Abstract
A consensus meeting on autoimmune pancreatitis (AIP) was held in Seoul on August 31, 2007. Many Korean and Japanese gastroenterologist interested in AIP participated in the joint symposium, and issues related to histology, radiology, clinical manifestation, serology, and diagnostic criteria were discussed. This joint meeting indicated the need for unified diagnostic criterion for AIP in Korea and Japan. Here, we provide a summary of the symposium presentations and discussions.
Keywords: Autoimmune pancreatitis, Consensus, Korea, Japan
INTRODUCTION
Speaker: Jae Bock Chung: A concept of autoimmune pancreatitis (AIP) was proposed firstly by Japanese investigators in 1995. Thereafter many AIP cases have been reported mostly from Japan and Korea. We are now familiar with radiologic findings, biochemical markers, clinical manifestations, histologic findings, and extrapancreatic lesions of AIP. And also we know that steroid therapy is effective for AIP.
However, we still do not know the cause, actual pathogenesis, natural course and long term prognosis of AIP at present. There are several diagnostic criteria of AIP reported from Japan, Korea, and Western countries.1-3 However, there are some limitations of each criterion to satisfy every case of AIP and controversial point about the response to steroid therapy including as a diagnostic criteria.
Today we will discuss about the characteristic features of AIP, and will compare the diagnostic criteria for AIP of Japan, USA and Korea, and finally we will discuss about the ideal diagnostic criteria for AIP to make Asian criteria.
It is not easy to make ideal diagnostic criteria for AIP by today's symposium within six hours. But I believe and hope that all the participants and speakers can make the ideal diagnostic criteria for AIP by active discussion at the end of this symposium.
PRESENTATIONS
1. Imaging findings of AIP
Speaker: Jae Hoon Lim: Imaging findings of AIP are fairly characteristic.4,5 In an appropriate clinical setting, a constellation of imaging features is very helpful in the diagnosis of AIP. The abnormal findings are reversible with steroid therapy and thus imaging can be used as an evaluation of treatment response.
The typical findings of computed tomography (CT) are diffuse enlargement of the entire pancreas with homogenous texture. The normal tiny cysts or serration along the pancreatic duct is lost and becomes smooth. The enlarged pancreas is covered by a thin, low density rim and the surface is pencil sharp. In some patients, there may be peripancreatic stranding and thickening of renal fascia. Frequently, the involved lesion may be focal or segmental, and the involved segment may be enlarged mimicking a pancreatic mass. Usually, there is no calcification, peripancreatic fluid collection or vascular involvement.
Ultrasonography shows diffuse homogenous enlargement of the pancreas. Magnetic resonance imaging (MRI) also reveals diffuse enlargement with rim enhancement. Magnetic resonance cholangiopancreaticography (MRCP) reveals diffuse narrowing and minute ductal irregularity along the main pancreatic duct. Sometimes, there may be a focal stricture. Endoscopic retrograde cholangiopancreaticography (ERCP) shows characteristic diffuse narrowing with irregularity or serration along varying segment of the main pancreatic duct. Focal stricture may be present. The intrapancreatic segment of the common bile duct may be focally or segmentally narrowed resulting in dilation of the proximal bile ducts. Infrequently the proximal extrahepatic ducts or intrahepatic bile ducts may be involved resulting in various degree of narrowing. By administrating steroid therapy, those abnormal findings resolve soon and become completely normal.
2. AIP: Serology
Speaker: Shigeyuki Kawa and Hideaki Hamano: Various serum markers in AIP are associated with diagnosis, disease activity and pathogenesis. The utility of IgG4 for the diagnosis of AIP has been accepted worldwide. Sensitivity is variable from 73% to 92%,6-8 and the difference in sensitivity is partly due to the differences of diagnostic criteria applied by individual countries. Sensitivities for antinuclear antibody and rheumatoid factor were 60% and 20%.9 However, SS-A and SS-B, and anti-mitochondrial antibody were scarcely found in sera of AIP patients. We investigated the outcome of long-term follow up of 51 patients with AIP, and found 21 patients (41%) with recurrences. Serum immune complex levels at onset were significantly higher in recurrence group than non-recurrence group. If the levels of immune complex at onset are over 10 g/dL, probability for recurrence is 60%. AIP is complicated with various extra-pancreatic involvements. Serum IgG4 level was well correlated with the number of extra-pancreatic involvements, and lachrymal and salivary gland lesions and hilar lymphadenopathy are significantly associated with high serum levels of IgG4 and immune complex. Decreased serum levels of C3 and C4 were found in 35% and 37% of AIP patients at onset,10 indicating that complement activation system is operating in the pathogenesis of this disease. Elevated serum levels of immune complex determined by C1q assay were significantly related to increased serum levels of IgG1 and decreased levels of C4, with a tendency toward decreased levels of C3, indicating that classical pathway may be operating in the pathogenesis of AIP.
3. AIP: Histology and immunostaining
Speaker: Se Jin Jang: AIP is a newly established entity defined by the following 3 characteristic clinicopathologic findings: (i) radiologic features of irregular narrowing of the main pancreatic duct and diffuse enlargement of the pancreas, (ii) laboratory findings such as elevation of serum IgG or the presence of autoantibodies, and (iii) histopathologic features of lymphoplasmacytic sclerosing pancreatitis (LPSP).3,11 Among them, histologic findings are generally known to be gold standard for the diagnosis of the disease. However, since histologic components of normal pancreas including acini, islets, ducts, blood vessels and connective tissue stroma respond to inflammatory stimuli with limited patterns, histologic features of AIP may overlap with other causes of chronic pancreatitis. Furthermore, as the chronic pancreatitis presents as repeated bouts of acute pancreatitis, the time of histologic examination during the disease process is another important variable to evaluate the histologic features.
Acinar changes in AIP are characterized by loss of exocrine cells with lymphoplasmacytic infiltration and replacement by loose collagenous stroma (intralobular fibrosis). Atrophy of the islets is less prominent. Sometimes, lobular architectures are preserved even though there are complete losses of acini. Ductal changes are most characteristic features of AIP. Intense lymphoplasmacytic infiltration in and around the wall of main pancreatic and medium-sized interlobular ducts with sclerosis and luminal narrowing, which has been named as LPSP, is considered as a histologic hallmark of AIP.12 Sometimes, active chronic inflammation, characterized by granulocytic infiltration in the ductal epithelium without denudation (granulocytic epithelial lesion, GEL) can be seen. Although the inflammatory reaction is intense, tissue necrosis by autodigestion is usually absent. Changes of blood vessels always appear in the intralobular small venules and interlobular veins. Perivenular lymphoplasmacytic infiltration with luminal obstruction forming an obliterative phlebitis is considered as a characteristic feature. Arteries and arterioles are always preserved. Those pathologic features are variable according to severity of the disease or stage of the disease. Therefore, sometimes immunostainings are helpful. Demonstration of high density IgG4 positive cells by immunostaining is most widely accepted as typical finding of AIP. Characteristic lobular changes, intralobular fibrosis with relatively preserved reticulin frameworks, can be visualized by immunostaining of collagen type IV. To distinguish from lymphoproliferative disorders, demonstration of polyclonal nature by immunostaining of CD3, CD20, CD4, and CD8 will be helpful. In summary, LPSP with high density (20>HPF) IgG4 positive cells and sclerosis with preserved lobular feature are diagnostic features of AIP.
4. Other organ involvement in AIP
Speaker: Tetsuhide Ito: The accumulation of clinical data in AIP patients has revealed a variety of extra-pancreatic involvement, including sclerosing cholangitis, lacrimal and salivary gland swelling, hypothyroidism, hilar lymphadenopathy, retroperitoneal fibrosis, interstitial pneumonia, and interstitial nephritis.12-14 Most patients with AIP and sclerosing sialadenitis show negativity for both anti-SSA and snti-SSB antibodies, suggesting that AIP differs from Sjogren's syndrome. Sclerosing cholangitis-like lesions accompanying AIP and primary sclerosing cholangitis respond differently to steroid therapy and have different prognosis, suggesting that they are not the same disorder. In this paper, we reported the result of incidence of extra-pancreatic involvement based on 191 AIP patients collected from seven institutions in Japan. The most frequent extra-pancreatic lesions in Japan were sclerosing cholangitis (58.6%), followed by hilar lymphadenopathy (28.3%) and salivary gland lesion (24.6%). The frequency of other organ involvement was as follows; retroperitoneal fibrosis in 11.0%, chronic thyroiditis in 8.9%, pseudotumor of the lachrymal gland in 8.4%, interstitial nephritis in 3.7%, interstitial pneumonia in 1.6%, thrombocytopenic purpura in 0.5%, and prostatitis in 0.5%. Interestingly, the complication of inflammatory bowel disease was 0%. Extra-pancreatic lesions have a characteristic histological finding of abundant IgG4-bearing plasma cell infiltration, and respond favorably to steroid therapy.14 Diagnosing AIP is needed to distinguish it from pancreatic or biliary cancer. Furthermore, extra-pancreatic lesions with AIP should be carefully diagnosed to avoid inadequate steroid administration. The current concept of AIP including extra-pancreatic lesions and associated disorders suggests that AIP may be pancreatic manifestation of a systemic disorder. Recognition of these characteristic findings will help the correct diagnosis of this disease.
5. AIP: Response to steroid and recurrence
Speaker: Isao Nishimori, Masaru Koizumi, and Makoto Otsuki: Most patients with AIP show a dramatic response to steroid therapy.7 To establish the standard therapeutic method for AIP, we set two rounds of survey asking treatment and clinical course of AIP patients in Japan.
The first was the nation-wide survey for AIP patients, who fulfilled the Japanese AIP criteria 2002. Clinical remission rate in patients treated with steroid (123/125; 98.4%) was significantly higher than that in patients without steroid therapy (14/16; 87.5%) (p<0.005). Among patients with steroid therapy, the initial does of predonisolone was 40 mg/day in 31 patients and 30 mg/day in 54 patients (average: 0.6 mg/body weight kg/day).
The second survey was designed to ask therapeutic outcome in AIP patients, who fulfilled the Japanese AIP criteria 2006 and were clinically observed for at least 2 years. Among 38 patients who showed clinical relapse, relapse of pancreatic manifestations were seen in 19 patients (50.0%), extra-pancreatic manifestations in 11 (28.9%) and both in 8 (21.1%). The higher was the prednisolonemaintenance doses, the lower was the relapse rate. There was a significant difference in relapse rates between patients treated with 0-2.5 mg/day of prednisolone-maintenance dose (48.1%, n=52) and patients with 5.0-7.5 mg/day (26.5%, n=34) (p<0.05). In most patients, clinical relapse appeared until 3 years after the start of steroid therapy.
In conclusion, steroid therapy with oral prednisolone is recommended for AIP patients; 0.6 mg/body weight kg/day of the starting dose and 5.0-7.5 mg/day of the maintenance dose for totally at least three years.
6. Overview of diagnostic criteria for AIP
Speaker: Ji Kon Ryu: Although, Japan Pancreas Society proposed the diagnostic criteria for the first time in 2002,15 the diagnosis of AIP is still challenging. Recently, several other diagnostic criteria were proposed as followings: revised proposal of JPS criteria 2006,1 HISORt criteria by Mayo Clinic,3 Kim's criteria,2 Massachusetts General Hospital criteria.16 Therefore, the new diagnostic criteria made by worldwide consensus will lessen inter-observer variation and provide a strong base for the research.
Because AIP can mimic pancreatic cancer both clinically and radiologically, and because it is responsive to steroid therapy, the goal is to make a diagnosis before surgery to avoid unnecessary operation. The overall impact of a missed diagnosis of AIP is not trivial. In one large series, 11 (2.5%) of 442 Whipple resections were for AIP; all were thought to be malignant preoperatively.17
In 2005, Spainish group published their own diagnostic criteria using scoring system. The criteria included clinical manifestation, morphologic parameters, laboratory parameters (serum IgG and ANA) and definite diagnosis was made only by histopathologic findings.18 A total joint score of 0 or 1 was considered "probably not AIP", 2 as "possible AIP", and 3 as "probable AIP".
In Korea, Kim et al proposed Kim's criteria based on his experience in 28 patients with AIP at single center.2 Kim's criteria included pancreatic imaging, laboratory findings, histopathologic findings and responsiveness to steroid. In Japan, clinical diagnostic criteria of AIP were published as a revised proposal by the research committee of intractable disease of the pancreas supported by the Japan Pancreas Society.1 They focused on how to distinguish it from pancreatic or biliary cancer. The criteria contained three components the same as previous Japan criteria and some minor modifications were made. The Japanese criteria are based on the minimum consensus features of AIP to avoid the misdiagnosis of malignancy as much as possible, but not to pick up suspicious cases of AIP.
The HISORt criteria were proposed by the Mayo clinic based on the 29 patients who met histologic criteria for AIP at single center.3 The criteria included 5 categories: histology, imaging, serology, other organ involvement and response to steroid therapy. The authors pointed out that the Japanese criteria for AIP focussed heavily on characteristic pancreatic imaging and therefore lack sensitivity to diagnose the wide spectrum of manifestations of AIP. They insisted that HISORt criteria reflect the current understanding of AIP as a systemic steroid-responsive disorder characterized by tissue infiltration with IgG4-positive cells.
Another new diagnostic criteria were proposed by Brugge at Massachusetts General Hospital (MGH).16 They defined AIP as a type of chronic pancreatitis characterized by an autoimmune inflammatory process in which prominent lymphocyte infiltration with associated fibrosis of the pancreas causes organ dysfunction. The MGH criteria are modified from those of the Japan Pancreas Society and minor modifications were done to elevate diagnostic sensitivity. They also suggested the diagnostic and treatment algorithm for AIP according to their diagnostic criteria.
In Korea, new Korean diagnostic criteria are established by the Korean Society of Pancreatobiliary Diseases and based on the Kim's criteria including response to steroid therapy to improved the diagnostic sensitivity and specificity. Korean criteria considered the variety of imaging findings of AIP and low yield of histologic acquisition.
Making a diagnosis of AIP can be challenging but is important to prevent unnecessary surgery. Diagnostic criteria have been proposed to incorporate histologic, radiologic, serologic, and clinical information, including the presence of other associated diseases and response to steroid therapy. Because the distinction between AIP and pancreatic cancer is difficult to make in many cases, every attempt needs to be made to exclude the possibility of malignancy, even if it results in a pancreatic resection for benign disease in some patients. As more data are gathered on AIP across the world, the diagnostic criteria will be shifted.
7. Clinical diagnostic criteria of Japan compared with those of Korea and USA
Speaker: Kazuichi Okazaki: The concept of Japanese criteria-2006 is minimum consensus for the practical use to differentiate AIP from pancreatic or biliary malignancy as far as possible, but neither for differentiation from systemic disorders or screening AIP. In Pancreatic imaging, typical pancreatogram with CT or MRI is required in Japanese,1 Korean2 and Mayo's Group-B criteria,3 but not in Mayo's G-C. ERCP is mandatory in the Japanese, but MRCP is also available in Korean and Mayo's. In the blood test, presence of autoantibody in addition to high serum IgG4 is available in the criteria of Korea and Japan, but not in Mayo's. Irrespective of laboratory or radiological data, only the LPSP is definitive for diagnosis of AIP in the Mayo's G-A, but not in Japan and Korea. In Mayo's and Korean criteria, dense infiltration of IgG4 positive plasma cells in the pancreas specimen is useful for diagnosis when LPSP is not confirmed. Extra-pancreatic lesions are included in the Korean and Mayo, but not in Japanese criteria. Steroid trial for the pancreas and/or extra-pancreatic lesions is available in the Korean and Mayo's criteria, but not in Japanese criteria. We prospectively studied three criteria using 21 AIP cases in Kansai Medical University, in which the sensitivity of Japanese, Korean and Mayo' criteria was 71.4, 76.2, and 52.3%, respectively. Sensitivity of pancreas images (90-100%) and blood test (IgG; 94%, IgG4; 81%, auto-Abs; 47%) are high, but low in histological findings from biopsied pancreatic specimen. These findings suggest that if steroid trial and presence of autoantibody are included in the criteria, diagnostic sensitivity is increased. We experienced an 80 year-old male patient with AIP followed by pancreatic cancer 4 years later, which suggested pancreatic cancer may accompany with AIP. Although steroid trial may increase diagnostic sensitivity for AIP, efficacy of steroid should be carefully evaluated and facile therapeutic diagnosis is not recommended at this moment.
8. Korean criteria: comparison with those of Japan and USA
Speaker: Kyutaek Lee: The overwhelming majority of reports of AIP have come from Asian countries (e.g. Japan and Korea), and the prevalence of AIP in Japan was estimated to be 0.82 per 100,000.19-21 Therefore, it is reasonable to establish unified Asian diagnostic criteria.
The revised Japanese criteria1 (2006) do not include the response to steroid therapy as a diagnostic component because inclusion of steroid responsiveness in the criteria may encourage the use of easy therapeutic diagnostic techniques just to distinguish AIP from pancreatic cancer. However, the new Korean criteria2 (2007) recommend short term (2 weeks) steroid trial to diagnosis AIP in suspicious cases with strict regulation. Two weeks delay of operation is not critical for the prognosis of pancreatic cancer patients. The American HISORt criteria3 (2006) was originally based on the histopathologic finding instead of clinical and practical finding of Korean and Japan criteria. The histological diagnosis of LPSP has most commonly been made on specimens obtained by surgical resection. The criteria emphasizing the histological findings of LPSP may have some limitations due to requirement of a large tissue sample.
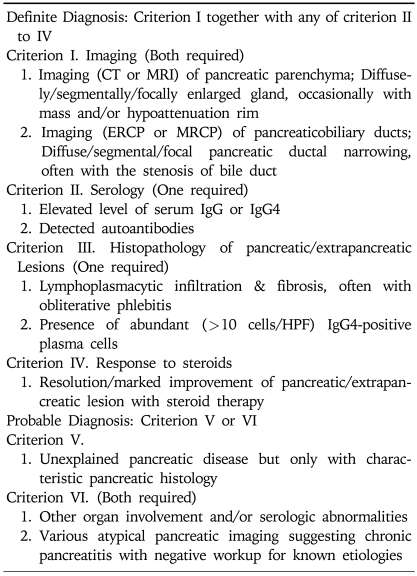
Korean AIP criteria 2007 (Table 1) are aimed high sensitivity to diagnose the wide spectrum of manifestations of AIP.
The diagnostic criteria for AIP are still evolving. Integrated diagnostic criteria are strongly advocated so that more patients can have an opportunity to receive medical treatment that will avoid any unnecessarily invasive procedure.
Table 1.
Korean Criteria for Autoimmune Pancreatitis (2007)
9. Ideal diagnostic criteria for AIP
Speaker: Myung-Hwan Kim: In theory, ideal diagnostic criteria should have 100% sensitivity and specificity; of course, such criteria do not exist in reality. Thus, the goal is to establish diagnostic criteria that have as high sensitivity as possible without sacrificing specificity. In practice, the differentiation of AIP from pancreatic cancer is the most important thing, so the high specificity of the criteria may have priority over high sensitivity.
When Japanese Pancreas Society published the diagnostic criterion of AIP for the first time in 2002,22 it was a criterion with emphasis on specificity. The strict criterion that specified more than one third of entire main pancreatic duct should be involved along with enlargement of whole pancreas came from a concern that some pancreas cancer may be erroneously included. However, some have pointed out that the criteria are too stringent and sensitivity is sacrificed excessively. Therefore, in 2006, Japan Pancreas Society has published the Revised Criteria.
On the other hand, Mayo clinic from US published a criteria3 (HISORt) in 2006 which has features that include diverse image presentation of AIP. This Criterion is notable for its more broad recognition of manifestation of AIP, but some consider specificity is sacrificed by including atypical imagings such as atrophy, calcification and stones. We present the new Korean criteria, which has combined features of both Japan and US criteria and is intended to maximize sensitivity while not sacrificing specificity.
Diagnostic criteria for AIP are evolving. With accumulation of clinical experience, development of newer diagnostic methods and change in disease concept, the criteria will need to be updated continuously. In this regard, consensus needs to be reached for unified criteria that could be shared internationally throughout the world, and it is our hope that the new Korean criteria can contribute in establishment of such criteria in the future.
10. Proposal of an Asian criteria for AIP
Speaker: Terumi Kamisawa: It is of utmost importance that AIP be differentiated from pancreatic cancer. The Japanese diagnostic criteria of AIP are based on minimum consensus features of AIP and aim to avoid misdiagnosis of malignancy. When response to steroid therapy is added to the criteria, the diagnostic sensitivity is increased. In my recently experienced 28 AIP cases, 6 seronegative cases could not be diagnosed in Japanese criteria, but they could be diagnosed by responsiveness to steroid therapy in Korean criteria.
However, the use of a steroid trial in cases where differentiation from malignancy is an issue may result in delaying pancreatic cancer surgery, which could lead to cancer progression in several cases. Diagnostic trial of steroid therapy should be done carefully only by pancreatologists familiar to AIP. Since general physicians use the diagnostic criteria of AIP, diagnostic steroid trial should not be easily recommended in the criteria.
I propose Asian diagnostic criteria of AIP (Table 2) and would stress that steroid should be given only to patients with a negative workup for pancreatobiliary cancer, and facile diagnostic steroid trial should not be used.
Table 2.
Asian Diagnostic Criteria for Autoimmune pancreatitis (Tentative)
*Steroid should be given only to patients with a negative work-up for pancreatobiliary cancer. Facile diagnostic steroid trial should not be used.
CONCLUSION
Speaker: Makoto Otsuki: At present, there are four diagnostic criteria for AIP including the Japanese, Korean, Mayo Clinic, and the Italian criteria. Diagnostic criteria should be simple enough for widespread use with ease, but stringent enough to avoid erroneous inclusion of malignant diseases as AIP. Diagnostic trial using short-term steroid harbors the risk of delaying diagnosis of pancreatic cancer and resulting in loss of chance for curative resection. Sometimes steroid can reduce the size of mass by suppressing inflammatory changes surrounding the pancreatic tumor. This is why Japanese gastroenterologists do not accept diagnostic trial of steroid.
It is not easy to making ideal diagnostic criteria fit for all cases of AIP and satisfying all gastroenterologists. It needs painstaking studies to further understand the disease entity both at the basic and clinical level. In this setting, it is desirable to open a way for Japanese and Korean gastroenterologists to collaborate, communicate with, and work together to reach at common diagnostic criterion. Sharing diagnostic criteria will further enhance communication and give birth to synergistic effect on understanding the AIP. I believe that further studies in collaboration between Japan and Korea will dissolve many unsolved questions. I would like to close this second Japan-Korea symposium on AIP and express my thanks to all participants and those who organized this meeting.
References
- 1.Okazaki K, Kawa S, Kamisawa T, et al. Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol. 2006;41:626–631. doi: 10.1007/s00535-006-1868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim KP, Kim MH, Kim JC, et al. Diagnostic criteria for autoimmune chronic pancreatitis revisited. World J Gastroenterol. 2006;12:2487–2496. doi: 10.3748/wjg.v12.i16.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chari ST, Smyrk TC, Levy MJ, et al. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010–1016. doi: 10.1016/j.cgh.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Sahani DV, Kalva SP, Farrel J, et al. Autoimmune pancreatitis: imaging features. Radiology. 2004;233:345–352. doi: 10.1148/radiol.2332031436. [DOI] [PubMed] [Google Scholar]
- 5.Yang DH, Kim KW, Kim TK, et al. Autoimmune pancreatitis: radiologic findings in 20 patient. Abd Imaging. 2006;31:94–102. doi: 10.1007/s00261-005-0047-8. [DOI] [PubMed] [Google Scholar]
- 6.Choi EK, Kim MH, Lee TY, et al. The sensitivity and specificity of serum immunoglobulin G and immunoglobulin G4 levels in the diagnosis of autoimmune pancreatitis: Korean experience. Pancreas. 2007;35:156–161. doi: 10.1097/MPA.0b013e318053eacc. [DOI] [PubMed] [Google Scholar]
- 7.Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732–738. doi: 10.1056/NEJM200103083441005. [DOI] [PubMed] [Google Scholar]
- 8.Kawa S, Hamano H. Assessment of serological markers for the diagnosis of autoimmune pancreatitis. J Jpn Pancreas Soc. 2003;17:607–610. [Google Scholar]
- 9.Kawa S, Hamano H. Clinical features of autoimmune pancreatitis. J Gastroenterol. 2007;42:9–14. doi: 10.1007/s00535-007-2044-x. [DOI] [PubMed] [Google Scholar]
- 10.Hamano H, Arakura N, Muraki T, et al. Prevalence and distribution of extrapancreatic lesions complicating autoimmune pancreatitis. J Gastroenterol. 2006;41:1197–1205. doi: 10.1007/s00535-006-1908-9. [DOI] [PubMed] [Google Scholar]
- 11.Chari ST. Diagnosis of autoimmune pancreatitis using its five cardinal features: introducing the Mayo Clinic's HISORt criteria. J Gastroenterol. 2007;42(Suppl 18):S39–S41. doi: 10.1007/s00535-007-2046-8. [DOI] [PubMed] [Google Scholar]
- 12.Kamisawa T, Funata N, Hayashi Y. Lymphoplasmacytic sclerosing pancreatitis is a pancreatic lesion of IgG4-related systemic disease. Am J Surg Pathol. 2004;28:1114. doi: 10.1097/01.pas.0000126634.43301.45. [DOI] [PubMed] [Google Scholar]
- 13.Kamisawa T, Funata N, Hayashi Y, et al. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:82–984. doi: 10.1007/s00535-003-1175-y. [DOI] [PubMed] [Google Scholar]
- 14.Kamisawa T. IgG4-positive plasma cells specifically infiltrate various organs in autoimmune pancreatitis. Pancreas. 2004;29:167–168. doi: 10.1097/00006676-200408000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Pearson RK, Longnecker DS, Chari ST, et al. Controversies in clinical pancreatology: autoimmune pancreatitis: does it exist? Pancreas. 2003;27:1–13. doi: 10.1097/00006676-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Finkelberg DL, Sahani D, Deshpande V, Brugge WR. Autoimmune pancreatitis. N Engl J Med. 2006;355:2670–2676. doi: 10.1056/NEJMra061200. [DOI] [PubMed] [Google Scholar]
- 17.Abraham SC, Wilentz RE, Yeo CJ, et al. Pancreaticoduodenectomy (Whipple resections) in patients without malignancy: are they all 'chronic pancreatitis'? Am J Surg Pathol. 2003;27:110–120. doi: 10.1097/00000478-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Aparisi L, Farre A, Gomez-Cambronero L, et al. Antibodies to carbonic anhydrase and IgG4 levels in idiopathic chronic pancreatitis: relevance for diagnosis of autoimmune pancreatitis. Gut. 2005;54:703–709. doi: 10.1136/gut.2004.047142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim KP, Kim MH, Song MH, Lee SS, Seo DW, Lee SK. Autoimmune chronic pancreatitis. Am J Gastroenterol. 2004;99:1605–1616. doi: 10.1111/j.1572-0241.2004.30336.x. [DOI] [PubMed] [Google Scholar]
- 20.Okazaki K. Autoimmune pancreatitis is increasing in Japan. Gastroenterology. 2003;125:1557–1558. doi: 10.1016/j.gastro.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Nishimori I, Tamakoshi A, Otsuki M. Prevalence of autoimmune pancreatitis in Japan from a nationwide survey in 2002. J Gastroenterol. 2007;42(Suppl 18):S6–S8. doi: 10.1007/s00535-007-2043-y. [DOI] [PubMed] [Google Scholar]
- 22.Members of the Criteria Committee for Autoimmune Pancreatitis of the Japan Pancreas Society. Diagnostic criteria for autoimmune pancreatitis 2002 by Japan Pancreas Society. J Jpn Pan Soc. 2002;17:585–587. [Google Scholar]