A comparison of the production of reactive oxygen species by suspended particulate matter and diesel exhaust particles with macrophages. Inhalation Toxicology. 2007;19:157–160. doi: 10.1080/08958370701496103. [DOI] [PubMed] [Google Scholar]
- Pereira CEL, Heck TG, Saldiva PHN, Rhoden CR. Ambient particulate air pollution from vehicles promotes lipid peroxidation and inflammatory responses in rat lung. Brazilian Journal of Medical and Biological Research. 2007;40:1353–1359. doi: 10.1590/s0100-879x2006005000164. [DOI] [PubMed] [Google Scholar]
- Rachakonda G, Xiong Y, Sekhar KR, Starner SL, Liebler DC, Freeman ML. Covalent modification at Cys151 dissociates the electrophile sensor Keap1 from the ubiquitin ligase CUL3. Chemical Research in Toxicology. 2008;21:705–710. doi: 10.1021/tx700302s. [DOI] [PubMed] [Google Scholar]
- Roberts ES, Malstrom SE, Dreher KL. In situ pulmonary localization of air pollution particle-induced oxidative stress. Journal of Toxicology and Environmental Health-Part a-Current Issues. 2007;70:1929–1935. doi: 10.1080/15287390701551357. [DOI] [PubMed] [Google Scholar]
- Rodriguez CE, Fukuto JM, Taguchi K, Froines J, Cho AK. The interactions of 9,10-phenanthrenequinone with glyceraldehyde-3-phosphate dehydrogenase (GAPDH), a potential site for toxic actions. Chemico-Biological Interactions. 2005;155:97–110. doi: 10.1016/j.cbi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Rumchev K, Spickett J, Bulsara M, Phillips M, Stick S. Association of domestic exposure to volatile organic compounds with asthma in young children. Thorax. 2004;59:746–751. doi: 10.1136/thx.2003.013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagrave JC, Berger J, Zielinska B, Sagebiel J, Rogers CF, McDonald JD, Mauderly JL. Comparative acute toxicities of particulate matter (PM) and semi-volarile organic compoun (SVOC) fractions of traffic tunnel air. Toxicologist. 2001;60:192. [Google Scholar]
- Seagrave JC, Mauderly JL, Seilkop SK. In vitro relative toxicity screening of combined particulate and semivolatile organic fractions of gasoline and diesel engine emissions. Journal of Toxicology and Environmental Health-Part A. 2003;66:1113–1132. doi: 10.1080/15287390390213881. [DOI] [PubMed] [Google Scholar]
- Shinyashiki M, Eiguren-Fernandez A, Schmitz DA, Di Stefano E, Li N, Linak WP, Cho SH, Froines JR, Cho AK. Electrophilic and redox properties of diesel exhaust particles. Environmental Research. 2009;109:239–244. doi: 10.1016/j.envres.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Shinyashiki M, Kumagai Y, HommaTakeda S, Nagafune J, Takasawa N, Suzuki J, Matsuzaki I, Satoh S, Sagai M, Shimojo N. Selective inhibition of the mouse brain Mn-SOD by methylmercury. Environmental Toxicology and Pharmacology. 1996;2:359–366. doi: 10.1016/s1382-6689(96)00070-1. [DOI] [PubMed] [Google Scholar]
- Shinyashiki M, Rodriguez CE, Di Stefano EW, Sioutas C, Delfino RJ, Kumagai Y, Froines JR, Cho AK. On the interaction between glyceraldehyde-3-phosphate dehydrogenase and airborne particles: Evidence for electrophilic species. Atmospheric Environment. 2008;42:517–529. [Google Scholar]
- Tolbert P, Klein M, Metzger K, Peel J, Flanders WD, Todd K, Mulholland J, Ryan PB, Frumkin H, Team AS. Particulate pollution and cardiorespiratory emergency department visits in Atlanta, August 1998–August 2000 (ARIES/SOPHIA studies) Epidemiology. 2001;12:S54–S54. [Google Scholar]
- Waidyanatha S, Troester MA, Lindstrom AB, Rappaport SM. Measurement of hemoglobin and albumin adducts of naphthalene-1,2-oxide, 1,2-naphthoquinone and 1,4-naphthoquinone after administration of naphthalene to F344 rats. Chemico-Biological Interactions. 2002;141:189–210. doi: 10.1016/s0009-2797(02)00048-0. [DOI] [PubMed] [Google Scholar]
- Xia T, Korge P, Weiss JN, Li N, Venkatesen MI, Sioutas C, Nel A. Quinones and aromatic chemical compounds in particulate matter induce mitochondrial dysfunction: Implications for ultrafine particle toxicity. Environmental Health Perspectives. 2004;112:1347–1358. doi: 10.1289/ehp.7167. [DOI] [PMC free article] [PubMed] [Google Scholar]