Abstract
Background/Aims
There have been several reports of thermal injury induced by argon plasma coagulation (APC) in animal models, but no follow-up studies have revealed the actual thermal injury.
Methods
APC was performed on the stomachs of two living minipigs with and without prior submucosal injection of normal saline. The power and argon gas flow were set to 60 watts and 2 L/min, respectively, and pulse durations of 5, 10, and 20 seconds were used. One of the minipigs was killed immediately thereafter and the other was killed 1 week later.
Results
The minipig killed immediately showed only subtle differences between noninjected and injected injuries under all the conditions, and the usefulness of prior submucosal injection was not obvious. However, the minipig killed 1 week later had a deep ulcer extending to the deeper muscle layer at the noninjected site where APC had been applied for 20 seconds, whereas tissue injury of the injected site was limited to the submucosal layer.
Conclusions
Unexpected tissue damage can occur even using a short-duration APC. Prior submucosal injection for APC might be a safer alternative technique, especially in a thinner and narrower gut wall.
Keywords: Argon, Submucosa, Injection, Animal model, Thermal
INTRODUCTION
The safety of argon plasma coagulation (APC) has been reported in clinical practice and the rate of severe complications such as perforation and stricture was less than 1%.1-7 Previous study using the fresh resected porcine models shows that tissue damage caused by APC may be limited up to the submucosal layer under the condition generally used,8,9 and prior submucosal injection may be further safe to prevent deep tissue destruction.9-11 However, the obtained results are only the facts of the resected materials or immediate euthanasia in an in vivo study. We sometimes experience unexpected deep ulceration or stenosis after APC at a follow-up endoscopic examination.3,6,12,13 In this study, we investigate tissue damage on the stomach of a living minipig to confirm the safety of APC and the usefulness of prior submucosal injection in a living body, including a follow-up case.
MATERIALS AND METHODS
1. Preparation of study animals
Two living minipigs (Sus scrofa; Miniature Swine) were used for this study, which were provided from Chugai Research Institute for Medical Science, Inc., Shizuoka, Japan, and the use for research purpose was fully approved by the institution.
2. Endoscopic procedures
Endoscopic procedures were performed on the minipigs under general anesthesia and mechanical ventilation after overnight fasting. The stomachs were sufficiently inflated with air after the residual food and mucus on the mucosal surface were washed out with tap water that was splashed from the instrumental channel of an endoscope. After these preparations, the application of APC over the mucosa was performed on the body of the fully-insufflated stomachs with or without prior submucosal injection of normal saline, at the setting of the power of 60W and the argon gas flow of 2 L/min. The pulse duration was changed as 5, 10, and 20 seconds. The unit used for application of APC was the standard APC equipment consisting of a high-frequency generator (Erbotom ICC 200), an automatically regulated argon source (APC 300), and a flexible APC applicator, 2.3 mm in diameter. All of them were products of ERBE Elektromedizin, Tubingen, Germany. A flexible APC applicator was inserted into the stomach through the endoscopic channel. Two milliliters of normal saline per site were injected into the submucosal layer for the testing of prior submucosal injection, by using a 23-gauge injection needle. Although it was difficult to apply APC at the same condition, we tried to keep a separation distance of an applicator and tissue approximately 2 mm and the angle 10 to 30 degree. After the coagulation was performed, one minipig was killed without delay and the other was killed after observation for one week.
3. Pathological analysis
The resected stomachs were cut on the points of coagulation and fixed with formalin and embedded in paraffin. A histological section was made from each block and stained with hematoxylin and eosin and examined about tissue damage microscopically.
RESULTS
1. Tissue damage of immediate euthanasia
A minipig killed without delay after APC application revealed that tissue damage without injection was extended deeper as the applied time increased and tissue damage with injection was limited to the shallower submucosal layer regardless of the applied time. However, the difference between noninjected area and injected area was subtle and the usefulness of prior submucosal injection was not revealed significantly (Fig. 1).
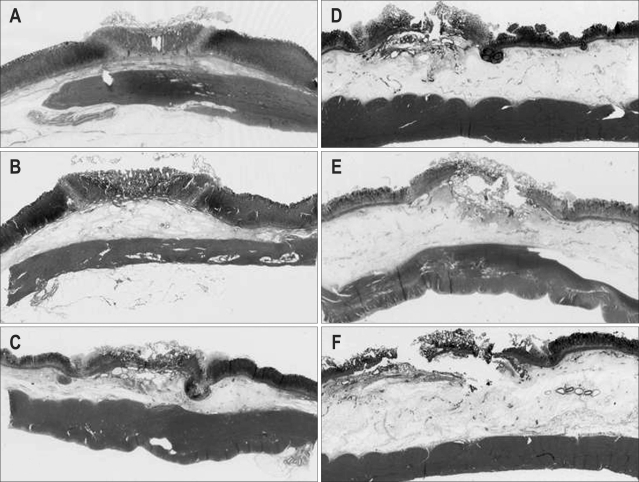
Fig. 1.
Tissue injury in a minipig killed immediately after argon plasma coagulation (APC). (A) APC (5 seconds) without prior submucosal injection. (B) APC (5 seconds) after submucosal injection. (C) APC (10 seconds) without prior submucosal injection. (D) APC (10 seconds) after submucosal injection. (E) APC (20 seconds) without prior submucosal injection. (F) APC (20 seconds) after submucosal injection. Tissue coagulation was limited to the deeper submucosal layer under all the conditions. The thermal effects tended to do deeper with a longer pulse duration and no prior saline injection. With prior saline injection, the increased thickness of the submucosal layer might prevent injury to the deeper submucosal layer.
2. Tissue damage of delayed euthanasia
A minipig killed after one week's follow-up revealed that granulomatous and fibrotic changes existed in the submucosal layer of the artificial ulcers at the pulse durations of 5 seconds and 10 seconds, regardless of submucosal injection. The difference between noninjected injury and injected injury, or between pulse durations of 5 seconds and 10 seconds was not obvious, and injury of the proper muscle layer was not observed in any of those conditions. On the contrary, the noninjected injury of the pulse duration of 20 seconds made the deep ulceration extended to the deeper proper muscle layer, whereas injected injury of the same duration did not extend to the proper muscle layer (Fig. 2).
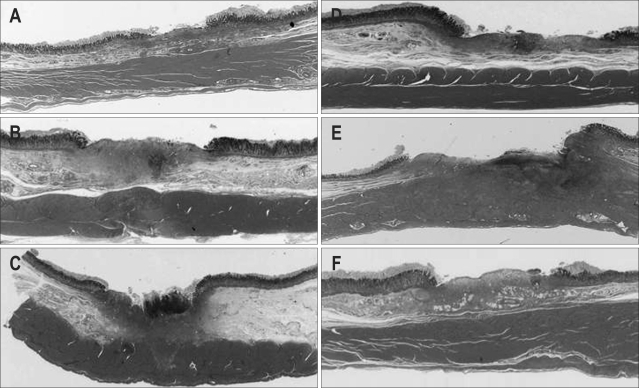
Fig. 2.
Tissue damage in a minipig killed 1 week after APC. (A) APC (5 seconds) without prior submucosal injection. (B) APC (5 seconds) after submucosal injection. (C) APC (10 seconds) without prior submucosal injection. (D) APC (10 seconds) after submucosal injection. (E) APC (20 seconds) without prior submucosal injection. (F) APC (20 seconds) after submucosal injection. Under all the conditions except for 20 seconds of APC without prior submucosal injection, granulomatous and fibrotic changes were evident in the submucosal layer of the artificial ulcer, although the actual muscle layer appeared intact. Deep ulceration that destroyed the muscle layer was evident for 20 seconds of APC without submucosal injection.
DISCUSSION
This study may give us two important messages. First, unexpected deep tissue destruction may possibly occur and follow-up study is necessary to find the true damaged area in a living body. Second, prior submucosal injection of normal saline may be useful to prevent deep tissue destruction in a living body and the resected stomach.
The reason why the discrepancy of tissue damage exists between the area with and without follow-up may be the limitations of histology in detectability of thermal damage. One of the characteristics of APC is reported that it uniformly creates deep zones of shrinking, desiccation, coagulation and devitalization, in turn, from the applied surface.14 However, histological investigation may not detect the zone of devitalization, which means that tissue damage is underestimated than the true one. Follow-up study is necessary to find the true damaged area. In this study, 20 seconds' application of APC resulted in damage limited to the submucosal layer when examined soon after application, but created a deep ulcer with destruction of the proper muscle layer when examined after one week. This result suggests that we have to be aware of late perforation, which may occur a few days to a few weeks after APC application.
Previous study using a resected stomach revealed the usefulness of prior submucosal injection.9 The present study using a living minipig also supports it. When APC was applied after submucosal injection, tissue damages were limited to up to submucosal layer regardless of time duration. Furthermore, the follow-up study for one week also revealed the same results. In practice, we sometimes experience an unexpected extension of tissue destruction, which may be affected by inevitable various factors; e.g. host factors (mucosal thickness, blood flow, inflammation, etc), technical factors (the extension of the gastric wall by inflated air, the applied angle, the distance between an applicator and tissue, etc).3,6,12,13 Therefore submucosal injection of normal saline may be essential to get the sufficient results at any encountered situation, without the fear of extensive damage up to the proper muscle layer. Since tissue damage up to submucosal layer is sufficient for treating most of lesions with APC, submucosal injection can become the standard preparation prior to APC application in humans. One recent case series of colonic angiodysplasia showed the safety and efficacy.15 Further prospective study, including a large number of patients with and without prior submucosal injection will be warranted to show the clinical impact.
ACKNOWLEDGEMENT
Part of this study was presented at the Digestive Disease Week, May 18 - 21, 2003, Orlando, Florida.
Footnotes
Koji Kashimura is a member of the Product Research Department, Kamakura Research Laboratories, Chugai Pharmaceutical Co., LTD., Kamakura, Kanagawa, Japan; Toyokazu Matsuura is a member of the Chugai Research Institute for Medical Science, INC., Gotenba, Shizuoka, Japan. No funding is related with this work
References
- 1.Grund KE, Straub T, Farin G. Clinical application of argon plasma coagulation in flexible endoscopy. Endosc Digest. 1998;10:1543–1554. [Google Scholar]
- 2.Grund KE, Storek D, Farin G. Endoscopic argon plasma coagulation (APC) first clinical experiences in flexible endoscopy. Endosc Surg Allied Technol. 1994;2:42–46. [PubMed] [Google Scholar]
- 3.Manner H, May A, Rabenstein T, et al. Prospective evaluation of a new high-power argon plasma coagulation system (hp-APC) in therapeutic gastrointestinal endoscopy. Scand J Gastroenterol. 2007;42:397–405. doi: 10.1080/00365520600898130. [DOI] [PubMed] [Google Scholar]
- 4.Sagawa T, Takayama T, Oku T, et al. Argon plasma coagulation for successful treatment of early gastric cancer with intramucosal invasion. Gut. 2003;52:334–339. doi: 10.1136/gut.52.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eickhoff A, Jakobs R, Schilling D, et al. Prospective non-randomized comparison of two modes of argon beamer (APC) tumor desobstruction: effectiveness of the new pulsed APC versus forced APC. Endoscopy. 2007;39:637–642. doi: 10.1055/s-2007-966571. [DOI] [PubMed] [Google Scholar]
- 6.Kitamura T, Tanabe S, Koizumi W, Mitomi H, Saigenji K. Argon plasma coagulation for early gastric cancer: technique and outcome. Gastrointest Endosc. 2006;63:48–54. doi: 10.1016/j.gie.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Manner H, May A, Faerber M, Rabenstein T, Ell C. Safety and efficacy of a new high power argon plasma coagulation system (hp-APC) in lesions of the upper gastrointestinal tract. Dig Liver Dis. 2006;38:471–478. doi: 10.1016/j.dld.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Sumiyama K, Kaise M, Kato M, et al. New generation argon plasma coagulation in flexible endoscopy: ex vivo study and clinical experience. J Gastroenterol Hepatol. 2006;21:1122–1128. doi: 10.1111/j.1440-1746.2005.04133.x. [DOI] [PubMed] [Google Scholar]
- 9.Fujishiro M, Yahagi N, Nakamura M, et al. Submucosal injection of normal saline may prevent tissue damage from argon plasma coagulation: an experimental study using resected porcine esophagus, stomach, and colon. Surg Laparosc Endosc Percutan Tech. 2006;16:307–311. doi: 10.1097/01.sle.0000213739.85277.3d. [DOI] [PubMed] [Google Scholar]
- 10.Fujishiro M, Yahagi N, Nakamura M, et al. Safety of argon plasma coagulation for hemostasis during endoscopic mucosal resection. Surg Laparosc Endosc Percutan Tech. 2006;16:137–140. doi: 10.1097/00129689-200606000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Goulet CJ, Disario JA, Emerson L, Hilden K, Holubkov R, Fang JC. In vivo evaluation of argon plasma coagulation in a porcine model. Gastrointest Endosc. 2007;65:457–462. doi: 10.1016/j.gie.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Farooq FT, Wong RC, Yang P, Post AB. Gastric outlet obstruction as a complication of argon plasma coagulation for watermelon stomach. Gastrointest Endosc. 2007;65:1090–1092. doi: 10.1016/j.gie.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Prost B, Poncet G, Scoazec JY, Saurin JC. Unusual complications of argon plasma coagulation. Gastrointest Endosc. 2004;59:929–932. doi: 10.1016/s0016-5107(04)01268-4. [DOI] [PubMed] [Google Scholar]
- 14.Farin G, Grund KE. Argon plasma coagulation in flexible endoscopy: the physical principle. Endosc Digest. 1998;10:1521–1527. [Google Scholar]
- 15.Suzuki N, Arebi N, Saunders BP. A novel method of treating colonic angiodysplasia. Gastrointest Endosc. 2006;64:424–427. doi: 10.1016/j.gie.2006.04.032. [DOI] [PubMed] [Google Scholar]