Abstract
Background/Aims
Glutathione S-transferase P1 (GSTP1) scavenges radicals via its peroxidase activity. The purpose of this study was to determine the association of GSTP1 genetic polymorphisms with the expression of H. pylori-associated gastroduodenal disease.
Methods
This study involved 1,911 subjects, comprising patients with four diseases (gastric cancer, dysplasia, benign gastric ulcer, and duodenal ulcer disease) and controls. Biallelic polymorphisms were genotyped by restriction fragment length polymorphism techniques.
Results
The frequency of the genetic polymorphism at nucleotide 313 of GSTP1 did not differ among the five study groups. However, when the gastric cancer group was subdivided into advanced gastric cancer (AGC) and early gastric cancer, the frequency of the G/G genotype was significantly higher in the AGC group than in all the control subgroups (OR: 1.2, 95% CI: 1.1-4.9). The frequency of this genotype differed significantly in the H. pylori-positive AGC group (OR: 2.7, 95% CI: 1.1-6.3) but not in the H. pylori-negative group. Furthermore, the difference was greater in the intestinal type, and was not found in diffuse types of disease.
Conclusions
This study found that genetic polymorphisms of GSTP1 were associated with H. pylori-associated gastric cancer only during the advanced stage of gastric cancer, with intestinal-type histology evident in H. pylori-positive subjects.
Keywords: Gastric cancer, GSTP1 protein, Polymorphism, Helicobacter pylori
INTRODUCTION
Interindividual differences in the cellular mechanisms of activation and detoxification of carcinogenic chemicals might confer different degrees of susceptibility to cancer.1 Several enzymes are involved in the detoxification of xenobiotic compounds. Glutathione S-transferase enzymes are phase II detoxifying molecules that catalyze the conjugation of reactive chemical intermediates, including the active metabolites of carcinogens to soluble glutathione.2 One member of the GST family, the GSTP1 enzyme, plays a central role in the inactivation of toxic and carcinogenic electrophiles.3 The GSTP1 gene is polymorphic in humans. Previous studies have shown that the A to G polymorphisms, at nucleotide 313 of the GSTP1 gene, cause an isoleucine (Ile) to valine (Val) change at residue 105 that reduces the catalytic activity of GSTP1.4-7 In contrast, several studies have shown that Val variants were more efficient with different substrates than were homozygous Ile/Ile genotypes.8 Moreover, several studies evaluating GSTP1 and cancer susceptibility have shown conflicting results. The 105 Val variant of GSTP1 has been correlated with the development of several types of cancer including bladder,9 testicular,9 lung cancer,10,11 breast cancer,12 esophageal,13 and oropharyngeal cancer,14 as well as a poor prognosis in patients with colorectal cancer.15 By contrast, other studies have shown opposite results16 or no association between GSTP1 polymorphisms and cancer type.17-19 Several studies have assessed the relationship between GSTP1 and gastric cancer and have found no association.10,20,21
One-half of the world's population is infected by H. pylori.22 This gram-negative bacterium, which produces superoxide radicals, is responsible for gastritis, duodenal ulcer (DU) disease and the majority of benign gastric ulcers (BGUs). H. pylori infection is also linked to gastric cancer (GC) and MALT-lymphoma.23-26 However, less than 20% of infected individuals present with clinical disease.27 The reasons for the variable clinical effects of H. pylori are perhaps determined by the interaction of the host, environmental factors and bacterial virulence. The production of superoxide radicals by H. pylori in the gastric mucosa may be associated with genetic polymorphisms of GSTP1 and influence the expression of H. pylori-associated gastroduodenal diseases such as GC. There are two histologically distinct GC to consider, the diffuse-type and the intestinal-type.28 The host genetic susceptibility is known to be an important factor in the development of GC.29 However, environmental factors also play an important role in gastric cancer carcinogenesis, especially in the intestinal type of GC.30 Therefore, the expression of genetic polymorphisms may differ depending on the gastric cancer tissue type.
The goal of this study was to determine whether GSTP1 polymorphisms were associated with different gastroduodenal diseases. Patients who were H. pylori-infected with GC (intestinal or diffuse type), dysplasia, BGU, or DU were compared to age, gender and H. pylori infected matched case-controls.
MATERIALS AND METHODS
1. Patients
The study included 1,911 subjects from Seoul and the vicinity in Korea. The study included controls (n=803), non-cardiac GC (n=400; intestinal type, n=232, diffuse type, n=168), those with dysplasia (n=100), BGU (n=334) and DU (n=274). All subjects were ethnic Koreans. The control group was recruited from healthy subjects that had a standard gastroscopy as part of a screening program for premalignant gastric mucosa or gastric cancer. Subjects (≥16 years) were included in the control group if the gastroscopy showed a normal gastric mucosa or gastritis; no control had significant gastroduodenal disease, such as gastric cancer, dysplasia, MALToma, peptic ulcer, or reflux esophagitis. In addition, the controls had no history of peptic ulcer disease. GC, BGU and DU were diagnosed by gastroscopy. GC tissue types were confirmed histologically and the stage such as early gastric cancer (EGC) or advanced gastric cancer (AGC) was determined by histological findings after surgery and/or a staging work up including computer tomography scanning and physical examination. The control group was matched with the gastric cancer group by age, gender and H. pylori positivity. All subjects provided informed consent, and the Ethical Committee of the Seoul National University Bundang Hospital approved the study. The results of the germline SNPs were not traceable to the patient's name to protect the genetic information of the individual subjects enrolled in this study.
2. H. pylori tests
Two biopsy specimens were taken from the greater curvature of the mid antrum and mid body of the stomach, and three from the lesser curvature of the mid antrum and mid body. Among these ten specimens, two from the mid antrum and two from the body were fixed in formalin then assessed for the presence of H. pylori (by modified Giemsa staining). Single specimens taken from the lesser curvature of the mid antrum and mid body were used for rapid urease testing (CLOtest, Delta West, Bentley, Australia), and two specimens from the mid antrum and mid body, were cultured. If any of these three tests produced a positive result, H. pylori infection was considered present. Anti-H. pylori immunoglobulin G quantification was performed using an enzyme-linked immunosorbent assay kit (Genedia H. pylori ELISA; Green Cross Medical Science Corp, Seoul, Korea) when the three above-mentioned H. pylori tests were all negative. Subjects were considered H. pylori-infected when the H. pylori IgG was positive.
3. Genotyping of GSTP1
Genomic DNAs from antral gastric mucosa without lesions were obtained using the standard proteinase K digestion and phenol/chloroform extraction technique. The nucleotide 313, of the GSTP1 A to G polymorphism, was measured by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). The following amplification primers were used: forward 5'-ACCCCAGGGCTCTATGGGAA-3' and reverse 5'-TGAGGGCACAAGAAGCCCCT-3' in a Perkin Elmer model 9600 (Perkin Elmer Co., Norwalk, CT, USA). The conditions were initially 95℃ for 10 min then 30 cycles at 94℃ for 30 sec, 55℃ for 30 sec, 72℃ for 30 sec, and 72℃ for 5 min. The PCR products of GSTP1 (GenBank accession number: NM_000852) were then digested overnight with 5 units of the restriction enzyme Alw26I (New England Biolabs, Ipswich, MA, USA), which distinguishes between the Ile and the Val alleles.9,15 The digestion products were separated in 3.5% agarose gels. This resulted in 176 bp (-GSTP1 A) or 85 and 91 bp (-GSTP1 G) products.
4. Statistical analyses
The data were analyzed using the χ2-test and logistic regression with the SAS statistical package. The control and gastric cancer groups were matched for age, gender and H. pylori status with a 2:1 ratio. In addition, multivariate analysis was performed adjusting for gender and age in tertiles. The risk for the presence of each condition, given the presence of GSTP1 alleles, was expressed as an odds ratio (OR) with a 95% confidence interval.31 A p value of <0.05 was considered statistically significant throughout.
RESULTS
1. Patient demographics
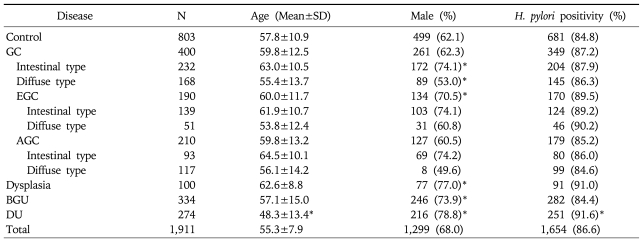
The mean ages and gender ratios of each group are shown in Table 1. The mean age for the patients with DU (48.3 years) was significantly lower than in the control group (57.8 years). There were significantly more men in the intestinal type GC, EGC, dysplasia, BGU, and DU groups than in the control group. The H. pylori-positive rates of the control, GC, dysplasia, BGU, and DU groups were 84.8% (681 of 803), 87.2% (349 of 400), 91.0% (91 of 100), 84.4% (282 of 334), and 91.6% (251 of 274), respectively, and a statistical difference was observed in the DU group compared to the controls.
Table 1.
Demographic Data of the Subjects
*versus controls: p<0.05.
SD, standard deviation; GC, gastric cancer; BGU, benign gastric ulcer; DU, duodenal ulcer.
2. Genetic polymorphism of GSTP1
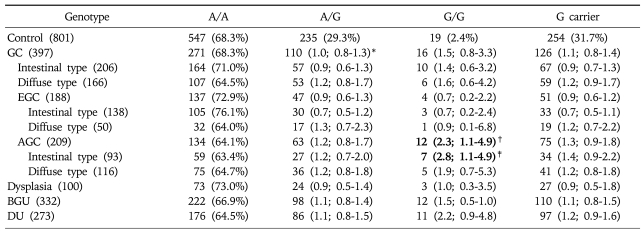
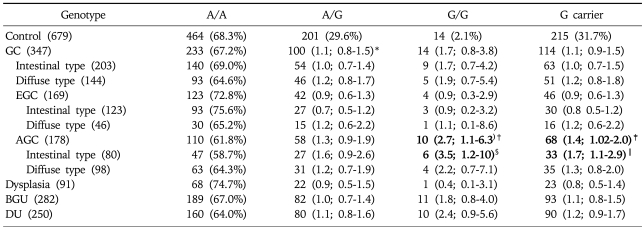
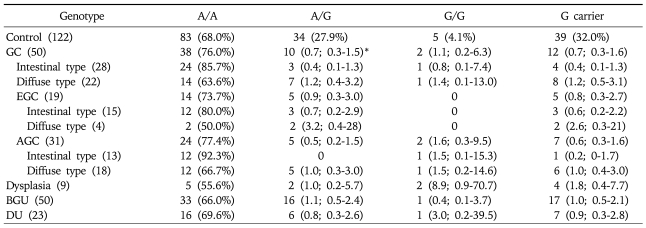
Genotyping of GSTP1 in the control group, regardless of H. pylori infection, revealed that 68.3% were A/A, 29.3% A/G and 2.4% G/G (Table 2). The frequency of the GSTP1 polymorphism at nucleotide 313 was not different in comparisons among the four disease groups including the GC group compared to the control group, regardless of H. pylori positivity. However, when the GC group was subdivided into AGC and EGC groups the frequency of the G/G allele was significantly higher in the AGC group than in the control group (OR: 1.2, 95% CI: 1.1-4.9, p=0.0309) (Table 2). Furthermore, when the AGC was subdivided into the intestinal and diffuse types the difference was more significant in the intestinal type (OR: 2.8, 95% CI: 1.1-7.3, p=0.0326) but disappeared in the diffuse type (Table 2). When the analysis was performed by H. pylori positivity, the statistical difference was greater in the H. pylori-positive AGC group (OR: 2.7, 95% CI: 1.1-6.3, p=0.0260) (Table 3) but it disappeared in the H. pylori-negative group (Table 4). The significant findings were associated with the frequency of the G carrier genotype in H. pylori-positive cases of the AGC group compared to the controls (OR 1.4, 95% CI; 1.0-2.0, p=0.0389). These findings differed from the results of the analyses of the total population (Table 3). However, similar to the results of the total population analyses, a statistical difference was found in the intestinal type (OR: 3.5, 95% CI: 1.2-10, p=0.0193) but disappeared in the diffuse type of GC in the H. pylori-positive group of patients (Table 3).
Table 2.
GSTP1 Genotype Frequencies in Each Group Irrespective of H. pylori Positivity
*adjusted for gender and age in tertiles. The figures set in bold indicate those with statistical significance.
†versus controls: p=0.0309.
‡versus controls: p=0.0326.
GC, gastric cancer; EGC, early gastric cancer; AGC, advanced gastric cancer; BGU, benign gastric ulcer; DU, duodenal ulcer.
Table 3.
GSTP1 Genotype Frequencies in Each of the H. pylori-positive Groups
*adjusted for gender and age in tertiles. The figures set in bold indicate those with statistical significance.
†versus controls: p=0.0260.
‡versus controls: p=0.0389.
§versus controls: p=0.0193.
∥versus controls: p=0.0299.
GC, gastric cancer; EGC, early gastric cancer; AGC, advanced gastric cancer; BGU, benign gastric ulcer; DU, duodenal ulcer.
Table 4.
GSTP1 Genotype Frequencies in Each of the H. pylori-negative Groups
*adjusted for gender and age in tertiles.
GC, gastric cancer; EGC, early gastric cancer; AGC, advanced gastric cancer; BGU, benign gastric ulcer; DU, duodenal ulcer.
DISCUSSION
Many investigators use genetic polymorphism studies to estimate the genetic contribution to the development of cancer. Possible cancer susceptibility genes have been sought among oncogenes, tumor suppressor genes, DNA repair genes, and genes encoding phase I and phase II enzymes.29 Large individual differences in the biotransformation of xenobiotics have been explained based on genetic polymorphisms of some detoxifying enzymes. Among these enzymes, GST is a large multigene family of phase II enzymes involved in the detoxification of potentially genotoxic chemicals.2 Five genetic polymorphisms of GST have been well documented. Total or partial deletions and (or) single nucleotide polymorphisms in the alleles encoding GSTM1, GSTM3, GSTPI, GSTT1, and GSTZ1 are associated with reduction of enzymatic activity of several substrates of different GST isoenzymes.32
GSTP1, a member of the glutathione S-transferase family, plays a central role in the inactivation of toxic and carcinogenic electrophiles.3 In addition, molecular epidemiology studies indicate that polymorphisms of GSTP1 appear to be associated with enzyme activity and to be linked to several types of cancer,9-15 although several studies have not confirmed these findings17-19 or have reported conflicting results.16 These differences are likely explained by variations in the studies with regard to the number of patients enrolled, the technical methods used to determine the genotypes and racial differences in the distribution of GSTP1 genotypes. The prevalence of different GSTP1 genotypes varies between different populations and ethnic groups. For example, in Western studies, 7-11% of the study populations have been reported to have the GSTP1 G/G genotypes.13,21 However, in Asia these genotypes have been reported to be present in 1.9-3%,20,33 similar to the 2.4% found in the control group of the present study.
To date, several studies have assessed the relationship between GSTP1 and GC, and have found no association.10,20,21 Recent studies also have shown no association between GSTP1 and precancerous lesions such as dysplasia or intestinal metaplasia in a Chinese population.34 In the present study, GC as a whole was not associated with GSTP1 genetic polymorphisms. However, when GC was subclassified by histological type and stage of cancer the presence of the GSTP1 G/G allele was found to be associated with the intestinal type of AGC. The significance of the G/G genotype of GSTP1 in the intestinal type but not in the diffuse type of GC might be explained by the influence of environmental factors in the development of GC, especially with the intestinal type. The reason why the GSTP1 G/G allele is associated with the intestinal type of AGC but not the EGC is not clear. However, toxic radicals may nonspecifically exert an influence on cancer progression, as the mechanisms of detoxification are lost during the progression of disease to gastric cancer with more rapid proliferation. This concept is supported by several previous reports that demonstrated that patients with AGC, who had the GSTP1 G/G genotype, were more sensitive to 5-FU/cisplatin based chemotherapy.31,35 However, further study is necessary to confirm this possibility.
Previous studies have shown that H. pylori infection leads to an increased production of reactive oxygen species within the gastric mucosa, which is thought to play a major role in the development of GC.36 The risk of lung cancer is increased in association with GST polymorphisms as well as other risk factors such as cigarette smoking.37 Similar to smoking, H. pylori might act to decrease the rate of detoxification causing higher levels of carcinogen-DNA adducts and more cytogenetic damage. However, most published studies have not reported on the H. pylori infection status of their subjects. Only an Italian report demonstrated that GSTP1 G/G genotypes were associated with a reduced risk of the diffuse type of GC in H. pylori negative subjects.38 In the present study, stratification of H. pylori infection was performed, and the GSTP1 G/G genotype was found to be significantly higher in the H. pylori-positive AGC group, particularly with the intestinal type; however, not in the H. pylori negative AGC group. These findings suggest that a patient with the G/G genotype of GSTP1 might be more susceptible to the development of the intestinal type of AGC in the presence of H. pylori infection. Our findings also suggest that histological or stage stratifications of GC are necessary for meaningful genetic analysis; even if the gastric cancer patients, as a group, do not show any significant difference in genetic polymorphisms.
The advantages of the present study are that: (a) a large population was enrolled with four distinct gastroduodenal diseases including dysplasia, GC, BGU, and DU. In addition, the study included age, gender and H. pylori status matched controls with gastric cancer, and (b) GC was subdivided by tissue type and cancer stage.
The results of this study showed that genetic polymorphisms of GSTP1 were associated with different stages of H. pylori-associated gastric cancer; that is, GSTP1 G/G correlated with the development of intestinal type AGC. This difference might reflect the different functions of the GSTP1 protein in the homeostasis of cells.
ACKNOWLEDGEMENTS
This work was supported by grant of the Korean Health 21 R&D Project, Ministry of Health & Welfare, Republic of Korea (#A060266).
References
- 1.van Iersel ML, Verhagen H, van Bladeren PJ. The role of biotransformation in dietary (anti)carcinogenesis. Mutat Res. 1999;443:259–270. doi: 10.1016/s1383-5742(99)00023-x. [DOI] [PubMed] [Google Scholar]
- 2.Strange RC, Spiteri MA, Ramachandran S, Fryer AA. Glutathione-S-transferase family of enzymes. Mutat Res. 2001;482:21–26. doi: 10.1016/s0027-5107(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 3.Hengstler JG, Arand M, Herrero ME, Oesch F. Polymorphisms of N-acetyltransferases, glutathione S-transferases, microsomal epoxide hydrolase and sulfotransferases: influence on cancer susceptibility. Recent Results Cancer Res. 1998;154:47–85. doi: 10.1007/978-3-642-46870-4_4. [DOI] [PubMed] [Google Scholar]
- 4.Zimniak P, Nanduri B, Pikula S, et al. Naturally occurring human glutathione S-transferase GSTP1-1 isoforms with isoleucine and valine in position 104 differ in enzymic properties. Eur J Biochem. 1994;224:893–899. doi: 10.1111/j.1432-1033.1994.00893.x. [DOI] [PubMed] [Google Scholar]
- 5.Watson MA, Stewart RK, Smith GB, Massey TE, Bell DA. Human glutathione S-transferase P1 polymorphisms: relationship to lung tissue enzyme activity and population frequency distribution. Carcinogenesis. 1998;19:275–280. doi: 10.1093/carcin/19.2.275. [DOI] [PubMed] [Google Scholar]
- 6.Ryberg D, Skaug V, Hewer A, et al. Genotypes of glutathione transferase M1 and P1 and their significance for lung DNA adduct levels and cancer risk. Carcinogenesis. 1997;18:1285–1289. doi: 10.1093/carcin/18.7.1285. [DOI] [PubMed] [Google Scholar]
- 7.Ali-Osman F, Akande O, Antoun G, Mao JX, Buolamwini J. Molecular cloning, characterization, and expression in Escherichia coli of full-length cDNAs of three human glutathione S-transferase Pi gene variants. Evidence for differential catalytic activity of the encoded proteins. J Biol Chem. 1997;272:10004–10012. doi: 10.1074/jbc.272.15.10004. [DOI] [PubMed] [Google Scholar]
- 8.Hu X, Ji X, Srivastava SK, et al. Mechanism of differential catalytic efficiency of two polymorphic forms of human glutathione S-transferase P1-1 in the glutathione conjugation of carcinogenic diol epoxide of chrysene. Arch Biochem Biophys. 1997;345:32–38. doi: 10.1006/abbi.1997.0269. [DOI] [PubMed] [Google Scholar]
- 9.Harries LW, Stubbins MJ, Forman D, Howard GC, Wolf CR. Identification of genetic polymorphisms at the glutathione S-transferase Pi locus and association with susceptibility to bladder, testicular and prostate cancer. Carcinogenesis. 1997;18:641–644. doi: 10.1093/carcin/18.4.641. [DOI] [PubMed] [Google Scholar]
- 10.Katoh T, Kaneko S, Takasawa S, et al. Human glutathione S-transferase P1 polymorphism and susceptibility to smoking related epithelial cancer; oral, lung, gastric, colorectal and urothelial cancer. Pharmacogenetics. 1999;9:165–169. [PubMed] [Google Scholar]
- 11.Harris MJ, Coggan M, Langton L, Wilson SR, Board PG. Polymorphism of the Pi class glutathione S-transferase in normal populations and cancer patients. Pharmacogenetics. 1998;8:27–31. doi: 10.1097/00008571-199802000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Helzlsouer KJ, Selmin O, Huang HY, et al. Association between glutathione S-transferase M1, P1, and T1 genetic polymorphisms and development of breast cancer. J Natl Cancer Inst. 1998;90:512–518. doi: 10.1093/jnci/90.7.512. [DOI] [PubMed] [Google Scholar]
- 13.Rossini A, Rapozo DC, Soares Lima SC, et al. Polymorphisms of GSTP1 and GSTT1, but not of CYP2A6, CYP2E1 or GSTM1, modify the risk for esophageal cancer in a western population. Carcinogenesis. 2007;28:2537–2542. doi: 10.1093/carcin/bgm222. [DOI] [PubMed] [Google Scholar]
- 14.Jourenkova-Mironova N, Voho A, Bouchardy C, et al. Glutathione S-transferase GSTM1, GSTM3, GSTP1 and GSTT1 genotypes and the risk of smoking-related oral and pharyngeal cancers. Int J Cancer. 1999;81:44–48. doi: 10.1002/(sici)1097-0215(19990331)81:1<44::aid-ijc9>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Sun XF, Ahmadi A, Arbman G, Wallin A, Asklid D, Zhang H. Polymorphisms in sulfotransferase 1A1 and glutathione S-transferase P1 genes in relation to colorectal cancer risk and patients' survival. World J Gastroenterol. 2005;11:6875–6879. doi: 10.3748/wjg.v11.i43.6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morita S, Yano M, Tsujinaka T, et al. Association between genetic polymorphisms of glutathione S-transferase P1 and N-acetyltransferase 2 and susceptibility to squamous-cell carcinoma of the esophagus. Int J Cancer. 1998;79:517–520. doi: 10.1002/(sici)1097-0215(19981023)79:5<517::aid-ijc12>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 17.Welfare M, Monesola Adeokun A, Bassendine MF, Daly AK. Polymorphisms in GSTP1, GSTM1, and GSTT1 and susceptibility to colorectal cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:289–292. [PubMed] [Google Scholar]
- 18.Saarikoski ST, Voho A, Reinikainen M, et al. Combined effect of polymorphic GST genes on individual susceptibility to lung cancer. Int J Cancer. 1998;77:516–521. doi: 10.1002/(sici)1097-0215(19980812)77:4<516::aid-ijc7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 19.Lin DX, Tang YM, Peng Q, Lu SX, Ambrosone CB, Kadlubar FF. Susceptibility to esophageal cancer and genetic polymorphisms in glutathione S-transferases T1, P1, and M1 and cytochrome P450 2E1. Cancer Epidemiol Biomarkers Prev. 1998;7:1013–1018. [PubMed] [Google Scholar]
- 20.Setiawan VW, Zhang ZF, Yu GP, et al. GSTP1 polymorphisms and gastric cancer in a high-risk Chinese population. Cancer Causes Control. 2001;12:673–681. doi: 10.1023/a:1011261602940. [DOI] [PubMed] [Google Scholar]
- 21.Wideroff L, Vaughan TL, Farin FM, et al. GST, NAT1, CYP1A1 polymorphisms and risk of esophageal and gastric adenocarcinomas. Cancer Detect Prev. 2007;31:233–236. doi: 10.1016/j.cdp.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hessey SJ, Spencer J, Wyatt JI, et al. Bacterial adhesion and disease activity in Heliocbacter associated chronic gastritis. Gut. 1990;31:134–138. doi: 10.1136/gut.31.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cover TL, Blaser MJ. Heliocbacter pylori infection, a paradigm for chronic mucosal inflammation: pathogenesis and implications for eradication and prevention. Adv Intern Med. 1996;41:85–117. [PubMed] [Google Scholar]
- 24.Dunn BE, Cohen H, Blaser MJ. Heliocbacter pylori. Clin Microbiol Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labigne A, de Reuse H. Determinants of Heliocbacter pylori pathogenicity. Infect Agents Dis. 1996;5:191–202. [PubMed] [Google Scholar]
- 26.Mobley HL. Heliocbacter pylori factors associated with disease development. Gastroenterology. 1997;113:S21–S28. doi: 10.1016/s0016-5085(97)80006-6. [DOI] [PubMed] [Google Scholar]
- 27.Pounder RE, Ng D. The prevalence of Heliocbacter pylori infection in different countries. Aliment Pharmacol Ther. 1995;9:S33–S39. [PubMed] [Google Scholar]
- 28.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez CA, Sala N, Capella G. Genetic susceptibility and gastric cancer risk. Int J Cancer. 2002;100:249–260. doi: 10.1002/ijc.10466. [DOI] [PubMed] [Google Scholar]
- 30.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process-First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 31.Ruzzo A, Graziano F, Kawakami K, et al. Pharmacogenetic profiling and clinical outcome of patients with advanced gastric cancer treated with palliative chemotherapy. J Clin Oncol. 2006;24:1883–1891. doi: 10.1200/JCO.2005.04.8322. [DOI] [PubMed] [Google Scholar]
- 32.Reszka E, Wasowicz W. Significance of genetic polymorphisms in glutathione S-transferase multigene family and lung cancer risk. Int J Occup Med Environ Health. 2001;14:99–113. [PubMed] [Google Scholar]
- 33.Hong SH, Kim JW, Kim HG, et al. Glutathione S-transferases (GSTM1, GSTT1 and GSTP1) and N-acetyltransferase 2 polymorphisms and the risk of gastric cancer. J Prev Med Pub Health. 2006;39:135–140. [PubMed] [Google Scholar]
- 34.You WC, Hong JY, Zhang L, et al. Genetic polymorphisms of CYP2E1, GSTT1, GSTP1, GSTM1, ALDH2, and ODC and the risk of advanced precancerous gastric lesions in a Chinese population. Cancer Epidemiol Biomarkers Prev. 2005;14:451–458. doi: 10.1158/1055-9965.EPI-04-0311. [DOI] [PubMed] [Google Scholar]
- 35.Goekkurt E, Hoehn S, Wolschke C, et al. Polymorphisms of glutathione S-transferases (GST) and thymidylate synthase (TS)--novel predictors for response and survival in gastric cancer patients. Br J Cancer. 2006;94:281–286. doi: 10.1038/sj.bjc.6602891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matthews GM, Butler RN. Cellular mucosal defense during Heliocbacter pylori infection: a review of the role of glutathione and the oxidative pentose pathway. Helicobacter. 2005;10:298–306. doi: 10.1111/j.1523-5378.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- 37.Schneider J, Bernges U, Philipp M, Woitowitz HJ. GSTM1, GSTT1, and GSTP1 polymorphism and lung cancer risk in relation to tobacco smoking. Cancer Lett. 2004;208:65–74. doi: 10.1016/j.canlet.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Ruzzo A, Canestrari E, Maltese P, et al. Polymorphisms in genes involved in DNA repair and metabolism of xenobiotics in individual susceptibility to sporadic diffuse gastric cancer. Clin Chem Lab Med. 2007;45:822–828. doi: 10.1515/CCLM.2007.143. [DOI] [PubMed] [Google Scholar]