Abstract
Background/Aims
Adenosine is an endogenous modulator of nociception. Its role in visceral nociception, particularly in visceral hyperalgesia, has not been studied. The aim of this study was to determine the effects of adenosine receptor agonists in a model of visceral hyperalgesia.
Methods
The visceromotor response (VMR) in rats to colorectal distension (CRD; 80 mmHg, 20 seconds) was quantified by electromyographic recordings from the abdominal musculature. Three hours after the intracolonic administration of zymosan (25 mg/mL, 1 mL), VMRs to CRD were measured before and after either subcutaneous or intrathecal administration of an adenosine receptor agonist.
Results
Subcutaneous injection of 5'-N-ethylcarboxyamidoadenosine (NECA; an A1 and A2 receptor agonist), R(-)-N6-(2-phenylisopropyl)-adenosine (R-PIA; a selective A1 receptor agonist), or CGS-21680 hydrochloride (a selective A2a receptor agonist) dose-dependently (10-100 mg/kg) attenuated the VMR to CRD, although hindlimb weakness occurred at the higher doses tested. Intrathecal administration of NECA or R-PIA dose-dependently (0.1-1.0 µg/kg) decreased the VMR, whereas CGS-21680 hydrochloride was ineffective over the same concentration range. Higher intrathecal doses of the A1/A2 receptor agonist NECA produced motor weakness.
Conclusions
Adenosine receptor agonists are antihyperalgesic, but also produce motor weakness at high doses. However, activation of the spinal A1 receptor significantly attenuates the VMR to CRD without producing motor weakness.
Keywords: Adenosine, Visceral, Hyperalgesia
INTRODUCTION
Adenosine has been known as one of the neurotransmitter and it exerts multiple influences on pain transmission. Several previous studies already proved there is an endogenous adenosine in the peripheral nerve ending and spinal cord.1,2 The endogenous compound of adenosine is present in all cells. It may be released from cells directly or via degradation of ATP and is involved in many regulatory mechanisms both in physiological and pathophysiological conditions.3,4 It affects both sensory and motor neuron. It acts as an endogenous moduator of nociception. Because of its potential as a new analgesic, interests in adenosine are increasing among physicians and pharmacologists. The various pain modulating effects of adenosine and its analogs in animal models has been known for more than 10 years. Adenosine has effects in peripheral and central nervous system, mediated through specific cell surface associated receptors. With the development of selective adenosine agonists and antagonists, its role in pain perception is being elucidated.
Adenosine receptors are found in the peripheral nerve ending and spinal cord. Adenosine has been suggested to have both pre- and postsynaptic effects within the dorsal horn indicating possible multiple sites of action of adenosine in the control of sensory events related to pain. Adenosine shows variable influences on pain transmission at peripheral and spinal sites according to its receptor.5 Adenosine A1 receptor activation in rodents, produces antinociception at peripheral nerve terminals and spinal cord level in somatic pain model. Activation of adenosine A2 receptor (A2a, A2b) exerts multiple responses according to the concentration that was administrated and the site of action. At the peripheral nerve endings in rodents, adenosine A2 receptor activation is known to induce pronociceptive or pain enhancing properties. However, in the spinal cord, adenosine A2 receptor activation at lower doses reduces pain perception and at higher doses it causes motor weakness.
All those findings were observed in somatic pain models, such as acute nociceptive, inflammatory or neuropathic pain model. Its role in visceral nociception, and particularly visceral hyperalgesia, has not been studied. However, experimental and clinical data are pointing at the adenosine system as an interesting target in relation to hypersensitivity states. The aim of this study was to evaluate the effects of adenosine receptor agonists in a model of visceral hyperalgesia.
MATERIALS AND METHODS
1. Animals
Adult male Sprague-Dawley rats (400-425 g; Harlan, Indianapolis, IN) were used. Rats were housed one per cage in the animal care facility at the university of Iowa (approved by the American Association for Accreditation of Laboratory Animal Care), allowed free access to food and water, and maintained on a 12-hour light-dark cycle (lights on between 6 am and 6 pm). All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Iowa.
2. Surgical Preparation
The procedure was described earlier.6 Rats were deeply anesthetized with pentobarbital sodium (45 mg/kg, Nembutal; Abbott Laboratories, North Chicago, IL) administered intraperitoneally. Electrodes (Teflon-coated stainless steel wire; Cooner Wire Sales, Chatworth, CA) were stitched into the external oblique musculature, just superior to the inguinal ligament, for electromyographic (EMG) recording. The electrode leads were tunneled subcutaneously and exteriorized at the nape of the neck for future access. An intrathecal catheter (polyethylene 10 tubing, 8.5 cm long) was inserted through the dura overlying the atlanto-occipital junction into the spinal subarachnoid space and guided to the lumbar enlargement. The catheter was surgically anchored to the musculature at the back of the neck and externalized with EMG leads. Animals exhibiting motor deficits after surgery due to spinal cord injury during intrathecal catheter insertion were not used. After surgery, rats were housed separately and allowed to recuperate for at least 7 days before testing. At the end of the experiments, rats were killed with an overdose of intraperitoneal pentobarbital.
3. Behavioral Testing
The stimulus used has been described previously.7,8 The descending colon and rectum were distended by pressure-controlled air inflation of a 6-7-cm-long, 2-3-cm diameter, flaccid, flexible latex balloon tied around a flexible tube (Tygon). The balloon was lubricated (Surgilube; E. Fougera & Co., Melville, NY), inserted into the colon via the anus, and anchored by taping the balloon catheter to the base of the tail. Noxious phasic CRD (80 mm Hg, 20 seconds, 5 minutes apart) were achieved by opening a solenoid gate to a constant pressure air reservoir. Intracolonic pressure was continuously monitored with the aid of a pressure control device (Bioengineering, University of Iowa, Iowa city, IA). The response quantified was the VMR, a contraction of the abdominal and hindlimb musculature. EMG activity produced by contraction of the external oblique musculature was quantified by recording the number of discharges crossing a preset voltage threshold (baseline). Each distention trial lasted 40 seconds, and EMG activity was quantified in 1-second bins for 10 seconds before distention, 20 seconds during distention, and 10 seconds after distention. The increase in total number of recorded counts during distention (above baseline) was defined as the response.
4. Drugs
Because adenosine is rapidly metabolized by endothelium within 10 seconds when administered systemically, we used adenosine analogues. Drugs used were 5'-N-ethylcarboxyamidoadenosine (NECA), an A1 and A2 receptor agonist, R(-)-N6-(2-phenylisopropyl)-adenosine (R-PIA), a selective A1 receptor agonist, or CGS-21680 hydrochloride (CGS 21680), a selective A2a receptor agonist. Stock solutions were freshly prepared by dissolving the drugs in dimethyl sulfoxide solution and followed by adding sterile preservative-free saline.
5. Experimental Protocol
On the day of testing, four stable control responses to CRD (80 mm Hg, 20 seconds, 5 minutes apart) were obtained in conscious, unsedated rats before any treatment. And then, while the animals were briefly anesthetized with halothane, zymosan (25 mg/mL, 1 mL; Sigma Chemical Co., St. Louis, MO), was instilled into the colon through a gavage needle inserted into the colon to a depth of about 7-8 cm. Three hours after intracolonic treatment, four control responses to CRD were obtained to establish whether rats were hyperalgesic (i.e., gave significantly increased responses to 80 mm Hg CRD). Two minutes after these distension, drug (or vehicle) was administered systemically through subcutaneous injection or inthrathecally to the lumbar enlargement through the indwelling catheter. All drugs that administered systemically were in a volume of 0.1 mL/100 g of rat body weight, and all drugs that administered intrathecally were in a volume of 5 µL followed by a flush with 10 µL of sterile, preservative-free saline over a period of 1 minute. The progress of the injection was continuously monitored by following the movement of an air bubble in the tubing. Absence of drug leakage during injection was also confirmed. Dose-response curves were generated using a cumulative dosing paradigm. The first intrathecal injection was made 2 minutes after documenting the presence of hyperalgesia. Subsequent doses of drugs were injected 20 minutes apart, thus allowing four distentions after each dose of drug. Data were reported as the average response to the four distentions. Because there was no change in VMR to CRD in group of animals that received saline subcutaneously or intrathecally, these data were pooled (vehicle).
6. Data Analysis
Data were represented as mean±SEM. Data for VMRs were normalized as percentage of control, calculated as percent of mean of four responses to CRD obtained in the same animal. Baseline VMR, VMR 3 hour after intracolonic zymosan instillation, and VMR after subcutaneously or intrathecal drug injections were compared. Changes in VMR after drug administration were statistically analized by repeated-measures analysis of variance (ANOVA), one-way ANOVA followed by the Student modified t test with the Bonferroni correction for multiple comparisons. p values of <0.05 were considered statistically significant in all tests.
RESULTS
1. Effect of Subcutaneous Administration of Adenosine Agonists and Antagonist on Zymosan-Induced Visceral Hyperalgesia
All experimental groups consisted of at least 6-8 animals. All the animals became hyperalgesic after intracolonic instillation of zymosan. The overall mean increase in the VMR three hours after intracolonic instillation of zymosan was 124±6% compared to that of baseline.
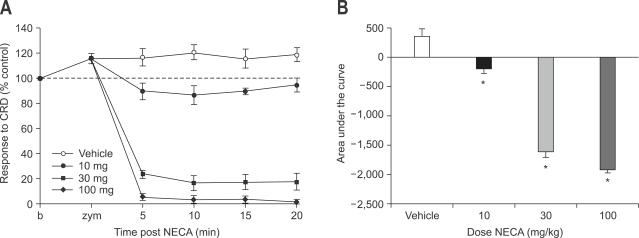
In this series of experiments, effects of adenosine agonists were evaluated at variable doses (10, 30, 100 mg). Subcutaneous injection of 5'-N-ethylcarboxyamidoadenosine (NECA), an A1 and A2 receptor agonist, 3 hours after intracolonic treatment with zymosan attenuated VMR to CRD dose dependently (p<0.05). At low dose (10 mg), NECA slightly decreased VMR to CRD without motor weakness compared to response after vehicle administration. VMR to CRD was average only 90% of baseline and that meant mild analgesic effect. However, at higher doses (30, 100 mg), NECA markedly decreased VMR to CRD with the development of motor weakness (Fig. 1). VMR to CRD was less than 10% of baseline at 100 mg. Hindlimb became flaccid and rat could not move with hindlimb, but it was recovered later completely. The effect of NECA on zymosan-produced visceral hyperalgesia was fast (5 minutes after administration) and last more than 20 minutes.
Fig. 1.
Effect of subcutaneous administration of NECA on zymosan-induced visceral hyperalgesia (A) and result of statistical analysis (B). Subcutaneous injection of NECA attenuated VMR to CRD dose dependently. At low dose (10 mg), NECA slightly decreases VMR to CRD. However, at higher doses (30, 100 mg), NECA markedly decreases VMR to CRD with the development of motor weakness (*p<0.05).
NECA, 5-N-ethylcarboxyamidoadenosine; VMR, visceromotor response; CRD, colorectal distension.
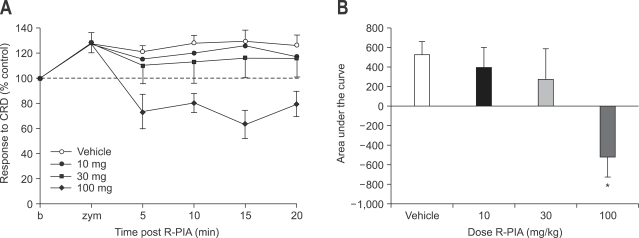
Subcutaneous administration of R(-)-N6-(2-phenylisopropyl)-adenosine, (R-PIA), a selective A1 receptor agonist, did not attenuated VMR to CRD at doses of 10 and 30 mg. At higher dose (100 mg), R-PIA decreased VMR to CRD (p<0.05) without development of motor weakness (Fig. 2). VMR to CRD was less than 80% of base line.
Fig. 2.
Effect of subcutaneous administration of R-PIA on zymosan-induced visceral hyperalgesia (A) and result of statistical analysis (B). R-PIA does not attenuated VMR to CRD at dose of 10 mg and 30 mg. At 100 mg, R-PIA decreases VMR to CRD without development of motor weakness (*p<0.05).
R-PIA, R(-)-N6-(2-phenylisopropyl)-adenosine; VMR, visceromotor response; CRD, colorectal distension.
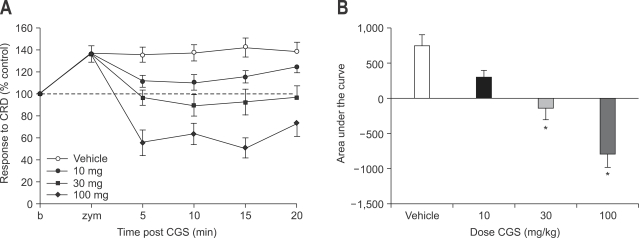
CGS-21680 hydrochloride (CGS 21680), a selective A2a receptor agonist, attenuated VMR to CRD at doses of 30 mg and 100 mg (p<0.05) but not at 10 mg (Fig. 3). At the higher dose (100 mg), hindlimb weakness was produced, but it was also recovered completely.
Fig. 3.
Effect of subcutaneous administration of CGS-21680 on zymosan-induced visceral hyperalgesia (A) and result of statistical analysis (B). CGS-21680 attenuates VMR to CRD at doses of 30 mg and 100 mg (p<0.05) but not at 10 mg. At high dose, hindlimb weakness is produced (*p<0.05).
VMR, visceromotor response; CRD, colorectal distension.
2. Effect of Subcutaneous Administration of Adenosine Antagonist on Zymosan-Induced Visceral Hyperalgesia
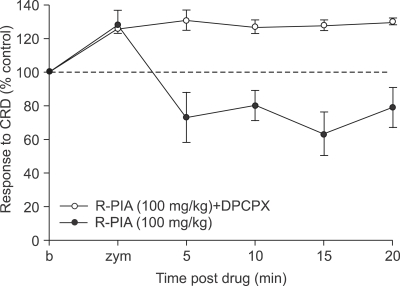
8-Cyclopentyl-1,3-dipropylxanthine (DPCPX), a selective A1 receptor antagonist was administered subcutaneously at a dose 100 mg 5 minutes before the subcutaneous administration of R-PIA. Even though effective dose of R-PIA (100 mg) was injected, DPCPX prevented the decrease in VMR to CRD by R-PIA (Fig. 4). Furthermore, there was no evidence of pain facilitation or side effect.
Fig. 4.
Effect of subcutaneous administration of DPCPX on zymosan-induced visceral hyperalgesia. DPCPX, when injected subcutaneously 5 minutes before the subcutaneous administration of R-PIA, prevents the decrease in VMR to CRD by R-PIA.
DPCPX, 8-cyclopentyl-1,3-dipropylxanthine; R-PIA, R(-)-N6-(2-phenylisopropyl)-adenosine; VMR, visceromotor response; CRD, colorectal distension.
3. Effect of Intrathecal Administration of Adenosine Agonists on Zymosan-Induced Visceral Hyperalgesia
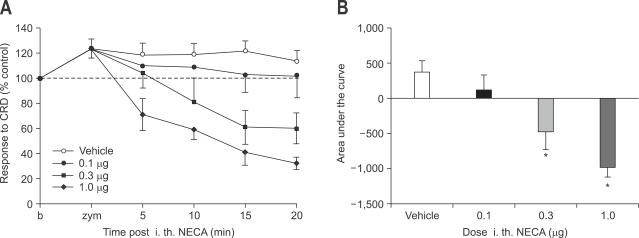
Effects of intrathecal adenosine agonists were investigated at variable doses (0.1, 0.3, 1.0 µg). Intrathecal administration of NECA attenuated VMR to CRD at dose of 0.3 µg and 1.0 µg (p<0.05) but not at 0.1 µg. Both 0.3 µg, 1.0 µg of NECA produced motor weakness (Fig. 5). Flaccidity of hindlimb disappeared later completely.
Fig. 5.
Effect of intrathecal administration of NECA on zymosan-induced visceral hyperalgesia (A) and result of statistical analysis (B). NECA attenuates VMR to CRD at dose of 0.3 µg and 1.0 µg (p<0.05) but not at 0.1 µg. Both 0.3 µg, 1.0 µg of NECA produces motor weakness (*p<0.05).
NECA, 5-N-ethylcarboxyamidoadenosine; VMR, visceromotor response; CRD, colorectal distension.
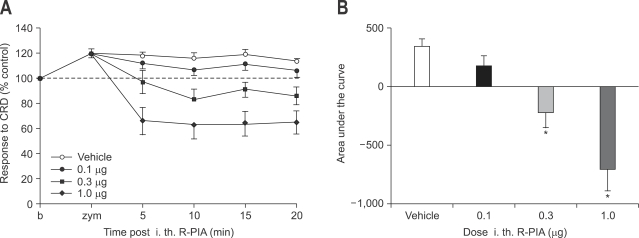
Intrthecal administration of R-PIA at doses of 0.1 µg did not induce decrease in VMR to CRD. However, 0.3 µg and 1.0 µg of intrathecal R-PIA produced decrease in VMR to CRD (p<0.05) without development of motor weakness (Fig. 6).
Fig. 6.
Effect of intrathecal administration of R-PIA on zymosan-induced visceral hyperalgesia (A) and result of statistical analysis (B). R-PIA at doses of 0.1 µg does not induce decrease in VMR to CRD. However, 0.3 µg and 1.0 µg of intrathecal R-PIA produces decrease in VMR to CRD without development of motor weakness (*p<0.05).
R-PIA, R(-)-N6-(2-phenylisopropyl)-adenosine; VMR, visceromotor response; CRD, colorectal distension.
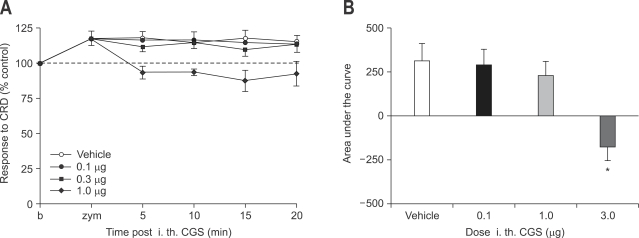
Intrthecal CGS 21680 administration (0.1-1.0 µg) was ineffective. Higher dose of CGS 21680 (3.0 µg) showed mild decrease in VMR to CRD (Fig. 7).
Fig. 7.
Effect of intrathecal administration of CGS 21680 on zymosan-induced visceral hyperalgesia (A) and result of statistical analysis (B). CGS 21680 (0.1-1.0 µg) is ineffective. Only higher dose of CGS 21680 (3.0 µg) shows slight decrease in VMR to CRD.
VMR, visceromotor response; CRD, colorectal distension.
4. Effect of Intrathecal Administration of Adenosine Antagonist on Zymosan-Induced Visceral Hyperalgesia
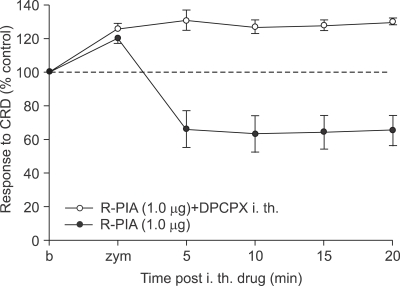
DPCPX, was administered intrathecally at a dose 1 µg 5 minutes before the intrathecal administration of R-PIA. Even though effective dose of R-PIA (1 µg) was injected intrathecally, DPCPX also prevented the decrease in VMR to CRD by intrathecal R-PIA (Fig. 8).
Fig. 8.
Effect of intrathecal administration of DPCPX on zymosan-induced visceral hyperalgesia. DPCPX, when injected intrathecally 5 minutes before the intrathecal administration of R-PIA, prevents the decrease in VMR to CRD by R-PIA.
DPCPX, 8-cyclopentyl-1,3-dipropylxanthine; R-PIA, R(-)-N6-(2-phenylisopropyl)-adenosine; VMR, visceromotor response; CRD, colorectal distension.
DISCUSSION
This study showed that adenosine can modulate visceral hyperalgesia. Several animal studies have shown that adenosine or adenosine analogues have important roles in the control of nociception4,5 and they may have a potential role in treatment of pain in humans.9,10 The most interesting was the potential to modulate the pathological states, such as hyperexcitability associated with injuries to the peripheral and central nervous systems. Adenosine was effective to decrease pain perception in neuropathic pain model created by ligation of nerve in rats.11,12 Clinically adenosine became a new candidate of analgesic for the treatment of patients with neuropathic pain.13-15 This study was performed in visceral hypersensitivity model and the results revealed adenosine and its analogues can have important role in the treatment of functional gastrointestinal disorders. Furthermore, there is an evidence of pain relief by adenosine in patients with noncardiac chest pain.16
As mentioned earlier, adenosine has at least two kinds of receptor, A1 and A2 receptor. At peripheral nerve terminals in rodents, adenosine A1 receptor activation by direct local administration of adenosine A1 receptor agonist produces antinociception, while adenosine A2 receptor activation produces pronociceptive or pain enhancing properties.5 In the spinal cord, recent studies using more selective agonists and antagonists have implicated primarily A1 receptor in antinociception and A2 receptor in muscle relaxation.17,18 For both receptor subtypes, dorsal spinal cord levels exceed ventral spinal cord level, and adenosine A1 receptors are selectively concentrated in the substantia gelatinosa.19-22 In our study, antihyperalgesic and analgesic effect of adenosine upon visceral pain was mainly mediated via A1 receptor. Systemic administration of A1 receptor agonist decreased VMR to CRD. Furthermore, even systemic administration of A2a agonist showed decreased VMR rather than increased VMR. But decrease in VMR after systemic administration of A2a agonist seems not to reflect decreased pain perception. That is thought to be caused by motor weakness not by analgesic effect. Spinal administration of A1 agonist in this visceral pain model induced decrease in VMR without motor weakness. That means spinal A1 receptor has important role for antinociception in visceral hyperalgesia. A recent study insisted that adenosine A1 agonists inhibited both pain sensation and muscle contraction, but nociceptive responses were inhibited at lower doses.23 However, in this study, even high doses of A1 receptor agonist given subcutaneously or intrathecally did not show motor weakness. Therefore, A1 receptor agonist can be a candidate of antihyperalgesic agent for the treatment of visceral hyperalgesia in functional gastrointestinal disorders. Strictly speaking, to confirm the selectivity of action on inflammation-induced visceral hypersensitivity, the influence of these adenosine agonists has to be tested on basal visceral sensitivity using intracolonic saline instillation before zymosan injection, and furthermore, experiments with a lower pressure of distention to determine a possible more selective action of adenosine analogues on components of colorectal hypersensitivity linked to inflammation to observe the role of low threshold receptors may be also needed.
In our study, we used adenosine analogues rather than adenosine itself. That is because adenosine, when given systemically, is so rapidly metabolized by endothelia cells (within 10 seconds). It is known that the potency of adenosine agonists in suppressing sensory transmission is greatest in selective A1 agonist, and then both A1 & A2 agonist, and selective A2 agonist in order.23 However, in this experiment, both A1 & A2 agonist induced more decrease in VMR to CRD than decrease in VMR by selective A1 agonist or A2 agonist alone. That means the amount of decrease in VMR by both A1 & A2 agonist was the summation of analgesic effect by A1 agonist and muscle relaxing effect by A2 agonist. That can be explained by the fact that the dorsal spinal cord contains both A1 and A2 receptors.19-22 However, it is regretful that the role of endogenous adenosine could not be evaluated due to lack of information but seemed to be trivial in the study because of its short duration of action compared to relatively long duration of balloon distention. The role of gut neural and non-neural A1, A2 or A3 receptors that might play a role in the subcutaneous hyperalgesic responses also could not be determined exactly in this protocol.
The concentration of intrathecally administrated adenosine to achieve analgesic effect was quite lower compared to that of systemically administrated adenosine. That means the main action site of adenosine is spinal cord. Usually adenosine A1 receptors are absent in the peripheral sensory terminal and they are present on the cell body of dorsal root ganglion cells and on the central terminals of primary afferent neurons.24,25 Only under the specific circumstances, those receptors migrate to the peripheral aspect of this nerve. Meanwhile, adenosine-like immunoreactivity is exhibited by terminals in the substantia gelatinosa of the dorsal horn of the spinal cord where high levels of adenosine receptors are found including A1 receptor.26 In addition adenosine, perhaps originating from ATP, is released from spinal synaptosomes.4 All these findings indicate that adenosine may play a role in the modulation of nociceptive transmission at the spinal level.
In conclusion, adenosine receptor agonists are antihyperalgesic, but also produce motor weakness. Activation of the spinal A1 receptor, however, significantly attenuates the VMR to CRD without producing motor weakness.
References
- 1.Pelleg A, Porter S. The pharmacology of adenosine. Pharmacotherapy. 1990;10:157–417. [PubMed] [Google Scholar]
- 2.Grundy D, Al-Chaer ED, Aziz Q, et al. Fundamentals of neurogastroenterology: basic science. Gastroenterology. 2006;130:1391–1411. doi: 10.1053/j.gastro.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 3.Abbracchio MP, Burnstock G. Purinergic signalling: pathophysiological roles. Jpn J Pharmacol. 1998;78:113–145. doi: 10.1254/jjp.78.113. [DOI] [PubMed] [Google Scholar]
- 4.Sawynok J, Sweeney MI. The role of purines in nociception. Neuroscience. 1989;32:557–569. doi: 10.1016/0306-4522(89)90278-9. [DOI] [PubMed] [Google Scholar]
- 5.Sawynok J. Adenosine receptor activation and nociception. Eur J Pharmacol. 1998;317:1–11. doi: 10.1016/s0014-2999(97)01605-1. [DOI] [PubMed] [Google Scholar]
- 6.Ness TJ, Gebhart GF. Colorectal distention as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- 7.Gebhart GF, Sengupta JN. Evaluation of visceral pain. In: Gaginella TS, editor. Handbook of methods in gastrointestinal pharmacology. Boca Raton, FL: CRC; 1996. pp. 359–374. [Google Scholar]
- 8.Coutinho SV, Gebhart GF. A role for spinal nitric oxide in mediating visceral hyperalgesia in the rat. Gastroenterology. 1999;116:1399–1408. doi: 10.1016/s0016-5085(99)70504-4. [DOI] [PubMed] [Google Scholar]
- 9.Poulsen SA, Quinn RJ. Adenosine receptors: new opportunities for future drugs. Bioorganic & Medicinal Chemistry. 1998;6:619–641. doi: 10.1016/s0968-0896(98)00038-8. [DOI] [PubMed] [Google Scholar]
- 10.Guieu R, Dussol B, Halimi G, et al. Adenosine and the nervous system: pharmacological data and therapeutic perspectives. Gen Pharmacol. 1998;31:553–561. doi: 10.1016/s0306-3623(98)00071-8. [DOI] [PubMed] [Google Scholar]
- 11.Lavand'homme PM, Eisenach JC. Exogenous and endogenous adenosine enhance the spinal antiallodynic effects of morphine in a rat model of neuropathic pain. Pain. 1999;80:31–36. doi: 10.1016/s0304-3959(98)00193-6. [DOI] [PubMed] [Google Scholar]
- 12.Lynch JJ, 3rd, Jarvis MF, Kowaluk EA. An adenosine kinase inhibitor attenuates tactile allodynia in a rat model of diabetic neuropathic pain. Eur J Pharmacol. 1999;364:141–146. doi: 10.1016/s0014-2999(98)00840-1. [DOI] [PubMed] [Google Scholar]
- 13.Rae CP, Mansfield MD, Dryden C, Kinsella J. Analgesic effect of adenosine on ischemic pain in human volunteers. Br J Anaesth. 1999;82:427–428. doi: 10.1093/bja/82.3.427. [DOI] [PubMed] [Google Scholar]
- 14.Belfrage M, Segerdahl M, Arner S, Sollevi A. The safety and efficacy of intrathecal adenosine in patients with chronic neuropathic pain. Anesth Analg. 1999;89:136–142. doi: 10.1097/00000539-199907000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Sollevi A. Adenosine for pain control. Acta Anaesthesiol Scand. 1997;110:S135–S136. doi: 10.1111/j.1399-6576.1997.tb05532.x. [DOI] [PubMed] [Google Scholar]
- 16.Achem SR. New frontiers for the treatment of noncardiac chest pain: the adenosine receptors. Am J Gastroenterol. 2007;102:939–941. doi: 10.1111/j.1572-0241.2007.01102.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee YW, Yaksh TL. Pharmacology of the spinal adenosine receptor which mediates the antiallodynic action of intrathecal adenosine agonists. J Pharmacol Exp Ther. 1996;277:1642–1648. [PubMed] [Google Scholar]
- 18.Sjolund KF, von Heijne M, Hao JX, Xu XJ, Sollevi A, Wiesenfeld-Hallin Z. Intrathecal administration of the adenosine A1 receptor agonist R-phenylisopropyl adenosine reduces presumed pain behavior in a rat model of central pain. Neurosci Lett. 1998;243:89–92. doi: 10.1016/s0304-3940(98)00092-5. [DOI] [PubMed] [Google Scholar]
- 19.Choca JI, Proudfit HK, Green RD. Identification of A1 and A2 adenosine receptors in the spinal cord. J Pharmacol Exp Ther. 1987;242:905–910. [PubMed] [Google Scholar]
- 20.Choca JI, Green RD, Proudfit HK. Adenosine A1 and A2 receptors of the substantia gelatinosa are located predominantly on intrinsic neurons: an autoradiography study. J Pharmacol Exp Ther. 1988;247:757–764. [PubMed] [Google Scholar]
- 21.Geiger JD, Labella FS, Nagy JI. Characterization and localization of adenosine receptors in rat spinal cord. J Neurosci. 1984;4:2303–2310. doi: 10.1523/JNEUROSCI.04-09-02303.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodman RR, Snyder SH. Autoradiographic localization of adenosine receptors in rat brain using [3H]cyclohexyladenosine. J Neurosci. 1982;2:1230–1241. doi: 10.1523/JNEUROSCI.02-09-01230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura I, Ohta Y, Kemmotsu O. Characterizartion of adenosine receptors mediating spinal sensory transmission related to nociceptive information in the rat. Anesthesiology. 1997;87:577–584. doi: 10.1097/00000542-199709000-00018. [DOI] [PubMed] [Google Scholar]
- 24.MacDonald RL, Skerritt JH, Werz MA. Adenosine agonist reduce voltage-dependent calcium conductance of mouse sensory neurons in cell culture. J Physiol. 1986;370:75–90. doi: 10.1113/jphysiol.1986.sp015923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santicioli P, Del Bianco E, Maggi CA. Adenosine A1 receptors mediate the presynaptic inhibition of calcitonin gene-related peptide release by adenosine in the rat spinal cord. Eur J Pharmacol. 1993;231:139–142. doi: 10.1016/0014-2999(93)90695-e. [DOI] [PubMed] [Google Scholar]
- 26.Braas KM, Newby AC, Wilson VS, Snyder SH. Adenosine-containing neurons in the brain localized by immunocytochemistry. J Neurosci. 1986;6:1952–1961. doi: 10.1523/JNEUROSCI.06-07-01952.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]