Abstract
Background/Aims
The incidence of colorectal cancer is increasing in Korea, but the epidemiology of colorectal neoplasm is not clearly defined. We aimed to elucidate the prevalence of colorectal neoplasm in average-risk Koreans and explore the underlying risk factors.
Methods
A large-scale, multicenter, prospective study was conducted. Of the 19,460 subjects who underwent colonoscopy at 11 university hospitals, we analyzed 3,951 consecutive asymptomatic adults with no risk factors for colorectal cancer.
Results
The subjects were aged 52.1±11.6 years (mean±SD) and 60.1% of them were men. The prevalences of colorectal neoplasm and advanced neoplasm were 33.3% and 2.2%, respectively. The prevalence of a neoplasm increased with age (trend: p<0.001) and was higher in males (p<0.001). The prevalence of a proximal neoplasm was higher in subjects with a distal neoplasm than in those without a distal neoplasm (11.9% vs. 5.4%, p<0.001). However, 150 (52.1%) of the 288 subjects with a proximal neoplasm had no distal neoplasm.
Conclusions
The overall prevalence of colorectal neoplasm in asymptomatic average-risk Koreans is comparable with that in Western countries. Being male and older are associated with a higher risk of colorectal neoplasm. Over half of proximal neoplasms are not associated with any distal sentinel lesions.
Keywords: Prevalence, Colonoscopy, Colorectal neoplasms, Korea, Mass screening
INTRODUCTION
Colorectal cancer is one of the most common malignancies in Western countries and the second leading cause of cancer death in the United States.1 Colorectal cancer incidence rates appear to be increasing in Asian countries.2-4 There has been remarkable increase of colorectal cancer and cancers of the lung, the liver, the stomach, and the colorectum, in that order, are the most common causes of cancer death in Koreans.5 Cancers of the stomach and the liver have traditionally predominated in Korea, but the incidence rates of those cancers have been declining gradually, while that of colorectal cancer has continued to increase.
Because most colorectal cancers develop from adenomas, the incidence and mortality of colorectal cancer can be reduced when precursor adenomas are removed.6,7 Based upon this adenoma-carcinoma sequence and epidemiologic data about colorectal neoplasm, guidelines for the prevention, early diagnosis and treatment of colorectal cancer have been recently published in Western countries.8-10 In Korea, the Korean Society of Coloproctology and National Cancer Center recommended a guideline for colorectal cancer screening and surveillance in 2001.11 However, the guideline was made without the support of robust data about the epidemiology of colorectal neoplasm in Korean. Accordingly, the usefulness of flexible sigmoidoscopy as a screening tool or the relevance of the start of colorectal cancer screening at age 50 years in asymptomatic average-risk Korean was not evaluated enough.
We conducted a prospective, multicenter colonoscopy survey to evaluate the prevalence of colorectal neoplasm in average-risk Koreans and explore risk factors for colorectal neoplasm.
MATERIALS AND METHODS
1. Study population
We aimed to prospectively include consecutive asymptomatic Korean adults who were undergoing colonoscopy as a screening procedure in 11 university hospitals from July 2003 to June 2004. A total of 19,460 colonoscopies were performed during the study period. Subjects were excluded if they met one of the following criteria: (i) symptoms of lower gastrointestinal tract disease, including rectal bleeding, changes in bowel habits, or lower abdominal pain that required medical evaluation; (ii) family history of colorectal cancer or adenomatous polyps in first-degree relatives; (iii) personal history of colorectal cancer, polyps, or inflammatory bowel disease; or (iv) history of colorectal examination (including sigmoidoscopy, colonoscopy, or barium enema) within the previous 10 years, or colorectal surgery. In addition, subjects whose colonoscopy failed to reach the cecum were excluded from the analysis. Finally, 3,951 asymptomatic subjects who had a complete colonoscopy as the primary screening procedure were analyzed in this study. Informed consent was obtained from all subjects and this study was carried out in accordance with the Helsinki Declaration.12
2. Study procedures
Colonoscopies were performed at the participating centers by experienced colonoscopists. Examinations were considered complete when a colonoscope reached the cecum. During the examination, all polypoid lesions were documented for location and size. The distal colon was defined as the rectum, sigmoid colon and descending colon. The proximal colon was defined as the splenic flexure and the more proximal portions of the colon. The size of each polyp was estimated by the use of an 8-mm-diameter open-biopsy forceps.
All the polyps were biopsied or polypectomized, and the obtained specimens were sent to pathology laboratories at the participating centers for the histologic examination.
Colorectal neoplasm was defined as an adenoma or invasive cancer. Invasive cancer was defined as the invasion of malignant cells beyond the muscularis mucosa. Advanced adenoma was defined as an adenoma with a diameter of 10 mm or more, a villous adenoma (i.e., at least 25% villous architecture), or an adenoma with high grade dysplasia. Advanced neoplasm was defined as an advanced adenoma or invasive cancer. Patients with multiple neoplasms were categorized on the basis of their most advanced lesion to determine the prevalence of the pathologic features. For example, a patient with a villous adenoma and a tubular adenoma was categorized as having a villous adenoma. Finding such as hyperplastic or inflammatory polyp was regarded as non-neoplastic lesion.
All the data collected prospectively at the participating centers were recorded in Microsoft Access database designed by the Korean Association for the Study of Intestinal Diseases (KASID). After the end of the study period, all the database at each center were collected and merged for the analyses.
3. Statistical analysis
Statistical analyses were performed with SPSS version 12.0 (SPSS Inc, Chicago, IL, USA). When appropriate, results were expressed as proportions, means and standard deviations (SD). The Pearson χ2 test, Fisher's exact test, and the likelihood ratio test for trend were used to assess relationship between age, gender, and prevalence of neoplasm. A p-value less than 0.05 was considered statistically significant.
RESULTS
1. Characteristics of study population
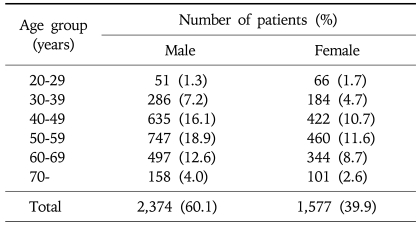
A total of 3,951 subjects who met the study criteria were included for the analyses. Among them, 2,374 were men (60.1%). The mean age (±SD) was 52.1±11.6 years (range, 20-86 years). In this cohort, 2,307 subjects (58.4%) were 50 years or older. Demographic data is shown in Table 1.
Table 1.
Age Distribution of the 3,951 Subjects
2. Prevalence of colorectal neoplasm
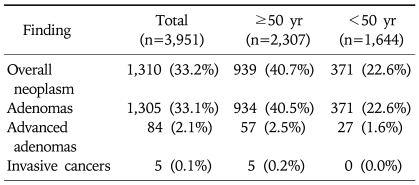
Among the 3,951 asymptomatic subjects, 1,310 (33.2%) were found to have colorectal neoplasm (non-advanced adenoma 31.0%; advanced adenoma 2.1%; invasive cancer 0.1%) (Table 2). When the analysis was restricted to the 2,307 subjects who were 50 years or older, the prevalence of colorectal neoplasm increased to 40.7% (non-advanced adenoma 38.0%; advanced adenoma 2.5%; invasive cancer 0.2%).
Table 2.
Colonoscopy Findings according to the Most Advanced Lesion
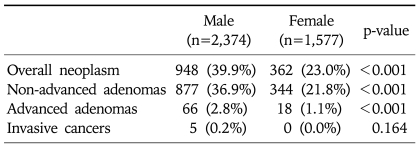
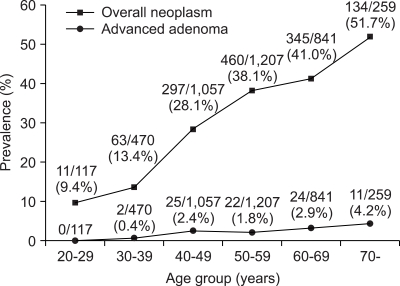
The prevalence of colorectal neoplasm was higher in men (948/2,374, 39.9%) than in women (362/1,577, 23.0%) (p<0.001) (Table 3). The prevalence of overall colorectal neoplasm significantly increased with age (trend: p<0.001) (Fig. 1). Also, the prevalence of advanced adenoma increased with age (trend: p<0.001).
Table 3.
Prevalence of Colorectal Neoplasm according to Gender
Fig. 1.
Prevalence of colorectal neoplasm according to age group. The prevalence of colorectal neoplasm increased with age (trend: p<0.001).
3. Distribution of colorectal neoplasm
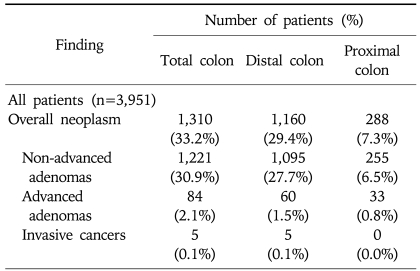
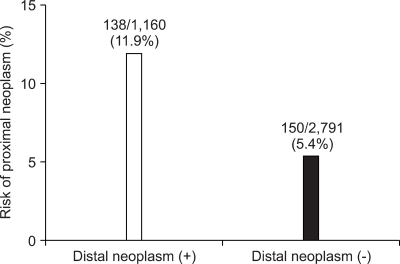
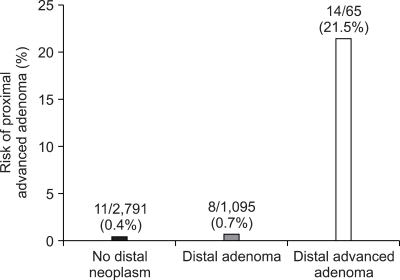
Of the 1,310 patients with colorectal neoplasm, 1,160 (88.5%) had their neoplasm in the distal colon, 288 (22.0%) in the proximal colon, and 138 (10.5%) had their neoplasm found in both the proximal and the distal colon (Table 4). One hundred thirty-eight subjects (11.9%) out of 1,160 with distal neoplasm had proximal neoplasm as well, while only 150 of 2,791 subjects (5.4%) without colorectal neoplasm in the distal colon had colonic neoplasm in the proximal colon (p<0.001) (Fig. 2). Of the 288 patients with proximal neoplasm, 138 (47.9%) had their neoplasm in the distal colon as well, but, 150 (52.1%) had no distal neoplasm. Of the 33 patients with proximal advanced neoplasm, 11 (33.3%) did not have any neoplasm in the distal colon. The prevalence of proximal advanced neoplasm increased as the histologic grade of distal neoplasm advanced (trend: p<0.001) (Fig. 3).
Table 4.
Colonoscopy Findings in the Distal and Proximal Colon
Fig. 2.
Risk of proximal neoplasm according to distal-colon findings (p<0.001).
Fig. 3.
Risk of proximal advanced adenoma according to distal-colon findings (trend: p<0.001).
DISCUSSION
The incidence of colorectal cancer has been recently rising in South Korea because of the westernization of lifestyles and dietary habits, and the colorectal cancer screening is a growing concern. As a result, the National Cancer Center recommended a guideline for colorectal cancer screening and the National Health Insurance Corporation started colorectal cancer screening with annual fecal occult blood test since 2004.13 However, these guidelines were established based on the data in Western countries because of a lack of reliable epidemiological data in Korea.14,15 Although a large cohort colonoscopy survey in asymptomatic Korean was recently reported, this survey was a single center study which cannot represent the status of general Korean population.16 Therefore, we conducted a prospective, multicenter colonoscopy survey to evaluate the prevalence of colorectal neoplasm in average- risk Koreans and explore risk factors for colorectal neoplasm. We, therefore, suggest that the results of this study have important implications as the baseline data for the preparation of Korean guideline of colorectal cancer screening.
In the present study, we found that the prevalence of overall colorectal neoplasm and advanced neoplasm among asymptomatic Koreans were 33.2% (1,310/3,951) and 2.2% (89/3,951), respectively. The prevalence of overall colorectal neoplasm ranged from 20.4% to 37.5% and that of advanced neoplasm ranged from 4.9% to 10.5% in Western studies.17-19 These results indicate that the prevalence of advanced neoplasm in average-risk Koreans may be slightly lower than that of westerners while the prevalence of overall colorectal neoplasm in average-risk Koreans may be similar to that of westerners. The reason why there is a discrepancy about advanced and non-advanced neoplasm between Korean and westerners is uncertain and it may be multifactorial including both genetic and environmental issues. Considering the continuous increase of colorectal cancer in Korea, one can imagine that it may be now a transition period from low to high prevalence of advanced neoplasm in Korea. Putting this result and assumption together with already high prevalence of non-advanced neoplasm, colorectal cancer screening is certainly necessary and should be strategically launched and performed in Korea.
It has been reported that the prevalence of adenomas and colorectal cancers is about 1.3- to 1.5-fold higher among men than among women in Western studies.20,21 Similarly, the prevalence of overall neoplasm in men was 1.7-fold higher than that in women in this study. It, also, is well known that old age is a demographic risk factor for colorectal neoplasm in Western studies.22 In this study, the prevalence of overall colorectal neoplasm and advanced adenoma significantly increased with age. This is consistent with reports from the West.18,22-24 The fact that male sex and old age were independent risk factors for colorectal neoplasm in average-risk Koreans, therefore, might help in the selection of suitable candidates for screening. The current guidelines in Western countries recommend that men and women of average-risk should be offered screening beginning at age 50 years.8-10 Our results showed that the prevalence of colorectal neoplasm was 28.1% in age 40 to 49 years. When considering these results, we suggest that primary screening may be started at age 40, especially in men. However, this study recruited asymptomatic subjects who "self referred" for colonoscopy screening. In addition, the participating centers were university hospitals which were not distributed evenly throughout the nation and were located mainly in urban area. Therefore this cohort was not the ideal representation of the general population. We think that it is necessary to derive the optimal starting age for screening based on more strictly nationwide studies with representative cases. In addition, the goal of screening is the reduction of the death from advanced colorectal cancer, not the detection of colorectal adenoma. All the invasive cancers were detected in those over 50 years in this study. Therefore, the cost-effectiveness of earlier detection of early colorectal adenomas in young subjects and their colonoscopic resection must also be thoroughly analyzed in order that the uniform application of nationwide screening from the age less than 50 years could be started.
It is well known that the presence of distal neoplasm is significant predictor for proximal neoplasm, and this is a reason why sigmoidoscopy can be included as a screening tool.15,23,25-27 Our results were similar to those of the West. In our cohort, 52.1% (150/288) of subjects with proximal colorectal neoplasm had no distal neoplasm and 33.3% (11/33) of subjects with proximal advanced neoplasm did not have any neoplasm in the distal colon. These are similar to the value of 52-62% reported in corresponding studies from the West and Korea.17,23,28 Therefore, from the viewpoint of effectiveness of the detection of colorectal neoplasm based on current data, a strategy that a full colonoscopy is performed only in patients with distal neoplasm detected by prior sigmoidoscopy might be insufficient in Korea as well as in Western countries. Rather, a primary colonoscopy screening appears to be more effective. In addition, if we assume that sigmoidoscopy in real practice can investigate only rectum and sigmoid colon and adopt the strategy of whole colonoscopy only in those with distal neoplasm in initial sigmoidoscopy, we would miss more proximal neoplasm than the results of our present study which adopted the definition of distal neoplasm as rectum, sigmoid colon and descending colon. Therefore, the value of a primary colonoscopy screening may be larger than that based upon the analysis in our present study. However, to adopt colonoscopy as a primary screening tool in whole population requires several prerequisites to be solved. First, quality of colonoscopy should be controlled. Second, safety concern should be settled. Third, acceptance rate for screening colonoscopy by general population should be increased. Finally, most of all, thorough investigation of cost-effectiveness of primary colonoscopy screening should be preceded.
This study has several limitations. First, a possible selection bias exists as previously described because of the inclusion of self-referred asymptomatic subjects and uneven distribution of participating centers. They are likely to be the more health-conscious and higher socioeconomic group. Second, other known risk factors for colorectal cancer have not been assessed, such as cigarette smoking, alcohol consumption and dietary habits. Finally, as in multicenter study, interobserver variation among colonoscopists may affect the findings. It is possible that pathologists in the local centers may also vary in their interpretation of the severity of dysplasia and, hence, the diagnosis of advanced lesions. In this study, actually, the prevalence of proximal neoplasm and advanced neoplasm tended to be lower than those seen in previous studies in Korea.14-16 It may be partly explained by the fact that there were both interobserver variation among colonoscopists and pathologists.
Despite these limitations, we believe that this study is valuable because it represents the first consorted effort in Korea to investigate the prevalence of colorectal neoplasm in average-risk Koreans in a prospective manner at 11 university hospitals. We found that the prevalence of colorectal neoplasm in asymptomatic average-risk Koreans was similar to the reported prevalence in corresponding studies of westerners while the prevalence of advanced neoplasm was lower than those observed in westerners. We also identified that male and advancing age were risk factors for colorectal neoplasm. In conclusion, we suggest that the epidemiology of colorectal neoplasm in Korea is not much different from westerners and strategic colorectal cancer screening should be settled.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Yuen ST, Chung LP, Leung SY, et al. Colorectal carcinoma in Hong Kong: epidemiology and genetic mutations. Br J Cancer. 1997;76:1610–1616. doi: 10.1038/bjc.1997.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ji BT, Devesa SS, Chow WH, Jin F, Gao YT. Colorectal cancer incidence trends by subsite in urban Shanghai, 1972-1994. Cancer Epidemiol Biomarkers Prev. 1998;7:661–666. [PubMed] [Google Scholar]
- 4.Muto T, Kotake K, Koyama Y. Colorectal cancer statistics in Japan: data from JSCCR registration, 1974-1993. Int J Clin Oncol. 2001;6:171–176. doi: 10.1007/pl00012102. [DOI] [PubMed] [Google Scholar]
- 5.Korea National Statistical Office. Seoul: Korea National Statistical Office; 2006. Annual Report on the cause of death statistics, 2005. Available from: http://www.nso.go.kr. [Google Scholar]
- 6.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 7.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343:1603–1607. doi: 10.1056/NEJM200011303432203. [DOI] [PubMed] [Google Scholar]
- 8.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenteroloy. 2008;134:1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Rhodes JM. Colorectal cancer screening in the UK: joint position statement by the British Society of Gastroenterology, the Royal College of Physicians, and the Association of Coloproctology of Great Britain and Ireland. Gut. 2000;46:746–748. doi: 10.1136/gut.46.6.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WGO-OMGE position statement: colorectal cancer screening and surveillance. [accessed 2008 Apr 16]. Available from: http://omge.org/globalguidelines/statement03/statement3.htm.
- 11.Jung SY. Screening guidelines for the early detection of colorectal cancer. Panel discussion. Korean J Gastrointest Endosc. 2002;24:317–320. [Google Scholar]
- 12.World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925–926. [PubMed] [Google Scholar]
- 13.National cancer screening program, 2004. Ministry of Health and Welfare, National Cancer Center. Available from: http://www.ncc.re.kr.
- 14.Kim TS, Kang YS, Jung SY, Cho HJ, Kim DS, Lee DH. Prospective evaluation of colorectal polyps in 1,683 consecutive colonoscopies. Korean J Gastrointest Endosc. 1999;19:887–896. [Google Scholar]
- 15.Choe JW, Yang SK, Kim YM, et al. Prediction of proximal colonic adenomas in patients with distal colonic polyps found on sigmoidoscopy: a controlled study. Korean J Gastroenterol. 2000;36:644–651. [Google Scholar]
- 16.Choe JW, Chang HS, Yang SK, et al. Screening colonoscopy in asymptomatic average-risk Koreans: analysis in relation to age and sex. J Gastroenterol Hepatol. 2007;22:1003–1008. doi: 10.1111/j.1440-1746.2006.04774.x. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman DA, Weiss DG, Bond JH, et al. Veterans Affairs Cooperative Study Group 380. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. N Engl J Med. 2000;343:162–168. doi: 10.1056/NEJM200007203430301. erratum in: N Engl J Med 2000;343:1204. [DOI] [PubMed] [Google Scholar]
- 18.Betes M, Munoz-Navas MA, Duque JM, et al. Use of colonoscopy as a primary screening test for colorectal cancer in average risk people. Am J Gastroenterol. 2003;98:2648–2654. doi: 10.1111/j.1572-0241.2003.08771.x. [DOI] [PubMed] [Google Scholar]
- 19.Schoenfeld P, Cash B, Flood A, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med. 2005;352:2061–2068. doi: 10.1056/NEJMoa042990. [DOI] [PubMed] [Google Scholar]
- 20.Stevens T, Burke CA. Colonoscopy screening in the elderly: when to stop? Am J Gastroenterol. 2003;98:1881–1885. doi: 10.1111/j.1572-0241.2003.07576.x. [DOI] [PubMed] [Google Scholar]
- 21.Rex DK, Khan AM, Shah P, Newton J, Cummings OW. Screening colonoscopy in asymptomatic average-risk African Americans. Gastrointest Endosc. 2000;51:524–527. doi: 10.1016/s0016-5107(00)70283-5. [DOI] [PubMed] [Google Scholar]
- 22.Rex DK, Lehman GA, Ulbright TM, et al. Colonic neoplasia in asymptomatic persons with negative fecal occult blood tests: influence of age, gender, and family history. Am J Gastroenterol. 1993;88:825–831. [PubMed] [Google Scholar]
- 23.Imperiale TF, Wagner DR, Lin CY, et al. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med. 2000;343:169–174. doi: 10.1056/NEJM200007203430302. [DOI] [PubMed] [Google Scholar]
- 24.Imperiale TF, Wagner DR, Lin CY, et al. Using risk for advanced proximal colonic neoplasia to tailor endoscopic screening for colorectal cancer. Ann Intern Med. 2003;139:959–965. doi: 10.7326/0003-4819-139-12-200312160-00005. [DOI] [PubMed] [Google Scholar]
- 25.Levin TR, Palitz A, Grossman S, et al. Predicting advanced proximal colonic neoplasia with screening sigmoidoscopy. JAMA. 1999;281:1611–1617. doi: 10.1001/jama.281.17.1611. [DOI] [PubMed] [Google Scholar]
- 26.Lewis JD, Ng K, Hung KE, et al. Detection of proximal adenomatous polyps with screening sigmoidoscopy: a systematic review and meta-analysis of screening colonoscopy. Arch Intern Med. 2003;163:413–420. doi: 10.1001/archinte.163.4.413. [DOI] [PubMed] [Google Scholar]
- 27.Pennazio M, Arrigoni A, Risio M, Spandre M, Rossini FP. Small rectosigmoid polyps as markers of proximal neoplasms. Dis Colon Rectum. 1993;36:1121–1125. doi: 10.1007/BF02052260. [DOI] [PubMed] [Google Scholar]
- 28.Gannon CJ, Malone DL, Royal RE, Schreiber M, Bass BL, Napolitano LM. Advanced proximal colon cancer. Surg Endosc. 2002;16:446–449. doi: 10.1007/s00464-001-8304-6. [DOI] [PubMed] [Google Scholar]