Abstract
Background/Aims
Interferon beta (IFN-β) has been shown to have antiviral activity, and thus could be useful in treating viral infections. Therefore, we compared the efficacy and safety of recombinant IFN-β (IFN-β-1a) plus oral ribavirin versus interferon alpha (IFN-α) plus ribavirin therapy for the treatment of chronic hepatitis C (HCV).
Methods
Twenty treatment-naïve patients were randomized into two equal-sized treatment groups. Both IFN-β-1a (44 µg) and IFN-α (3 MIU) were given subcutaneously three times a week, while ribavirin was given orally at 1,000-1,200 mg/day. Patients were treated for 24 weeks and followed for an additional 24 weeks.
Results
After 24 weeks of treatment, six (60%) and four patients (40%) in the IFN-β-1a group and IFN-α groups, respectively, achieved viral clearance. The sustained virological response (SVR) at the end of the observation period was similar in both groups (40%). However, the baseline viral load was significantly higher (p=0.034) in the IFN-β-1a group than in the IFN-α group, and there were more HCV genotype 1 patients in the IFN-β-1a group (eight versus seven). The IFN-β-1a group was associated with similar adverse events in terms of frequency and severity.
Conclusions
The SVR rate and safety profile were similar for the combination of IFN-β-1a and ribavirin and that of IFN-α and ribavirin.
Keywords: Hepatitis C, Treatment outcome, Interferons, Prospective studies
INTRODUCTION
Since the isolation of hepatitis C virus (HCV) genome back in 1989 and the establishment of consequences of the infection, treatment modalities have undergone several changes over the last decade.1-4 From the early era when interferon alpha (IFN-α) monotherapy was given at 3 MIU for 24 week with a sustained virological response (SVR) rate of less than 10% to the current treatment of 48-week pegylated IFN-α plus ribavirin combined where 56% of SVR rate could be obtained, significant progresses have been achieved to counter this chronic infectious disease.5-7
Despite this achievements, there remains a large cohort of patients who do not have their viruses cleared even after 48 weeks of treatment with pegylated IFN-α plus ribavirin therapy. This includes approximately 50% of the difficult-to-treat HCV genotype 1 cohort and about 20% of the HCV genotype type 2 or 3 cohort.8 Re-treatment with similar regimen after the initial failure is not an optimal choice as the outcome is relatively poor.9,10 Furthermore, the IFN-α based treatment is not without side effects. Patients often have to discontinue treatment due to intolerable side effects or reduce the treatment dosages to minimize such effects.11 Indeed, there is an urgent need to investigate other treatment options to close the gaps on efficacies and safety profiles of the current regimens.
Interferon beta (IFN-β) is classified under the same type I IFN family with IFN-α since both molecules share a common cell surface receptor.12,13 Due to its antiviral activities, natural IFN-β produced by fibroblast cells has been used extensively in Japan for the treatment of HCV infection.14-17 Unfortunately, the intravenous administration route of the current natural IFN-β did not offer the same convenience compared to the subcutaneous route used in the IFN-α administration. Hence, the usage of natural IFN-β has largely been confined to Japan.
Recombinant IFN-β (IFN-β-1a), produced by mammalian cells, has a similar structure and glycosylation as the naturally occurring IFN-β.18 Early study in HCV infection showed that it had similar antiviral properties as IFN-α monotherapy.19 Recently, it has been shown to be safe and efficacious when combined with ribavirin.20 Another recent report has highlighted its potential therapeutic properties in Chinese population.21 However, all of these studies had been performed without a comparative IFN-α treatment arm.
We had conducted a prospective, randomized, comparative pilot study with two arms consisting of IFN-β-1a plus ribavirin versus IFN-α plus ribavirin in Korean population to investigate the efficacy and safety of both combination therapies in the same clinical setting.
MATERIALS AND METHODS
1. Patients
We included patients at the age 18 or above with chronic HCV infection confirmed by HCV-RNA reverse transcriptase-polymerase chain reaction (RT-PCR). Patients should have an elevated serum alanine aminotransferase (ALT) with level between 1.5 times and 10 times the upper limit of normal. All had adequate bone marrow reserve and organ function.
Patients were excluded if they had undergone previous treatment with an IFN, clinical evidence of liver cirrhosis defined by a Child-Pugh score of 7 and above, history of hepatic failure, other viral hepatitis, history of immunologically mediated disease, chronic renal impairment, history of cancer.
2. Study design
This was an open, randomized, comparative pilot study conducted in a gastroenterology unit of a university-affiliated hospital in Korea. The study was reviewed and approved by the hospital Ethics Committee. Each patient was provided with his/her written consent prior to the start of the study.
After confirmation of patient's eligibility, he/she would be randomized to receive IFN-β-1a (Rebif®, Serono international, Geneva, Switzerland) plus ribavirin or IFN-α (Intermax-alpha®, LG Biotech, Taejeon, Korea) plus ribavirin. IFN-β-1a at 44 mcg per dose and IFN-α at 3 MIU per dose were given three times a week subcutaneously. Ribavirin was given orally twice a day with a total daily dosage of 1,000-1,200 mg depending on the weight of the patient. The treatment duration was 24 weeks followed by a 24-week observation phase. However, at treatment week 12, patient who did not achieve a virological response, defined as a decrease of at least 2 log viral load from the baseline, was removed from the treatment but would be included in the 24-week observation phase. Sustained virological response (SVR) was defined as a viral clearance, measured by a qualitative HCV RNA assay, at both the end of treatment and end of observation phase.
Baseline assessments included hematology, blood chemistry, HCV genotyping and serum HCV RNA measurement. Virological responses were assessed at treatment week 12, treatment week 24 and end of the observation period using both qualitative and quantitative HCV RNA assays. Qualitative HCV RNA measurement was performed using a polymerase chain reaction assay with primers specific for the 5' untranslated region of the HCV genome (COBAS Amplicor, version 2.0; Roche Diagnostics, Branchburg, NJ, USA) with a lower sensitivity of 50 IU/ml while the quantitative HCV RNA measurement was done using the COBAS Monitor Amplicor HCV 2.0 (Roche Diagnostics, Meylan, France) with a lower detection limit of 600 IU/mL. Safety was assessed by monitoring the adverse events and changes in the laboratory parameters at each study visits
3. Statistical analysis
Data from both groups were analyzed and presented in a descriptive manner in terms of percentages and ranges. The assessments of baseline characteristics were performed for the total study population. HCV genotype and gender were assessed by Chi-square test. Baseline parameters, for example, ALT and HCV RNA levels were assessed by the nonparametric test Mann-Whitney U-test. The two-tailed significance level was set at 5%.
RESULTS
1. Patient characteristics
A total of 20 patients, 10 for each treatment group, were enrolled and completed the study between December 2003 and March 2005. One patient with genotype 1b from each arm was considered as a non-responder at treatment week 12. In the IFN-α group, another patient decided to withdraw her consent after 8 weeks of the treatment. In total, 19 patients completed the study with 17 completed the 24 weeks of treatment followed by 24 weeks of observation.
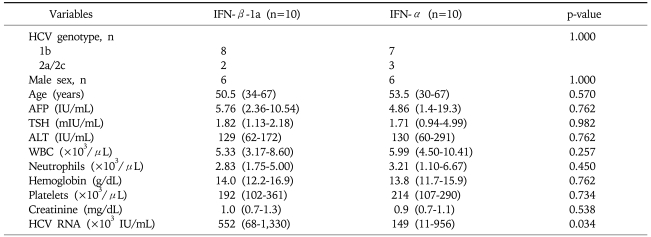
All baseline parameters, except viral load, were found to be similar between the two groups (Table 1). IFN-β-1a group had a significantly higher (p=0.034) viral load as compared to that in the IFN-α group. IFN-β-1a had one additional patient with HCV genotype 1 infection than IFN-α group.
Table 1.
Baseline Characteristics of the Patients
Qualitative variables are expressed as the median (25th-75th percentile).
IFN-β, interferon beta; IFN-α, interferon-alpha; AFP, alpha-fetoprotein; TSH, thyroid-stimulating hormone; ALT, alanine aminotransferase; WBC, white blood cells; HCV, hepatitis C virus.
2. Efficacy
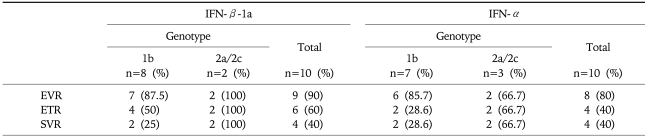
The early virological response (EVR, treatment week 12), end-of-treatment response (ETR, treatment week 24) and sustained virological response (SVR, the 24-week observation phase) are similar between the 2 groups (Table 2). Nine out of 10 patients and 8 out of 10 patients had an EVR in the IFN-β-1a and IFN-α group respectively. The ETR was lower for both groups as compared to the EVR. At the end of the study, SVR was obtained in 4 out of 10 patients (40%) in both groups. Especially, the SVR was lower for patients with genotype 1b as compared to that with the genotype 2a/2c.
Table 2.
Overall Virological Responses
IFN-β, interferon beta; IFN-α, interferon-alpha; EVR, early virological response (treatment week 12); ETR, end-of-treatment response (treatment week 24); SVR, sustained virologic response (the 24-week observation phase).
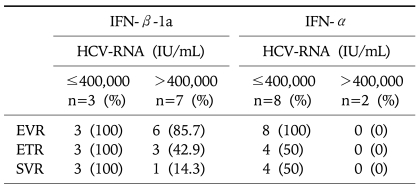
By categorizing the virological response in terms of viral load, those greater than 400,000 IU/mL, none of the 2 patients in the IFN-α group showed any virological response in the 3 time points measured. In the IFN-β-1a group, 3 out of 7 patients achieved ETR though only one patient eventually achieved SVR (Table 3).
Table 3.
Virological Responses in the Cohort with a High Baseline Viral Load
IFN-β, interferon beta; IFN-α, interferon-alpha; HCV, hepatitis C virus; EVR, early virological response (treatment week 12); ETR, end-of-treatment response (treatment week 24); SVR, sustained virologic response (the 24-week observation phase).
3. Safety and tolerability
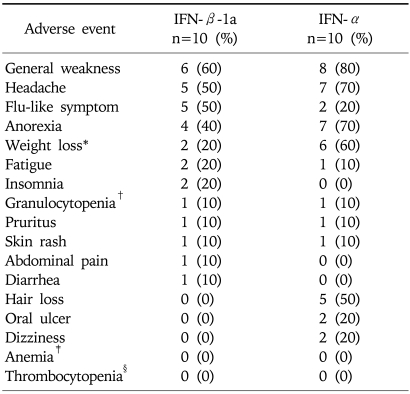
19 of the 20 patients completed the study. One patient with genotype 2a/2c in the IFN-α group decided to discontinue the study after 8 weeks of treatment due to general weakness and fatigue. In general, both treatments were well tolerated and the adverse events were mild to moderate (Table 4). There was no clinically significant differences in the laboratory parameters between the two groups at all the time points. No serious adverse event attributed to the study treatment was reported.
Table 4.
Incidence of Adverse Events during the Treatment Period
*Weight loss>2 kg/month.
†WBC<750/µL.
‡Hgb<10 g/dL.
§Platelet<50,000/uL.
DISCUSSION
Currently, IFN-α based antiviral therapies are well-accepted and approved regimens for the treatment of chronic HCV infection. Despite the modification of the IFN-α molecule from the conventional form to the pegylated form and the extension of treatment duration from 24 weeks to 48 weeks, there is still a substantial proportion of patients who did not achieve a SVR.4 The outlook is still disappointing for the approximately 50% of patients with HCV genotype 1 infection. Despite the encouraging breakthroughs in search of the next solution for HCV infection, it may still take several years before any new treatment can be approved for widespread use.8,22 In view of the situation, IFN-β appears to be an attractive alternative to be investigated since it too has antiviral activities and binds to the same IFN type 1 receptor as IFN-α.
Although natural IFN-β has been used to treat HCV infection in Japan for quite a while and its virological response is similar to IFN-α, its intravenous route of administration is probably the main obstacle preventing it from becoming a treatment of choice.23,24 In an early study, natural IFN-β given 6 MIU 3 times a week intramuscularly had been compared to IFN-α in patients with HCV infection not responding to previous IFN-α therapy. The virological response was poorer in the IFN-β group.25 Another report looked into the use natural IFN-β via subcutaneous injection. In this study, 6 MIU of natural IFN-β was given subcutaneously at 3 times a week for 6 months. Though the response was not optimum, the authors suggested that a higher dose might be used based on the excellent tolerance.26
Another form of IFN-β produced by recombinant DNA technology, recombinant IFN-β (IFN-β-1a), had been approved for the treatment of multiple sclerosis for nearly 10 years.27 Habersetzer et al.19 were the first group to study the use of IFN-β-1a in HCV treatment. In their pilot study using IFN-β-1a 3 times a week for 24 weeks, the low-dose arm (9 MIU) had a better outcome than the high-dose arm (12 MIU). Apparently, the baseline viral load was twice in the high-dose arm. This might contribute to the poor response in the high-dose group. The authors strongly suggested further evaluation of IFN-β-1a, especially in combination with ribavirin, in the treatment of chronic HCV infection.
Recently, two other studies using IFN-β-1a had been reported. Cheng et al.21 reported results of IFN-β-1a monotherapy in 270 patients who had HCV infection that was non-responsive to IFN-α therapy. Although the overall SVR was merely 3.4%, a sub-group analysis provided the interesting finding that Chinese population achieved a significantly higher SVR (21.7%) suggesting an ethnic factor at the work. Pellicano et al.20 were the first to report the use of IFN-β-1a plus ribavirin combination therapy. Their study, conducted in treatment-naïve Italian population, compared IFN-β-1a monotherapy and IFN-β-1a plus ribavirin combination therapy. IFN-β-1a was administered subcutaneously daily at 6 MIU (22 mcg) for 24 weeks. The SVR rate obtained were 21.6% and 27.5% in the monotherapy and combination therapy, respectively. They noted both HCV genotype and baseline viral load as predictive factors of response.20
Our pilot study was the first to report IFN-β-1a administered subcutaneously 3 times a week plus daily ribavirin combination therapy and compare it with IFN-α plus ribavirin in treatment-naïve patients with the same clinical setting. Our SVR results (40%) for IFN-β-1a combination therapy were better than that reported Pellicano et al.20 Although in their study, IFN-β-1a was given daily instead of 3 times a week, the total dosages and treatment duration were similar to ours. One possibility of the better response seen in our study could be the ethnic factor suggested by Cheng et al.21 According to their analysis, Caucasian population seemed to response poorly with IFN-β-1a therapy. Such finding has also been observed in IFN-α therapy.28 Recently, Chan et al.29 strongly supported the use of IFN-β-1a as an alternative to the IFN-α for Asian patients with chronic hepatitis C. The present study was more consistent with that from Cheng et al.21 since we would expect a much better response from the treatment-naïve population.
The other interesting finding in our study was the trend toward a better response for IFN-β-1a therapy in the patients with higher baseline viral load. As pointed out by Castro et al.26, investigation of higher doses of IFN-β-1a were legitimate based on its safety profile. Although the results from Habersetzer et al.19 may seem puzzling with the low-dose group giving a better response, we believe this was probably due to the significantly higher baseline viral load in the high-dose group. It is well recognized today that baseline viral load, especially in HCV genotype 1, is a strong predictor of treatment response. Our results indicated that IFN-β-1a combination therapy might be more effective in patients with high viral load as compared to IFN-α combination therapy.
In our study, IFN-β-1a group appeared to be associated with similar adverse events in terms of frequency and severity when compared to IFN-α group. Although there was no significant difference between two groups, two severe adverse events (headache and general weakness) were reported in the IFN-α group but none reported in the IFN-β-1a group. A recent review had confirmed the better safety profile of IFN-β-1a in the treatment of chronic HCV infection.30 High dose IFN-β-1a of 24 MIU (88 mcg) given daily for 48 weeks had been investigated previously in HCV infected patients and the dosage was found to be well tolerated.21 Though IFN-α has an acceptable safety profile in HCV infected population, it does have some severe potential side effects and we do not foresee a higher dose of IFN-α as a viable option.31 In this aspect, IFN-β-1a can be a potential candidate in the cohort of patients who cannot tolerate IFN-α therapy. Recently, the phase I study of pegylated IFN-β-1a is ongoing in the patients with multiple sclerosis in Europe. In addition, the availability of pegylated IFN-β-1a in the near future may offer an alternative to the current pegylated IFN-α therapy.32
The limitation of this study was due to its small sample size. Thus the results of this pilot study need to be confirmed in a larger patient population. If the efficacy of IFN-β-1a could be confirmed in a larger number of patients, IFN-β-1a would offer an alternative to the IFN-α therapy in patients with chronic hepatitis C. As another perspective, our study couldn't elucidate the effect of the IFN-β-1a for HCV patients with genotype 1. In general, a full 48-week therapy is required of the patients infected with genotype 1. Therefore, it is likely that 24 weeks of treatment might have been insufficient for those infected with genotype 1.
In conclusion, our pilot study provided IFN-β-1a combination therapy might be considerably advantageous for patients with chronic hepatitis C in terms of efficacy as well as safety. A larger comparative study is needed to confirm these encouraging findings especially in Asian populations.
ACKNOWLEDGEMENTS
This study was supported by a research grant from Serono International SA, Geneva, Switzerland.
References
- 1.Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes of Health. National Institutes of Health Consensus Development Conference Statement: Management of Hepatitis C: 2002--June 10-12, 2002. Hepatology. 2002;36:S3–S20. doi: 10.1053/jhep.2002.37117. [DOI] [PubMed] [Google Scholar]
- 3.Davis GL, Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transpl. 2003;9:331–338. doi: 10.1053/jlts.2003.50073. [DOI] [PubMed] [Google Scholar]
- 4.Strader DB, Wright T, Thomas DL, Seeff LB American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2004;39:1147–1171. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 5.Myers RP, Regimbeau C, Thevenot T, et al. Interferon for interferon naive patients with chronic hepatitis C. Cochrane Database Syst Rev. 2002;(2):CD000370. doi: 10.1002/14651858.CD000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thévenot T, Regimbeau C, Ratziu V, Leroy V, Opolon P, Poynard T. Meta-analysis of interferon randomized trials in the treatment of viral hepatitis C in naive patients: 1999 update. J Viral Hepat. 2001;8:48–62. doi: 10.1046/j.1365-2893.2001.00271.x. [DOI] [PubMed] [Google Scholar]
- 7.Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 8.Pawlotsky JM. Hepatitis C: it's a long way to new therapy, it's a long way to go. Gastroenterology. 2004;127:1629–1632. doi: 10.1053/j.gastro.2004.09.059. [DOI] [PubMed] [Google Scholar]
- 9.San Miguel R, Guillén F, Cabasés JM, Buti M. Meta-analysis: combination therapy with interferon-alpha 2a/2b and ribavirin for patients with chronic hepatitis C previously non-responsive to interferon. Aliment Pharmacol Ther. 2002;16:1611–1621. doi: 10.1046/j.1365-2036.2002.01328.x. [DOI] [PubMed] [Google Scholar]
- 10.Cammà C, Bruno S, Schepis F, et al. Retreatment with interferon plus ribavirin of chronic hepatitis C non-responders to interferon monotherapy: a meta-analysis of individual patient data. Gut. 2002;51:864–869. doi: 10.1136/gut.51.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36:S237–S244. doi: 10.1053/jhep.2002.36810. [DOI] [PubMed] [Google Scholar]
- 12.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodbourn S, Didcock L, Randall RE. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J Gen Virol. 2000;81:2341–2364. doi: 10.1099/0022-1317-81-10-2341. [DOI] [PubMed] [Google Scholar]
- 14.Kaito M, Yasui-Kawamura N, Iwasa M, et al. Twice-a-day versus once-a-day interferon-beta therapy in chronic hepatitis C. Hepatogastroenterology. 2003;50:775–778. [PubMed] [Google Scholar]
- 15.Suzuki F, Chayama K, Tsubota A, et al. Twice-daily administration of interferon-beta for chronic hepatitis C is not superior to a once-daily regimen. J Gastroenterol. 2001;36:242–247. doi: 10.1007/s005350170110. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda K, Arase Y, Saitoh S, et al. Interferon beta prevents recurrence of hepatocellular carcinoma after complete resection or ablation of the primary tumor-A prospective randomized study of hepatitis C virus-related liver cancer. Hepatology. 2000;32:228–232. doi: 10.1053/jhep.2000.9409. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi Y, Watanabe S, Konishi M, et al. Quantitation and typing of serum hepatitis C virus RNA in patients with chronic hepatitis C treated with interferon-beta. Hepatology. 1993;18:1319–1325. [PubMed] [Google Scholar]
- 18.Buchwalder PA, Buclin T, Trinchard I, Munafo A, Biollaz J. Pharmacokinetics and pharmacodynamics of IFN-beta 1a in healthy volunteers. J Interferon Cytokine Res. 2000;20:857–866. doi: 10.1089/10799900050163226. [DOI] [PubMed] [Google Scholar]
- 19.Habersetzer F, Boyer N, Marcellin P, et al. A pilot study of recombinant interferon beta-1a for the treatment of chronic hepatitis C. Liver. 2000;20:437–441. doi: 10.1034/j.1600-0676.2000.020006437.x. [DOI] [PubMed] [Google Scholar]
- 20.Pellicano R, Craxi A, Almasio PL, et al. Interferon beta-1a alone or in combination with ribavirin: a randomized trial to compare efficacy and safety in chronic hepatitis C. World J Gastroenterol. 2005;11:4484–4489. doi: 10.3748/wjg.v11.i29.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng PN, Marcellin P, Bacon B, et al. Racial differences in responses to interferon-beta-1a in chronic hepatitis C unresponsive to interferon-alpha: a better response in Chinese patients. J Viral Hepat. 2004;11:418–426. doi: 10.1111/j.1365-2893.2004.00514.x. [DOI] [PubMed] [Google Scholar]
- 22.De Francesco R, Migliaccio G. Challenges and successes in developing new therapies for hepatitis C. Nature. 2005;436:953–960. doi: 10.1038/nature04080. [DOI] [PubMed] [Google Scholar]
- 23.Omata M, Yokosuka O, Takano S, et al. Resolution of acute hepatitis C after therapy with natural beta interferon. Lancet. 1991;338:914–915. doi: 10.1016/0140-6736(91)91774-o. [DOI] [PubMed] [Google Scholar]
- 24.Barbaro G, Di Lorenzo G, Soldini M, et al. Intravenous recombinant interferon-beta versus interferon-alpha-2b and ribavirin in combination for short-term treatment of chronic hepatitis C patients not responding to interferon-alpha. Multicenter Interferon Beta Italian Group Investigators. Scand J Gastroenterol. 1999;34:928–933. doi: 10.1080/003655299750025426. [DOI] [PubMed] [Google Scholar]
- 25.Pérez R, Pravia R, Artímez ML, et al. Clinical efficacy of intramuscular human interferon-beta vs interferon-alpha 2b for the treatment of chronic hepatitis C. J Viral Hepat. 1995;2:103–106. doi: 10.1111/j.1365-2893.1995.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 26.Castro A, Carballo E, Domínguez A, et al. Tolerance and efficacy of subcutaneous interferon-beta administered for treatment of chronic hepatitis C. J Interferon Cytokine Res. 1997;17:65–67. doi: 10.1089/jir.1997.17.65. [DOI] [PubMed] [Google Scholar]
- 27.Murdoch D, Lyseng-Williamson KA. Spotlight on subcutaneous recombinant interferon-beta-1a (Rebif) in relapsing-remitting multiple sclerosis. BioDrugs. 2005;19:323–325. doi: 10.2165/00063030-200519050-00005. [DOI] [PubMed] [Google Scholar]
- 28.Hepburn MJ, Hepburn LM, Cantu NS, Lapeer MG, Lawitz EJ. Differences in treatment outcome for hepatitis C among ethnic groups. Am J Med. 2004;117:163–168. doi: 10.1016/j.amjmed.2004.02.043. [DOI] [PubMed] [Google Scholar]
- 29.Chan HL, Ren H, Chow WC, Wee T. Interferon beta-1a Hepatitis C Study Group. Randomized trial of interferon beta-1a with or without ribavirin in Asian patients with chronic hepatitis C. Hepatology. 2007;46:315–323. doi: 10.1002/hep.21683. [DOI] [PubMed] [Google Scholar]
- 30.Festi D, Sandri L, Mazzella G, et al. Safety of interferon beta treatment for chronic HCV hepatitis. World J Gastroenterol. 2004;10:12–16. doi: 10.3748/wjg.v10.i1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bagheri H, Fouladi A, Barange K, et al. Follow-up of adverse drug reactions from peginterferon alfa-2b-ribavirin therapy. Pharmacotherapy. 2004;24:1546–1553. doi: 10.1592/phco.24.16.1546.50947. [DOI] [PubMed] [Google Scholar]
- 32.Mager DE, Neuteboom B, Jusko WJ. Pharmacokinetics and pharmacodynamics of PEGylated IFN-beta 1a following subcutaneous administration in monkeys. Pharm Res. 2005;22:58–61. doi: 10.1007/s11095-004-9009-z. [DOI] [PubMed] [Google Scholar]