Abstract
OBJECTIVE
Intraventricular hemorrhage remains an important problem among very low birth weight infants and may result in long-term neurodevelopmental disabilities. Neonatologists have been unable to accurately predict impending intraventricular hemorrhage. Because alterations in the autonomic nervous system’s control of heart rhythm have been associated with intraventricular hemorrhage after its development, we sought to determine if early subtle alterations of heart rhythm could be predictive of impending intraventricular hemorrhage in very low birth weight infants.
METHODS
This case-control study included 10 newborn very low birth weight infants with intraventricular hemorrhage (5 grade IV, 4 grade III, and 1 grade II) and 14 control infants without intraventricular hemorrhage. Heart rhythm data from the first day of life before the development of intraventricular hemorrhage were evaluated. Detrended fluctuation analysis, a nonlinear fractal heart rate variability method, was used to assess the fractal dynamics of the heart rhythm. Fractal scaling exponents were calculated by using this analysis.
RESULTS
Twenty-four infants (mean ± SD, birth weight: 845 ± 213g: gestational age: 26.1 ± 1.9 weeks) participated in the study. The short-term scaling exponent was significantly larger in infants who later developed intraventricular hemorrhage compared with those who did not (0.60 ± 0.1 vs 0.45 ± 0.1). A value of 0.52 resulted in 70% sensitivity and positive predictive value and 79% specificity and negative predictive value. The short-term scaling exponent was the only significant predictor of intraventricular hemorrhage.
CONCLUSIONS
Fractal dynamics of the heart rhythm is significantly altered in very low birth weight infants before developing intraventricular hemorrhage and may be predictive of impending intraventricular hemorrhage.
Keywords: intraventricular hemorrhage, prematurity, very low birth weight infants, heart rate variability fractal analysis, detrended fluctuation analysis
Despite advances in obstetrics and neonatal intensive care, the prevalence of severe intraventricular hemorrhage (IVH) among very low birth weight (VLBW; birth weight ≤1500 g) infants has not improved over the last 15 years.1–6 Long-term neurodevelopmental abnormalities such as behavioral and learning disabilities, mental retardation, seizures, and cerebral palsy can be seen as a result of IVH.7 A conservative cost estimate of the lifetime care expenses for a yearly cohort of surviving VLBW infants with severe IVH affected with adverse neurodevelopmental sequelae is $3 billion.8,9 Given this important public health problem, it would be quite valuable to identify VLBW infants who are at highest risk for developing IVH, before they actually develop it, so that they may benefit from prevention and intervention strategies. Unfortunately, accurate prediction methodologies of impending IVH have not been developed.
Altered autonomic function has been observed by heart rate variability (HRV) analysis in premature infants after IVH.10–12 HRV refers to the beat-to-beat (R-R interval from the electrocardiogram) fluctuation of heart rate and reflects the balance between parasympathetic and sympathetic impulses to the heart that is under central nervous system control. Researchers have used HRV analysis as a tool to estimate autonomic neural control of the heart and for predicting impending arrhythmia13 and long-term outcome of VLBW infants.11,14
The objective of this study was to determine if impending IVH in VLBW infants may be predicted by altered autonomic function. We hypothesized that autonomic dysfunction precedes the development of IVH in VLBW infants. We used a nonlinear method, called detrended fluctuation analysis (DFA),11,15 to examine the HRV characteristics of VLBW infants at risk for IVH. Identification of noninvasive predictors of IVH and potential preventive measures may help in reducing the burden of IVH in VLBW survivors of neonatal intensive care.
METHODS
Study Sample
Inborn ventilated VLBW newborn infants without complex congenital anomalies or chromosomal abnormalities admitted to the University of Arkansas for Medical Sciences NICU between June 2002 and June 2005 were eligible for this study. All enrolled infants had normal cranial ultrasound findings on the first day of life, before study procedures. Case infants included those diagnosed with IVH (>grade I) after the first day of life. The control infants consisted of similar infants (based on birth weight and gestational age) who never developed IVH. Our case–control study was nested within a larger investigation of the development of cerebral autoregulation in VLBW infants.16,17
Continuous Noninvasive Heart Rate and Rhythm Monitoring
Three radiolucent monitoring electrodes were placed via adhesive backing onto the chest and abdomen of each infant (red dot neonatal/infant prewired monitoring electrodes [3M, St Paul, MN]), according to recommendations of the manufacturer. The leads were connected to an electrocardiogram cable that was attached to a cardiorespiratory monitor to continuously monitor heart rate and the electrocardiogram.
Experimental Protocol
Informed consent was obtained from a parent before study participation. Continuous monitoring (~1 hour) of cerebral blood flow velocity, blood pressure, heart rate (~10 000 data point segments from the electrocardiogram), and arterial blood gases were obtained from VLBW infants before, during, and after indicated neonatal intensive care procedures twice during the first day of life. Baseline monitoring began 15 minutes before surfactant administration or tracheal suctioning, when infants were supine, quiet, and not undergoing any other procedures, and continued for 45 minutes after the procedure. Analog signals from the cardiorespiratory monitor for heart rate (100 samples per second) were collected with a data acquisition system (PowerLab 8 channel [ADInstruments, Mountain View, CA]) and stored. R-R interval data were filtered offline by removing heart rate values of >195 and <20. Heart rate values exceeding the 195 limit were found in <0.1% of data in all subjects.
Infants had cranial ultrasounds performed on the first day of life before physiologic monitoring and twice more during the first week of life according to the discretion of the attending neonatologist. The University of Arkansas for Medical Sciences institutional review board approved the study protocol.
Data Collection
The infants’ medical charts were reviewed and the following data were recorded: birth weight, gestational age, race, gender, date and time of birth, cranial ultrasound findings, maternal demographics, delivery route, obstetrical history, maternal medications, labor and delivery complications, details of newborn stabilization, neonatal complications, type of ventilator and settings, and timing and number of surfactant doses.
Detrended Fluctuation Analysis
Conventional HRV methods perform only linear analysis and, therefore, the changes over time and nonlinear characteristics of the rhythm are ignored. Indeed heart rhythm has significant complexity and even demonstrates fractal-like (self-similar) structure.18,19 Loss of normal fractal complexity of heart beat dynamics has been observed in various diseases.20–22
DFA is a method to quantify the self-similar structure by determining the fractal dimension or scaling exponent in time series data. DFA is a modified root mean square analysis of a random walk and is applicable to biological data.15 The root mean square fluctuations of the integrated and detrended data are measured within the observation windows of various sizes, and then scales of fluctuation are plotted to fit a curve on a log-log plot. Self-similarity is assessed by the characteristics of the fitted curve. Usually, the best linear fit procedure (least squares algorithm) is used, and the slope of the fitted line defines the scaling exponent, which indicates the degree of self-similarity. A freeware source code from PhysioNet was used to compute the scaling exponents (www.physionet.org).23
Considering the multifractal nature of the heart rhythm data, 3 short-term scaling exponents for 4 to 11, 8 to 15, and 12 to 19 beats and also a long-term scaling exponent for 17 to 50 beats were calculated. A short-term scaling exponent is usually calculated within the range 4 and n beats, where n ≤ 16, and long-term scaling exponent is then calculated within the range n + 1 and 64.18 The 4- to 11-beat window selection for heart rhythm data has been an empirical selection decision based on data from normal adults.15 We speculated that it would be more appropriate to use a sliding window technique for determination of the short-term scaling exponent because the data are obtained from premature infants.
Statistical Analysis
Characteristics of subjects with and without IVH were compared by using appropriate statistical tests (t test, median test, χ2, and Fisher’s exact test). The results are expressed as percentage, mean ± SD, or median (interquartile range) where appropriate. Multiple logistic regression models were used to estimate odds ratios and the associated 95% confidence intervals of developing IVH by using a purposeful selection method. A univariate logistic regression model was fit for each of the clinically meaningful variables. All statistically significant univariate variables were included in a multiple logistic regression model. A reduced multiple logistic regression model was fit after removing the apparently statistically noninformative variables. Because of the sample size, we developed a regression model with 3 variables. The response variable was the presence or absence of IVH. Independent variables considered in the model were short-term scaling exponent (α1), gestational age (weeks), race, gender, presence or absence of hypotension, receipt of antenatal steroids, and age (hours) at monitoring.
RESULTS
Study Population
Twenty-four (14 female and 10 male) ventilated VLBW infants were included in the study. The birth weight and gestational age (mean ± SD) were 845 ± 213 g and 26.1 ± 1.9 weeks, respectively. Thirteen infants were white, 9 were black, and 2 were Hispanic. Eighteen (75%) were exposed to antenatal steroids. Seventeen (71%) were delivered by cesarean section. There was no statistical difference between the 10 infants with IVH and the 14 without IVH for birth weight, gestational age, race, gender, Apgar scores, route of delivery, receipt of antenatal steroids, receipt of antepartum magnesium, and monitoring time (Table 1). Of the infants with IVH, 5 had grade IV, 4 had grade III, and 1 had grade II IVH.24 Infants were monitored at 10 ± 3.8 hours of life.
TABLE 1.
Patient Characteristics
| Variable | IVH (N = 10) | No IVH (N = 14) | P |
|---|---|---|---|
| Birth weight, median (IQR), g | 766 (732–847) | 843 (660–1179) | .578 |
| Gestational age, median (IQR), wk | 24.5 (24–26) | 26.5 (25–28) | .229 |
| Black race, % | 30 | 42.9 | .553 |
| Male gender, % | 40 | 42.9 | .918 |
| Apgar score at 1 min, median (IQR) | 3 (2–5) | 3.5 (2–4) | .933 |
| Apgar score at 5 min, median (IQR) | 6 (5–7) | 6 (6–7) | .420 |
| Route (vaginal), % | 10 | 42.9 | .095 |
| Antenatal steroids, % | 60 | 85.7 | .172 |
| Magnesium, % | 40 | 71.4 | .141 |
| Age at monitoring time, mean ± SD, h | 11.4 ± 4.5 | 9.0 ± 3.0 | .139 |
| PDA, % | 70 | 28.6 | .095 |
| Indomethacin, % | 20 | 21.4 | ≥0.999 |
| RDS, % | 100 | 100 | ≥0.999 |
| Pneumothorax, % | 20 | 0 | .240 |
| Vasopressors, % | 80 | 42.9 | .104 |
IQR indicates interquartile range; PDA, patent ductus arteriosis (treated with indomethacin or ligation); RDS, respiratory distress syndrome (treated with surfactant).
Nonlinear Analysis Results
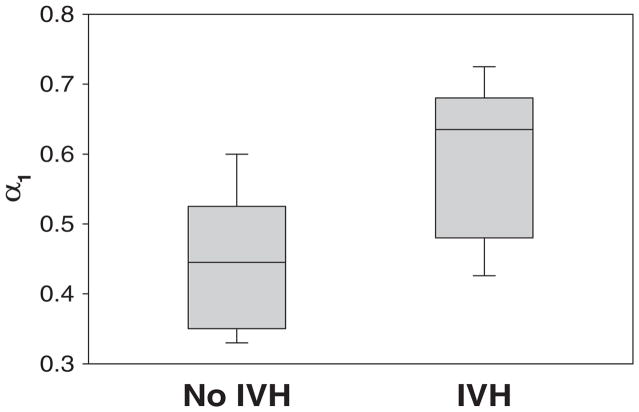
The short-range scaling exponents calculated for 4–11 beats, which is used in adults for short-term scaling exponent analysis, was not significantly different between the 2 groups (0.53 ± 0.09 vs 0.46 ± 0.12 [P = not significant], infants with IVH versus without IVH, respectively). However, the short-range scaling exponent calculated for 8–15 beats (hereafter referred to as “α1”) was significantly greater in infants with IVH compared with those without (0.60 ± 0.1 vs 0.45 ± 0.1 [P < .01]; Fig 1). Representative examples of log-log figures from DFA analysis of a study and control group patient are shown in Fig 2. The third window used for the short-term scaling exponent for 12–19 beats also failed to show any significant difference between the 2 groups (0.72 ± 0.16 vs 0.59 ± 0.15 [P = not significant]). The long-range scaling exponent, α2 (17–50 beats), did not show any difference between groups (1.02 ± 0.1 vs 0.99 ± 0.08 [P = not significant]).
FIGURE 1.
Box plot of short-term scaling exponent, α1, of heart rhythm data obtained from the VLBW infants during the first day of life according to whether they developed IVH.
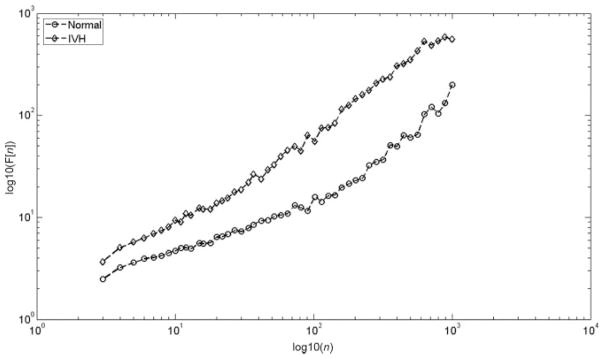
FIGURE 2.
A representative DFA log-log plot from a control and a study group patient is illustrated. The curves of these plots represent short-term and long-term scaling exponents.
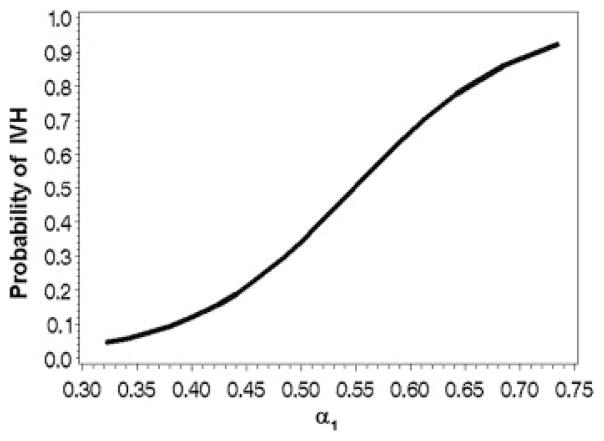
From multiple logistic regression analysis, by using 3 independent predictors (α1, gestational age, and receipt of antenatal steroids), α1 was the only statistically significant predictor of IVH (P = .02); Fig 3 shows the observed unadjusted predicted probability of IVH according to α1.
FIGURE 3.
Predicted probability of IVH with respect to short-term scaling exponent, α1.
The ability of α1 to discriminate between the 2 groups was also assessed by examining various cutoff values (between 0.32 and 0.73) by receiver operating characteristic curve analysis (area: 0.84 [95% confidence interval: 0.63–0.95]; P < .001). An α1 cutoff value of 0.52 (which corresponds to a predicted probability of .42) resulted in the highest likelihood ratio (3.2) with 70% sensitivity and positive predictive value, and 79% specificity and negative predictive value (P = .02) (Table 2).
TABLE 2.
Fractal Scaling Exponent α1(8–15 Beats) for Infants With and Without IVH (P = .02)
| No IVH | IVH | |
|---|---|---|
| α1 < .52 | 11 | 3 |
| α1 > .52 | 3 | 7 |
DISCUSSION
Altered autonomic function has been demonstrated with linear HRV analysis in premature infants after IVH.10–12 It was first observed in 1983 that premature infants with increasingly worse grades of IVH had progressively reduced HRV.12 Others also reported that premature infants with IVH had decreased HRV.10–12 To date, altered HRV has only been observed after IVH in these infants. Because increased grade of IVH has been associated with worse neurodevelopmental outcomes in premature infants,7,25–30 a reasonably reliable means of predicting impending IVH (> grade I) would potentially allow clinicians to provide prophylactic interventions to the identified high-risk infants. Early intervention may decrease IVH prevalence and severity and potentially improve long-term neurodevelopmental outcomes. Until now, the tools necessary to accurately predict which VLBW infants will develop IVH have been unavailable. To our knowledge, the present study is the first that takes advantage of a noninvasive method for predicting impending IVH in VLBW infants, with sufficient sensitivity and specificity as well as positive and negative predictive values. The fact that fractal pattern of the heart rhythm on the first day of life was altered before the onset of any significant IVH is very promising and suggests that DFA may be an excellent prospective predictor of IVH.
The pattern of development of the autonomic nervous system innervation correlates well with increased HRV analysis observed from infancy to adulthood. HRV studies examining autonomic function in the developing fetus and premature newborn suggest a predominance of sympathetic innervation, followed by gradual development of the parasympathetic nervous system.10–12,31–35 Because an increased short-term scaling exponent represents decreased parasympathetic function,36 our results suggest that VLBW infants with impending IVH have additional alterations of parasympathetic activity. Previous HRV studies (linear analysis) in premature infants with preexisting IVH also observed altered parasympathetic activity.10,12
We speculate that parasympathetic dysfunction in VLBW infants may lead to IVH by impairing appropriate cerebral blood flow and cerebral autoregulation. In animals, activation of parasympathetic cerebral arterial perivascular nerves has been observed to increase cerebral blood flow via vasodilation.37 In contrast, parasympathetic denervation decreases cerebral blood flow during hemorrhagic hypotension and attenuates cerebral vasodilation in hypertensive rats.38,39 In addition, it has been observed that the lower limit of the autoregulatory plateau is shifted toward the right in parasympathetically denervated rats,37 indicating that the cerebral circulation becomes pressure-passive at higher blood pressures. Thus, VLBW infants with impaired parasympathetic regulation may be at increased risk of IVH, because disturbances of cerebral blood flow and cerebral autoregulation have been associated with the development of IVH.40
In the current study, we assessed 3 short-term scaling exponents for 4–11, 8–15, and 12–19 beats. The notable difference was that the short-term scaling exponent for a window of 8–15 beats was significantly larger in infants who subsequently developed IVH, compared with no difference between infants for the 4–11 and 12–19 beat short-term scaling exponents. Because human heart rhythm has multifractal properties,41 a large number of scaling exponents may be necessary to characterize the scaling properties of heart rhythm dynamics. Traditional DFA ignores that to some extent by generating only 2 scaling exponents. Indeed, our approach was beneficial as we observed a significant alteration of short-term scaling exponents in infants with IVH for a window size selected for 8–15 beats. Therefore, one of the conclusions of this study is that assessing only 2 scaling exponents is probably ignoring part of the multifractal properties present in the heart beat data that may be different between adults and premature infants.
The major limitation of this case-control study is the small patient population. Although a larger population of infants will be necessary to assess the clinical usefulness of this noninvasive marker as a predictor of impending IVH, the results from this study are quite compelling and will serve as the foundation for a larger trial. Another potential problem is that human heart rate data are known to have multifractal patterns. DFA is limited in this regard and future studies will need to address the multifractal nature of heart rhythm data. Currently, however, there is no consensus regarding the proper nonlinear technique to use to examine heart rhythm data. A third potential limitation is that we did not correlate our nonlinear HRV results to respiratory sinus arrhythmia14 and other conventional linear HRV analyses.10,11,42 Of note, our overall results are similar to previous studies using linear HRV analyses, except that they examined premature infants after IVH developed.10,12 Lastly, we did not examine possible interventions (eg, prophylactic indomethacin)43 that could be used once infants at high risk of developing IVH were identified by using DFA. Although prophylactic indomethacin has been shown to decrease the prevalence of IVH among premature infants,43,44 long-term outcome was not improved.44 However, one can speculate that long-term benefits could have been realized if only the infants at highest risk for IVH, identified by DFA, would have been provided indomethacin.
CONCLUSIONS
Parasympathetic activity, as determined by fractal dynamics of the heart rhythm, is significantly altered in VLBW infants before the development of IVH. Thus, the short-term scaling exponent (8–15 beats) may be a useful noninvasive predictor of impending IVH. Additional prospective studies with larger infant numbers are warranted to evaluate the potential clinical usefulness of this noninvasive marker.
What’s Known on This Subject
Altered HRV has been observed in premature infants after the development of IVH.
What This Study Adds
Altered HRV in premature infants predicts the development of IVH.
Acknowledgments
Dr Kaiser was supported by National Institutes of Health grants 1 K23 NS43185, RR20146, and M01RR14288.
Abbreviations
- IVH
intraventricular hemorrhage
- VLBW
very low birth weight
- HRV
heart rate variability
- DFA
detrended fluctuation analysis
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
This work was presented in part at the 2007 Pediatric Academic Society, Society for Pediatric Research meeting; May 5–8, 2007; Toronto, Ontario, Canada.
References
- 1.Fanaroff AA, Wright LL, Stevenson DK, et al. Very-low-birth-weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, May 1991 through December 1992. Am J Obstet Gynecol. 1995;173(5):1423–1431. doi: 10.1016/0002-9378(95)90628-2. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson DK, Wright LL, Lemons JA, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1993 through December 1994. Am J Obstet Gynecol. 1998;179(6 pt 1):1632–1639. doi: 10.1016/s0002-9378(98)70037-7. [DOI] [PubMed] [Google Scholar]
- 3.Lemons JA, Bauer CR, Oh W, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1995 through December 1996. Pediatrics. 2001;107(1) doi: 10.1542/peds.107.1.e1. Available at: www.pediatrics.org/cgi/content/full/107/1/e1. [DOI] [PubMed]
- 4.Fanaroff AA, Hack M, Walsh MC. The NICHD Neonatal Research Network: changes in practice and outcomes during the first 15 years. Semin Perinatol. 2003;27(4):281–287. doi: 10.1016/s0146-0005(03)00055-7. [DOI] [PubMed] [Google Scholar]
- 5.Horbar JD, Badger GJ, Carpenter JH, et al. Trends in mortality and morbidity for very low birth weight infants, 1991–1999. Pediatrics. 2002;110(1 pt 1):143–151. doi: 10.1542/peds.110.1.143. [DOI] [PubMed] [Google Scholar]
- 6.Horbar JD, Carpenter J, Kenny J. Very Low Birth Weight Database Summary 2005. Burlington, VT: Vermont Oxford Network; 2006. [Google Scholar]
- 7.Pinto-Martin JA, Whitaker AH, Feldman JF, Van Rossem R, Paneth N. Relation of cranial ultrasound abnormalities in low-birthweight infants to motor or cognitive performance at ages 2, 6, and 9 years. Dev Med Child Neurol. 1999;41(12):826–833. doi: 10.1017/s0012162299001644. [DOI] [PubMed] [Google Scholar]
- 8.Ment LR, Allan WC, Makuch RW, Vohr B. Grade 3 to 4 intraventricular hemorrhage and Bayley scores predict outcome. Pediatrics. 2005;116(6):1597–1598. doi: 10.1542/peds.2005-2020. [DOI] [PubMed] [Google Scholar]
- 9.Honeycutt A, Dunlap L, Chen H, et al. Economic costs associated with mental retardation, cerebral palsy, hearing loss, and vision impairment—United States, 2003. MMWR Morb Mortal Wkly Rep. 2004;53(3):57–59. [PubMed] [Google Scholar]
- 10.van Ravenswaaij-Arts CM, Hopman JC, Kollee LA, et al. The influence of respiratory distress syndrome on heart rate variability in very preterm infants. Early Hum Dev. 1991;27(3):207–221. doi: 10.1016/0378-3782(91)90195-9. [DOI] [PubMed] [Google Scholar]
- 11.Hanna BD, Nelson MN, White-Traut RC, et al. Heart rate variability in preterm brain-injured and very-low-birth-weight infants. Biol Neonate. 2000;77(3):147–155. doi: 10.1159/000014209. [DOI] [PubMed] [Google Scholar]
- 12.Hörnchen H, Betz R, Kotlarek F, Roebruck P. Microprocessor-based long term cardiorespirography. II. Status evaluation in term and premature newborns. J Perinat Med. 1983;11(1):32–42. doi: 10.1515/jpme.1983.11.1.32. [DOI] [PubMed] [Google Scholar]
- 13.Tuzcu V, Nas S, Borklu T, Ugur A. Decrease in the heart rate complexity prior to the onset of atrial fibrillation. Europace. 2006;8(6):398–402. doi: 10.1093/europace/eul031. [DOI] [PubMed] [Google Scholar]
- 14.Doussard-Roosevelt JA, Porges SW, Scanlon JW, Alemi B, Scanlon KB. Vagal regulation of heart rate in the prediction of developmental outcome for very low birth weight preterm infants. Child Dev. 1997;68(2):173–186. [PubMed] [Google Scholar]
- 15.Peng CK, Buldyrev SV, Havlin S, et al. Mosaic organization of DNA nucleotides. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1994;49(2):1685–1689. doi: 10.1103/physreve.49.1685. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser JR, Gauss CH, Williams DK. Surfactant administration acutely affects cerebral and systemic hemodynamics and gas exchange in very-low-birth-weight infants. J Pediatr. 2004;144(6):809–814. doi: 10.1016/j.jpeds.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser JR, Gauss CH, Williams DK. The effects of hypercapnia on cerebral autoregulation in ventilated very low birth weight infants. Pediatr Res. 2005;58(5):931–935. doi: 10.1203/01.pdr.0000182180.80645.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng CK, Havlin S, Stanley HE, Goldberger AL. Quantification of scaling exponents and crossover phenomena in nonstationary heartbeat time series. Chaos. 1995;5(1):82–87. doi: 10.1063/1.166141. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi M, Musha T. 1/f fluctuation of heartbeat period. IEEE Trans Biomed Eng. 1982;29(6):456–457. doi: 10.1109/TBME.1982.324972. [DOI] [PubMed] [Google Scholar]
- 20.Goldberger AL, West BJ. Fractals in physiology and medicine. Yale J Biol Med. 1987;60(5):421–435. [PMC free article] [PubMed] [Google Scholar]
- 21.Peng CK, Havlin S, Hausdorff JM, et al. Fractal mechanisms and heart rate dynamics. Long-range correlations and their breakdown with disease. J Electrocardiol. 1995;28(suppl):59–65. doi: 10.1016/s0022-0736(95)80017-4. [DOI] [PubMed] [Google Scholar]
- 22.Mäkikallio TH, Hoiber S, Kober L, et al. Fractal analysis of heart rate dynamics as a predictor of mortality in patients with depressed left ventricular function after acute myocardial infarction. TRACE Investigators. TRAndolapril Cardiac Evaluation. Am J Cardiol. 1999;83(6):836–839. doi: 10.1016/s0002-9149(98)01076-5. [DOI] [PubMed] [Google Scholar]
- 23.Goldberger AL, Amaral LAN, Glass L, et al. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation. 2000;101(23):e215–e220. doi: 10.1161/01.cir.101.23.e215. [DOI] [PubMed] [Google Scholar]
- 24.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1 500 gm. J Pediatr. 1978;92(4):529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 25.Ambalavanan N, Nelson KG, Alexander G, et al. Prediction of neurologic morbidity in extremely low birth weight infants. J Perinatol. 2000;(8 pt 1):496–503. doi: 10.1038/sj.jp.7200419. [DOI] [PubMed] [Google Scholar]
- 26.Ancel PY, Livinec F, Larroque B, et al. Cerebral palsy among very preterm children in relation to gestational age and neonatal ultrasound abnormalities: the EPIPAGE cohort study. Pediatrics. 2006;117(3):828–835. doi: 10.1542/peds.2005-0091. [DOI] [PubMed] [Google Scholar]
- 27.Patra K, Wilson-Costello D, Taylor G, Mercuri-Minich N, Hack M. Grades I-II intraventricular hemorrhage in extremely low birth weight infants: effects on neurodevelopment. J Pediatr. 2006;149(2):169–173. doi: 10.1016/j.jpeds.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Hack M, Wilson-Costello D, Friedman H, et al. Neurodevelopment and predictors of outcomes of children with birth weights of less than 1000 g: 1992–1995. Arch Pediatr Adolesc Med. 2000;154(7):725–731. doi: 10.1001/archpedi.154.7.725. [DOI] [PubMed] [Google Scholar]
- 29.Vohr BR, Wright LL, Dusick AM, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Developmental Neonatal Research Network, 1993–1994. Pediatrics. 2000;105(6):1216–1226. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 30.Allan WC, Vohr B, Makuch RW, Katz KH, Ment LR. Antecedents of cerebral palsy in a multicenter trial of indomethacin for intraventricular hemorrhage. Arch Pediatr Adolesc Med. 1997;151(6):580–585. doi: 10.1001/archpedi.1997.02170430046010. [DOI] [PubMed] [Google Scholar]
- 31.Prietsch V, Knoepke U, Obladen M. Continuous monitoring of heart rate variability in preterm infants. Early Hum Dev. 1994;37(2):117–131. doi: 10.1016/0378-3782(94)90153-8. [DOI] [PubMed] [Google Scholar]
- 32.Clairambault J, Curzi-Dascalova L, Kauffmann F, Medigue C, Leffler C. Heart rate variability in normal sleeping term and preterm neonates. Early Hum Dev. 1992;28(2):169–183. doi: 10.1016/0378-3782(92)90111-s. [DOI] [PubMed] [Google Scholar]
- 33.Cabal LA, Siassi B, Zanini B, Hodgman JE, Hon EE. Factors affecting heart rate variability in preterm infants. Pediatrics. 1980;65(1):50–56. [PubMed] [Google Scholar]
- 34.Nakamura T, Horio H, Miyashita S, Chiba Y, Sato S. Identification of development and autonomic nerve activity from heart rate variability in preterm infants. Biosystems. 2005;79(1–3):117–124. doi: 10.1016/j.biosystems.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Van Ravenswaaij-Arts C, Hopman J, Kollee L, Stoelinga G, Van Geijn H. Spectral analysis of heart rate variability in spontaneously breathing very preterm infants. Acta Paediatr. 1994;83(5):473–480. doi: 10.1111/j.1651-2227.1994.tb13062.x. [DOI] [PubMed] [Google Scholar]
- 36.Tulppo MP, Kiviniemi AM, Hautala AJ, et al. Physiological background of the loss of fractal heart rate dynamics. Circulation. 2005;112(3):314–319. doi: 10.1161/CIRCULATIONAHA.104.523712. [DOI] [PubMed] [Google Scholar]
- 37.Morita Y, Hardebo JE, Bouskela E. Influence of cerebrovascular parasympathetic nerves on resting cerebral blood flow, spontaneous vasomotion, autoregulation, hypercapnic vasodilation and sympathetic vasoconstriction. J Auton Nerv Syst. 1994;49(suppl):S9–S14. doi: 10.1016/0165-1838(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 38.Koketsu N, Moskowitz MA, Kontos HA, Yokota M, Shimizu T. Chronic parasympathetic sectioning decreases regional cerebral blood flow during hemorrhagic hypotension and increases infarct size after middle cerebral artery occlusion in spontaneously hypertensive rats. J Cereb Blood Flow Metab. 1992;12(4):613–620. doi: 10.1038/jcbfm.1992.85. [DOI] [PubMed] [Google Scholar]
- 39.Talman WT, Dragon DN. Parasympathetic nerves influence cerebral blood flow during hypertension in rat. Brain Res. 2000;873(1):145–148. doi: 10.1016/s0006-8993(00)02490-2. [DOI] [PubMed] [Google Scholar]
- 40.Perlman JM, Goodman S, Kreusser KL, Volpe JJ. Reduction in intraventricular hemorrhage by elimination of fluctuating cerebral blood-flow velocity in preterm infants with respiratory distress syndrome. N Engl J Med. 1985;312(21):1353–1357. doi: 10.1056/NEJM198505233122104. [DOI] [PubMed] [Google Scholar]
- 41.Ivanov PC, Amaral LA, Goldberger AL, et al. Multifractality in human heartbeat dynamics. Nature. 1999;399(6735):461–465. doi: 10.1038/20924. [DOI] [PubMed] [Google Scholar]
- 42.Smith SL, Doig AK, Dudley WN. Characteristics of heart period variability in intubated very low birth weight infants with respiratory disease. Biol Neonate. 2004;86(4):269–274. doi: 10.1159/000080053. [DOI] [PubMed] [Google Scholar]
- 43.Ment LR, Oh W, Ehrenkranz RA, et al. Low-dose indomethacin and prevention of intraventricular hemorrhage: a multi-center randomized trial. Pediatrics. 1994;93(4):543–550. [PubMed] [Google Scholar]
- 44.Schmidt B, Davis P, Moddemann D, et al. Long-term effects of indomethacin prophylaxis in extremely low-birth-weight infants. N Engl J Med. 2001;344(26):1966–1972. doi: 10.1056/NEJM200106283442602. [DOI] [PubMed] [Google Scholar]