Abstract
Dielectrophoresis can discriminate distinct cellular identities in heterogeneous populations, and monitor cell state changes associated with activation and clonal expansion, apoptosis, and necrosis, without the need for biochemical labels. Demonstrated capabilities include the enrichment of haematopoetic stem cells from bone marrow and peripheral blood, and adult stem cells from adipose tissue. Recent research suggests that this technique can predict the ultimate fate of neural stem cells after differentiation before the appearance of specific cell-surface proteins. This review summarises the properties of cells that contribute to their dielectrophoretic behaviour, and their relevance to stem cell research and translational applications.
1. Introduction
Dielectrophoresis (DEP) refers to the motion of electrically polarisable particles induced by electric field gradients. It is an established technique which has been previously used to discriminate between distinct cellular identities in heterogeneous populations, notably haemotopoietic stem cells and differentiated derivatives in blood and mesenchymal stem cells in adipose tissue [1–3]. It has also been used to monitor changes in cell states associated with activation and clonal expansion, apoptosis, necrosis, and responses to chemical and physical agents [4–6]. In a recent report, Flanagan et al. [7] applied DEP to neural stem cell populations and concluded that the ultimate fate of cells after differentiation can be predicted by distinct changes in their dielectrophoretic properties before the presence of specific cell-surface proteins (antigens) can be detected. This study provides a timely reminder of as yet not fully exploited opportunities which DEP provides to selectively isolate target subpopulations of cells from other cells in suspension, without harm or the need for biochemical labels or other bioengineered tags. In this paper, we identify the properties of whole cells likely to contribute to their dielectrophoretic profile and how this information can be used to benefit stem cell research and translational applications.
Stem cells are immature cells characterised by a varying capacity for growth (“immortal” in the case of embryonic stem cells) and the ability to differentiate into one or more different derivatives with specialised function or maintain their stem cell phenotype (i.e., self-renewal). These capacities can vary depending on the in vivo origin of the stem cell populations, the in vitro environment, and the manipulation(s) to which they are subjected. The dynamic nature of stem cells and their susceptibility to environmental influences establish exacting requirements for technology to monitor, characterise, and manipulate living cells. Ideally, these methodologies should be sensitive (i.e., relatable to individual cells which might be sampled to represent larger populations); rapid and quantitative, providing real-time information on cell identity and fate which could be used to inform production processes; non- or minimally-invasive, so as not to consume or alter the behaviour of the cells being analysed; and scalable, for maximum analytical throughput.
Currently, the most common methods used to quantitatively characterise or positively/negatively select/purify cell populations for research or translational applications include flow cytometry (fluorescence activated cell sorting, FACS) or magnetic bead-coupled cell separation. These methods are dependent on the existence of specific cell-surface antigens and the formulation/availability of high-affinity probes to these antigens. Irreversible attachment of these probes to target cells also has the potential to influence cell behaviour. In the absence of a specific or unique marker or to avoid potentially confounding interactions of probes with cells, and to facilitate achieving the objectives of scalability and noninvasiveness mentioned above, alternative methods are required to identify and manipulate target cells in heterogeneous cell populations. This opens up potentially important applications of DEP as a tool to address an unmet need in stem cell research and therapy.
2. Dielectrophoresis (DEP)
DEP is the term used to describe the motion of particles when they are exposed to an electric field gradient. Unlike electrophoresis, the particle need not carry an electric charge, and alternating, radio frequency, electric signals rather than direct current voltages are usually employed to energise the electrodes. The factors controlling the DEP behaviour of a cell can be understood by considering first how the imposed electric field polarises the cell, and then evaluating what happens if the electrodes generate regions of high field gradient, characterised by rapid changes of electric potential as a function of distance.
2.1. Polarising Effect of the Field
A cell exposed to an electric field experiences mechanical (electrostrictive) forces arising from induced electric charges that accumulate at the various interfaces defined by the cell's structure, as for example, at the outer membrane of the cell and around structural components inside the cell. The amount of induced charges is small (typically much less than the charges occurring naturally on a cell surface)—but they are nonuniform in distribution and lend to the cell the properties of an electric dipole moment. Thus, although a cell is not polar in nature (i.e., it does not possess a permanent electric dipole moment), the applied field has the effect of polarising the cell into the form of an electric dipole.
We can derive the magnitude of the cell's induced dipole moment by modelling the cell as a sphere of radius r suspended in a fluid of absolute dielectric permittivity εm. For an applied electric field E, the effective induced dipole moment meff is given as
| (1) |
where p is the effective polarisability (per unit volume) of the cell [8–10]. This result takes into account a depolarisation factor of 1/3 to account for the fact that a spherical body distorts an external applied field, and that the electric field inside the sphere differs from the external field. The polarisability term p (known as the Clausius-Mossotti function) has values mathematically bounded by −0.5 ≤ p ≤ 1.0, and is determined by the frequency-dependent conductive and dielectric properties of the cell and its suspending fluid. A positive value for p will result in a dipole moment that aligns itself with the field. Negative values for p produce dipole moments of opposite polarity, namely, those that align themselves against the field. For DEP experiments on cells, the conductivity of the suspending solution is usually chosen to give a negative value for p at low frequencies, but a positive value at higher frequencies.
2.2. Effect of a Field Gradient
If the applied electric field is uniform, the cell may well reorient so as to minimise the energy of interaction between its induced dipole moment and the applied field, but it will not undergo lateral displacement. A uniform field can be produced between two parallel planar electrodes. If the electrodes are designed to produce a nonuniform field (a metal pin facing a flat metal plate will suffice), a polarised cell will find itself in a field gradient. In this case, there will be a net electrostrictive force acting on the cell, and it will move relative to its surroundings. Depending on the polarity of the induced dipole moment, the cell will either move towards regions of large spatial variation of the electric potential (an effect known as positive DEP) or away from such regions (negative DEP). The largest field gradients always occur at electrode edges, so that positive DEP results in the collection (trapping) of cells at the electrodes, whilst negative DEP results in cells being “pushed” away from the electrodes. Fluid flow can be used to remove the cells that are not trapped by positive DEP at the electrodes, and this is the basis for the selective separation or enrichment of target cells using DEP [1–3].
In an alternating current field, the time-averaged DEP force FDEP acting on a cell is thus proportional to the product of the induced dipole moment and the field gradient. This is mathematically expressed as
| (2) |
where the vector symbol ∇ (del) is used to define the field gradient. From equations (1) and (2), we have the following result:
| (3) |
The r3 term demonstrates that DEP is a ponderomotive effect—a term used to indicate that magnitude of the DEP force is proportional to the cell volume. Equation (3) also reveals an important experimental feature, namely, that the DEP force is proportional to the product of the local field and the local field gradient. The design of the microelectrodes is therefore an important exercise. The field generated should be large enough to polarise the cell with a significant induced dipole moment, but not so large as to cause electrical or thermal damage to the cell. The electrode geometry should also produce a highly nonuniform field to give a DEP force that overcomes the randomising effects of Brownian motion in the surrounding medium, and to cause the cell to move to a desired location.
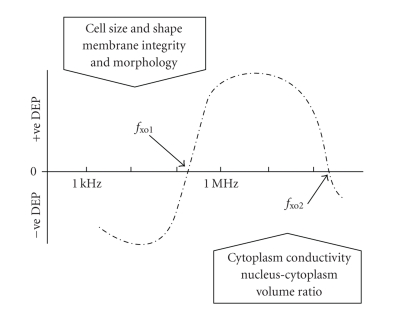
The DEP response exhibited by a typical mammalian cell, as a function of the frequency of the applied electric field, is shown in Figure 1. In early studies, it was observed that at low frequencies (less than ~1 kHz) the DEP behaviour is influenced by cell surface charge, implying that the effective polarisability of the cell is dominated by the electrical double layer that surrounds a charged cell [11, 12]. This was validated by neuraminidase treatment of erythrocytes, to reduce charge associated with membrane sialic acid residues, without changing the conductivity and permittivity of the membrane [12]. The integrity of the cytoplasmic membrane was also found to influence the low-frequency DEP response. Saponin treatment of erythroleukaemic cells, which permeabilised the membrane without causing a major loss of cytoplasmic protein, resulted in an increase of the positive DEP response of the cells in the frequency range from 10 Hz to 100 kHz [12]. This implied that the overall conductivity of the cells had increased slightly as a result of the saponin treatment.
Figure 1.
The DEP response exhibited by a viable cell under typical experimental conditions is often of the form shown here. The DEP force reverses polarity at two DEP cross-over frequencies, fxo1 and fxo2, determined by the various cell parameters listed in this figure. As indicated by (4), determination of fxo1 and cell diameter, along with the conductivity of the suspending solution, provides a measure of cell membrane capacitance. As yet, direct measurements of fxo2 have not been reported.
A transition from negative to positive DEP occurs at a well-defined frequency, fxo1, commonly referred to as the DEP “cross-over” frequency. For frequencies below fxo1, the polarisability factor p in (3) is dominated by the high resistance of the cell membrane, and has a negative value. The cell is repelled, under the action of negative DEP, from regions near electrode edges where the greatest spatial changes of the electric potential are generated. For frequencies above fxo1, the factor p in (3) attains a positive value and the cell is driven towards an electrode edge and trapped there. Theoretical representations of the effective cell polarisability (equivalent to modelling the DEP responses) across a full frequency spectrum are shown in Figures 2–4. These theoretical analyses employ the double-shell model of a cell, in which a shelled-sphere (the nucleus) is incorporated into a single-shell consisting of the cytoplasm surrounded by the cytoplasmic membrane [13]. Shelled models to describe the properties of cells carry uncertainties with respect to variations of cell phase dielectric parameters [14], but are useful in understanding the main DEP characteristics of suspended cells. Theoretical studies have also indicated that, with all cell phase dielectric properties remaining constant, a change in shape from a sphere to an ellipsoid can result in significant changes of a cell's dielectric properties [15]. However, it is usually the case that when cultured cells are suspended in solution they “round up” into spheres. The shapes (and diameters) of cells are usually noted during DEP experiments, and deviations from a spherical shape are not normally observed in studies of suspended mammalian cells (e.g., [16]).
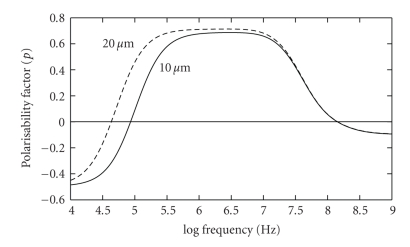
Figure 2.
Theoretical modelling of the polarisability factor p of (1) and (3), as a function of frequency, for a viable cell suspended in a solution of conductivity 40 mS/m. The modelling employs the double-shell representation of a nucleated cell [13]. The plot is normalized against the polarisability of a conducting sphere of the same diameter. With increasing frequency, the membrane capacitance electrically shorts out the membrane resistance, allowing the applied field to penetrate through the cytoplasmic membrane, and the cell's polarisability approaches that (p = 1) of a conducting sphere. Changes in cell diameter, from 10 μm to 20 μm, alter the polarisability for frequencies below ~1 MHz.
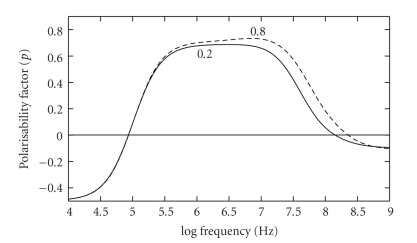
Figure 4.
Theoretical modelling of the polarisability factor p of (1), for two values of the nucleus-cytoplasm (N/C) ratio. A reduction of the N/C ratio from 0.8 to 0.2 influences the DEP response across a wide frequency range. The dielectric parameters derived by Asami et al. [13] for mouse lymphocytes have been used for this model.
As shown in Figure 2, the value of fxo1 is sensitive to the size of the cell, with all other factors remaining constant. We can understand this result by noting that the cross-over frequency fxo1 is inversely proportional to the characteristic time required for the outer cell membrane to fully polarise with its induced accumulation of charges. The bigger the cell, the longer it will take to charge the cell membrane using the fixed ion charge density available in the surrounding electrolyte. A longer period in the time-domain translates to a lower frequency in the frequency-domain. Thus, a large cell will exhibit a lower fxo1 value than a smaller cell, as shown in Figure 2 (and predicted by (4) below). A simple electrical representation of the charging of the cell membrane is a series RsCm circuit, where Rs represents the effective resistance of the surrounding electrolyte as a source of charging ions, and Cm is the capacitance of the cell membrane. The cell membrane acts as a capacitor because it is constructed like one—namely, a thin dielectric situated between two conductors (the outer and inner electrolytes). The relationship between frequency fxo1, cell radius r, surrounding electrolyte conductivity σs and membrane capacitance Cm is given by
| (4) |
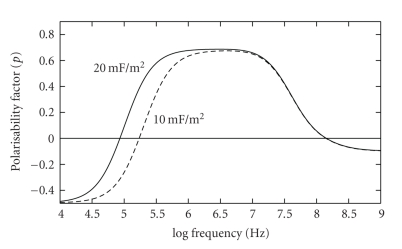
This equation assumes that the high resistance value of the cell membrane has not been impaired due to damage or the onset of cell death, for example, [4–6]. For a fixed cell radius, the effective membrane capacitance of a smooth cell will be less than that for a cell having a complex cell surface topography associated with the presence of microvilli, blebs, membrane folds, or ruffles, for example. This will influence the value observed for fxo1, and this effect is shown in Figure 3. An important practical application of the influence of cell size and membrane capacitance on the dielectric polarisability of a cell has recently been demonstrated by Holmes et al. [17], in the form of a microfluidic cytometer that counts white leukocytes and assigns them into the major subtypes on the basis of their electrical impedance (a measure of the membrane capacitance and effective conductance of a cell).
Figure 3.
The cytoplasmic membrane exhibits an electrical capacitance, whose value per unit membrane area is proportional to such structural features as membrane folding, microvilli, and blebs, for example. As indicated in this theoretical modelling, and demonstrated experimentally [4–6], changes in membrane surface features result in a shift of the DEP cross-over frequency fxo1. The example shown here, for typical membrane capacitance values, demonstrates the change expected for a doubling of the effective surface “roughness” of the membrane.
As shown in Figure 2, the second “cross-over” frequency fxo2 at ~100 MHz should not change with cell size. However, factors such as the cytoplasm conductivity and permittivity, nuclear envelope permittivity, and nucleus-cytoplasm volume (N/C ratio) ratio are expected to control the value of fxo2 [18]. A theoretical modelling of the effect of changes of the N/C ratio is shown in Figure 4. It can be seen that the N/C ratio has a great influence on a cell's polarisability for frequencies above fxo1, a result reflecting the fact that the dielectric properties of the nucleoplasm and nuclear envelop differ significantly from those of the cytoplasm and cytoplasmic membrane. The modelling for Figure 4 employed the dielectric parameters derived by Asami et al. [13] for mouse lymphocytes. The conductivity of the nucleoplasm (1.35 S/m) is modelled to be larger than that of the cytoplasm (0.32 S/m) because in general the nucleoplasm has a greater hydrated free ion content than the cytoplasm. The nuclear envelope is given a large specific conductance value (1.5 × 105 S/m2) to reflect the presence of nuclear pores [13], whereas the cytoplasmic membrane exhibits a much smaller conductance of ~100 S/m2 [18]. For frequencies above ~10 MHz, the polarisability factor p in (3) is dominated by the permittivity of the various cell components, whilst below this frequency the conductivity terms are dominant [18]. The transition from a positive to negative value of p at fxo2 implies that the relative permittivity of the whole cell becomes less than that (~70) of the aqueous suspending medium. For the results shown in Figure 4, the relative permittivity of the nucleoplasm was assigned the value of 52 [13]. Although caution should be exercised in placing too much confidence in theoretical models of a cell, we can understand why the effective relative permittivity of a cell might become less than that exhibited by bulk water by considering the likely dielectric properties of the nucleus. The DNA will exhibit a dielectric dispersion, arising from counter-ion fluctuations over small regions of the molecule [19], and the protein will exhibit restricted relaxations of polar groups [20]. The fall in permittivity associated with these dielectric dispersions, from a value above to one below that of water, occurs at a frequency below ~200 MHz [19, 20]. This, together with the influence of “bound” water, will result in an overall relative permittivity for the nucleus material being below that of bulk water at frequencies above ~100 MHz [16].
We are not aware of reported values for fxo2, a situation that reflects the fact that commercially available signal generators suitable for DEP measurements are not available for frequencies above around 30 MHz. Our present efforts are directed towards addressing this situation by constructing purpose built equipment. As discussed above, the effective conductivity and permittivity of the whole cell will be a function of the N/C ratio. The ability to characterise and separate cells on the basis of the fxo2 DEP cross-over frequency could therefore be particularly important for stem cell research, because the nucleus-to-cytoplasm (N/C) ratio generally decreases with cell differentiation and maturity [21]. For example, limbal epithelial stem cells exhibit an N/C ratio of 0.82, whilst for peripheral corneal epithelial cells the N/C ratio reduces to 0.17 [22].
DEP thus offers many distinct advantages as a tool for biomedical applications. In summary, these include the ability to:
monitor changes in cell viability,
isolate viable, culturable, cells with minimal or no biological damage,
monitor changes in the surface morphology or internal structure of cells,
separate cells to high specificity for their identification and enumeration,
separate cells without the need for biochemical labelling or modification,
separate rare target cells from heterogeneous samples, avoiding cell loss with a process that uses one procedure (namely DEP),
process samples at high cell-sorting rates (comparable to or faster than FACS).
All of these attributes can be used to further the basic and applied research of stem cells.
3. DEP Studies of Stem Cells
The number of reported DEP experiments on stem cells is small. The first studies [1, 2] demonstrated that DEP could be used to enrich haematopoetic stem cells (defined as those expressing the CD34 antigen) from a mixed cell population in bone marrow and peripheral blood, without the requirement for any cell manipulation such as antibody binding. The different cell fractions exhibited a spread of DEP characteristics, suggesting some form of heterogeneity within the CD34+ cell population. The separated cells were viable and data from colony forming assays correlated well with the percentage of CD34+ cells in each collected cell fraction. In more recent work, DEP has been used to obtain populations enriched from putative stem cells, as defined by expression of the stromal marker NG2 from enzyme-digested adipose tissue [3].
Of particular interest is the recent work of Flanagan et al. [7]. The objective of this study was to determine whether stem cells and their more differentiated progeny can be identified by means other than flow cytometry. DEP was investigated as a potentially nonbiased approach to probe the characteristics of an entire cell without relying on the expression of a certain set of markers on the cell surface. Mouse neural stem/precursor cells (NSPCs) and their differentiated derivatives (neurons and glia) were found to have distinct DEP signatures. Moreover, the DEP signatures were found to distinguish NSPCs from different developmental ages in a fashion that predicted their respective fate biases. This suggests that the developmental progression of progenitor cell populations can be revealed by the cells' dielectric properties. These results also highlight the fact that stem cell differentiation is a gradual process and that cells may begin to develop some characteristics of their more differentiated progeny before known cell surface markers can be detected and well before the cells become fully differentiated. Flanagan et al. [7] also concluded that DEP can be used to quantify the heterogeneity of a population of cells, providing another measure for characterising stem cell cultures.
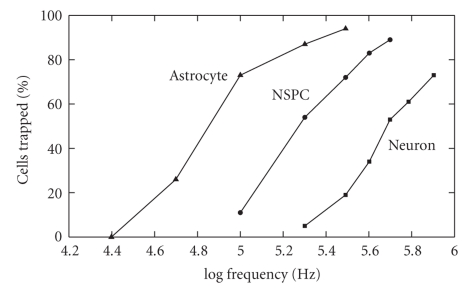
In the Flanagan et al. study, DEP signatures for the neural stem cells were obtained by monitoring the rate at which they collected at the electrodes [7]. Figure 5 highlights the important aspects of these results, to show that the three cell types investigated exhibited markedly different DEP signatures. This can also be deduced by extrapolating for values of the DEP cross-over frequency fxo1 for the cell types. In their DEP experiments Flanagan et al. [7] did not change the conductivity of the cell suspending electrolyte, and cell viability was checked at all times. From (4) we can deduce that the two cell properties responsible for the different characteristics shown in Figure 5 are cell size and membrane capacitance (possibly reflecting differences in cell surface topography [4, 6]). However, cell size was not determined (or at least reported) by Flanagan et al. [7], and so we are unable to relate the observed DEP differences to intrinsic properties related to surface properties of the cell membrane. Commitment of stem cells to different lineages is regulated by many cues, including cell shape. For example, it has been demonstrated that human mesenchymal stem cells (hMSCs) allowed to adhere, flatten, and spread out underwent osteogenesis, while unspread, round cells underwent adipogenesis [23]. The shape of hMSCs is also involved in the decision between chondrogenic or smooth muscle cell fates in response to TGFβ3 signalling [24]. It will be important to the DEP characterisation of stem cells to discover to what extent different cell shapes during cell culture translate to different cell surface complexities (e.g., extent of membrane folding or ruffles), and hence different measurable DEP properties after they have rounded up when suspended in DEP measurement media.
Figure 5.
DEP cell trapping efficiency curves for embryonic mouse neural stem/precursor cells (NSPC), neurons and astrocytes, based on the results reported by Flanagan et al. [7]. Extrapolating this (positive) DEP data to zero cell trapping indicates that these three different cell types exhibit different fxo1 cross-over frequencies. Such differences should enable the selective isolation of these different cell types from each other using DEP (e.g., [1–3]).
Determinations of the DEP cross-over frequencies fxo1 and fxo2 are also important for practical purposes—such as selective cell enrichment. At these cross-over frequencies, the effective dielectric properties of the cell exactly balance those of the suspending medium, so that the DEP force is zero. If we know, and can control the dielectric properties of the suspending medium, we can deduce and also control the DEP behaviour of the suspended cells. This has important implications for applying DEP to characterize and selectively isolate target cells from other cells [18, 25, 26]. Flanagan et al. [7] have reported data relevant to a determination of fxo1. Advancing to higher frequencies of DEP measurement to determine fxo2 would be another significant step, because now the nature of the cell interior could be explored. This will provide valuable information on such details as the ratio of nucleus:cytoplasm volumes, the presence and number of organelles such as the endoplasmic reticulum and even provide a measure of the overall “internal complexity” of the cell. One might expect these parameters to be lower in stem cells than in their differentiated progeny. These achievable steps will establish the dielectric properties of cells as an important factor to be included in stem cell research.
4. Opportunity to Apply DEP to Pluripotent Stem Cells
An as yet unexplored opportunity is the application of DEP to pluripotent stem cells (pSCs). Whether derived from embryos of varying stages of development [27, 28], or induced by expression of nuclear factors in somatic cells [29], pluripotent stem cell isolation and renewal are significantly challenged by the absence of noninvasive methods to discriminate and specifically promote the growth of this cell type either from limiting quantities of tissue (e.g., embryos) or amidst competing unreprogrammed somatic cells during induction protocols, respectively. This affects the efficiency of their isolation and interferes with the ability to achieve clonal cell lines. The propensity of pSCs to spontaneously differentiate and their unpredictability to commit to specific lineages underlines the need for sensitive and noninvasive methods to monitor and separate cell populations. This is especially the case when coculturing these cells with other cell types (e.g., feeders) supportive of self-renewal and/or differentiation. Alternatively, in a therapeutic context, DEP may provide us with a simple and noninvasive way to positively or negatively select for target or contaminating cell types prior to transplantation.
5. Conclusion
While as a technology DEP has been available for over three decades, its unique predictive power to define cellular properties on the basis of the motion of electrically polarisable particles is just beginning to be explored in the context of stem cell biology and medicine. The properties exploited by DEP are intimately associated with the cell's dimensions and physico-chemical properties, and the extent to which otherwise invisible differences between cells can be distinguished by DEP has yet to be fully understood. However, the rewards for doing so are great, providing the opportunity to distinguish between subpopulations of cellular phenotypes which is much needed to fully realise the promise of stem cells in regenerative medicine.
References
- 1.Talary MS, Mills KI, Hoy T, Burnett AK, Pethig R. Dielectrophoretic separation and enrichment of CD34+ cell subpopulation from bone marrow and peripheral blood stem cells. Medical and Biological Engineering and Computing. 1995;33(2):235–237. doi: 10.1007/BF02523050. [DOI] [PubMed] [Google Scholar]
- 2.Stephens M, Talary MS, Pethig R, Burnett AK, Mills KI. The dielectrophoresis enrichment of CD34+ cells from peripheral blood stem cell harvests. Bone Marrow Transplantation. 1996;18(4):777–782. [PubMed] [Google Scholar]
- 3.Vykoukal J, Vykoukal DM, Freyberg S, Alt EU, Gascoyne PRC. Enrichment of putative stem cells from adipose tissue using dielectrophoretic field-flow fractionation. Lab on a Chip. 2008;8(8):1386–1393. doi: 10.1039/b717043b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang X, Becker FF, Gascoyne PR. Membrane dielectric changes indicate induced apoptosis in HL-60 cells more sensitively than surface phosphatidylserine expression or DNA fragmentation. Biochimica et Biophysica Acta. 2002;1564(2):412–420. doi: 10.1016/s0005-2736(02)00495-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pethig R, Bressler V, Carswell-Crumpton C, et al. Dielectrophoretic studies of the activation of human T lymphocytes using a newly developed cell profiling system. Electrophoresis. 2002;23(13):2057–2063. doi: 10.1002/1522-2683(200207)23:13<2057::AID-ELPS2057>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 6.Pethig R, Talary MS. Dielectrophoretic detection of membrane morphology changes in Jurkat T-cells undergoing etoposide-induced apoptosis. IET Nanobiotechnology. 2007;1(1):2–9. doi: 10.1049/iet-nbt:20060018. [DOI] [PubMed] [Google Scholar]
- 7.Flanagan LA, Lu J, Wang L, et al. Unique dielectric properties distinguish stem cells and their differentiated progeny. Stem Cells. 2008;26(3):656–665. doi: 10.1634/stemcells.2007-0810. [DOI] [PubMed] [Google Scholar]
- 8.Jones TB. Electromechanics of Particles. Cambridge, UK: Cambridge University Press; 1995. [Google Scholar]
- 9.Morgan H, Green NG. AC Electrokinetics: Colloids and Nanoparticles. Baldock, UK: Research Studies Press; 2003. [Google Scholar]
- 10.Hughes MP. Nanoelectromechanics in Engineering and Biology. Boca Raton, Fla, USA: CRC Press; 2003. [Google Scholar]
- 11.Burt JPH, Al-Ameen TAK, Pethig R. An optical dielectrophoresis spectrometer for low-frequency measurements on colloidal suspensions. Journal of Physics E. 1989;22(11):952–957. [Google Scholar]
- 12.Burt JPH, Pethig R, Gascoyne PRC, Becker FF. Dielectrophoretic characterisation of Friend murine erythroleukaemic cells as a measure of induced differentiation. Biochimica et Biophysica Acta. 1990;1034(1):93–101. doi: 10.1016/0304-4165(90)90158-s. [DOI] [PubMed] [Google Scholar]
- 13.Asami K, Takahashi Y, Takashima S. Dielectric properties of mouse lymphocytes and erythrocytes. Biochimica et Biophysica Acta. 1989;1010(1):49–55. doi: 10.1016/0167-4889(89)90183-3. [DOI] [PubMed] [Google Scholar]
- 14.Ermolina I, Polevaya Yu, Feldman Yu. Analysis of dielectric spectra of eukaryotic cells by computer modeling. European Biophysics Journal. 2000;29(2):141–145. doi: 10.1007/s002490050259. [DOI] [PubMed] [Google Scholar]
- 15.Asami K, Hanai T, Koizumi N. Dielectric approach to suspensions of ellipsoidal particles covered with a shell in particular reference to biological cells. Japanese Journal of Applied Physics. 1980;(2):359–365. [Google Scholar]
- 16.Pethig R, Jakubek LM, Sanger RH, Heart E, Corson ED, Smith PJS. Electrokinetic measurements of membrane capacitance and conductance for pancreatic β-cells. IEE Proceedings. 2005;152(6):189–193. doi: 10.1049/ip-nbt:20050040. [DOI] [PubMed] [Google Scholar]
- 17.Holmes D, Pettigrew D, Reccius CH, et al. Leukocyte analysis and differentiation using high speed microfluidic single cell impedance cytometry. Lab on a Chip. 2009;9(20):2881–2889. doi: 10.1039/b910053a. [DOI] [PubMed] [Google Scholar]
- 18.Pethig R. Encyclopedia of Surface and Colloidal Science. New York, NY, USA: Taylor & Francis; 2006. Dielectrophoresis of biological cells; pp. 1719–1736. [Google Scholar]
- 19.Bone S, Lee RS, Hodgson CE. Dielectric studies of intermolecular interactions in native DNA. Biochimica et Biophysica Acta. 1996;1306(1):93–97. doi: 10.1016/0167-4781(95)00232-4. [DOI] [PubMed] [Google Scholar]
- 20.Pethig R, Kell DB. The passive electrical properties of biological systems: their significance in physiology, biophysics and biotechnology. Physics in Medicine and Biology. 1987;32(8):933–970. doi: 10.1088/0031-9155/32/8/001. [DOI] [PubMed] [Google Scholar]
- 21.Turgeon ML. Clinical Hematology: Theory and Procedures. 4th edition. Baltimore, Md, USA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 22.Arpitha P, Prajna NV, Srinivasan M, Muthukkaruppan V. High expression of p63 combined with a large N/C ratio defines a subset of human limbal epithelial cells: implications on epithelial stem cells. Investigative Ophthalmology and Visual Science. 2005;46(10):3631–3636. doi: 10.1167/iovs.05-0343. [DOI] [PubMed] [Google Scholar]
- 23.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental Cell. 2004;6(4):483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 24.Gao L, McBeath R, Chen CS. Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem Cell. 2010;28(3):564–572. doi: 10.1002/stem.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voldman J. Electrical forces for microscale cell manipulation. Annual Review of Biomedical Engineering. 2006;8:425–454. doi: 10.1146/annurev.bioeng.8.061505.095739. [DOI] [PubMed] [Google Scholar]
- 26.Vykoukal J, Vykoukal D. Dielectrophoretic methods for biomedical applications. In: Yih TC, Talpasanu I, editors. Micro and Nano Manipulations for Biomedical Applications. Norwood, Mass, USA: Artech House; 2008. pp. 179–213. [Google Scholar]
- 27.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 28.Klimanskaya I, Chung Y, Becker S, Lu S-J, Lanza R. Human embryonic stem cell lines derived from single blastomeres. Nature. 2006;444(7118):481–485. doi: 10.1038/nature05142. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]