Abstract
Background/Aims
RUNX3 (PEBP2αC/CBFA3/AML2) is a novel tumor suppressor gene in the human gastric carcinoma. The aims of this study were to determine the methylation of RUNX3 promoter and the association between RUNX3 methylation and the clinicopathological characteristics of patients with gastric carcinoma.
Methods
Seventy-nine patients with gastric carcinoma were studied prospectively from April 2005 to May 2007. The methylations of RUNX3 promoter on the gastric carcinoma specimens and the corresponding nonneoplastic mucosa were evaluated by methylation-specific polymerase chain reaction.
Results
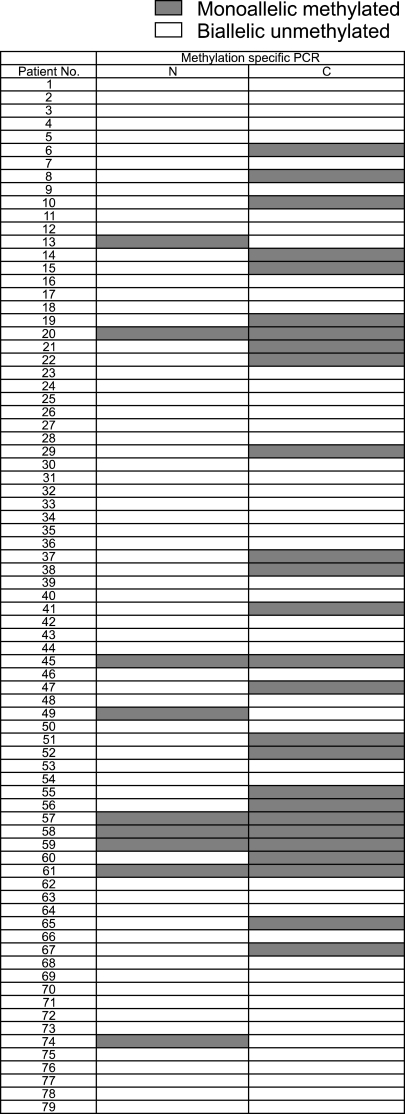
Comparison of the results with the clinicopathological characteristics identified RUNX3 monoallelic methylation in 32.9% (26/79) of the gastric carcinoma patients and in 11.4% (9/79) of those with nonneoplastic mucosa (p=0.053). The monoallelic methylated gastric carcinoma specimens predominantly consisted of well- and moderately differentiated carcinomas (44.7%), with the unmethylated group constituting 22.0% of them (p=0.031). Among the 48 patients (60.8%) who underwent gastrectomy, there was no correlation between the two groups with regard to Lauren's classification (p=0.235), depth of invasion (p=0.990), nodal status (p=0.601), stage (p=0.900), lymphatic invasion (p=0.537), and vascular invasion (p=0.815).
Conclusions
Methylation of the tumor suppressor gene RUNX3 might be one of the mechanisms involved in the pathogenesis of gastric carcinoma.
Keywords: Runx3 protein, Gastric cancers, Methylation
INTRODUCTION
Gastric carcinoma, the second most common cause of cancer-related deaths in the world,1,2 is the most common malignancy in Korea.3 Therefore, the roles of oncogenes and tumor suppressor genes in the pathogenesis of the gastric carcinoma have recently been receiving considerable attention,4,5 through studies of colorectal cancer, allelic deletions of the MCC (mutated in colon cancer), APC (adenomatous polyposis coli), and p53 tumor suppressor genes.6
A recent study revealed that RUNX3 (PEBP2αC/CBFA3/AML2), belonging to the Runt-domain family of the transcription factors, plays an important role in the genesis and the progression of human gastric cancer, and also functions as a tumor suppressor gene.7 The expression of RUNX3 and RUNX1, and their cofactor CBFB, are frequently downregulated at a significant proportion in the gastric cancer patients.8 Of the three known RUNX3 family members, RUNX3 has been shown to be involved in the neurogenesis of the dorsal root ganglia, T-cell differentiation, and tumorigenesis of the gastric epithelium.9 The gastric epithelium of RUNX3 null mice has been reported to show hyperplasia and epithelial proliferation with the suppression of the TGF-β-induced apoptosis.7 The mechanisms underlying the loss of RUNX3 expression are gene mutation or deletion, and aberrant methylation of the promoter CpG islands.10 Methylation of RUNX3 is frequently found in major types of the human cancers.11 These types include gastric cancer (64%), hepatocellular carcinoma (73%), lung cancer (46%), breast cancer (25%), and colon cancer (4.9%).12 However, RUNX3 methylation has also been found in the nonneoplastic tissues including the premalignant lesions of gastric carcinoma such as chronic gastritis (8.1%), intestinal metaplasia (28.1%), and gastric adenoma (27.3%).12 In addition, methylation of the multiple tumor suppressor genes including RUNX3 were found to increase with age in the nonneoplastic gastric epithelia.13
Although the absence of RUNX3 expression has been reported in several studies on the human gastric carcinoma,7,11,12,14,15 a recent study reported that the immunohistochemical staining detected the RUNX3 protein expression in the infiltrating leukocytes, but not in the gastric epithelium.16 Therefore, the low levels of RUNX3 expression in the gastric epithelium and the absence of downregulation of RUNX3 in the gastric cancer do not support the idea that RUNX3 functions as a gastric tumor suppressor gene.16 Overall, the role of RUNX3 as a possible gastric tumor suppressor gene remains unclear and controversial. In addition, the association of RUNX3 with the clinicopathological findings and prognosis of the patients with gastric cancer has not yet been determined. In the present study, the RUNX3 promoter methylation was investigated by methylation-specific polymerase chain reaction (PCR). In addition, the association between RUNX3 methylation and clinicopathological characteristics, and the prognostic value of RUNX3 in patients with gastric carcinoma were evaluated.
MATERIALS AND METHODS
1. Patients
Of 654 patients (mean age 65±13 years, 406 male and 248 female) who have been diagnosed or treated as gastric carcinoma in Ewha Womans University Mokdong Hospital from April 2005 to May 2007, biopsy specimens from 79 patients with gastric carcinoma were enrolled (50 males and 29 females) prospectively. All patients studied were histologically confirmed to have gastric carcinoma based on the diagnostic gastroscopic examination. Among the 79 patients, 48 underwent gastrectomy with curative or palliative intent. Histological classification was determined according to Lauren's classification system (intestinal type and diffuse type).17 Four biopsy specimens with the representative samples of the carcinoma and macroscopically nonneoplastic mucosa obtained from each patient were frozen and stored at -70℃ until used for the extraction of the genomic DNA. Clinical data of all patients with gastric carcinoma were analyzed. The median duration of the follow-up for the study population was, on the average, 10 months (1-25 months). At the last follow-up examination, 45 patients were still alive, 9 patients were lost to follow-up, and 25 patients were dead. The present study was approved by the Human Research Review Committee, and informed consents were obtained from all patients.
2. Bisulfite modification and methylation-specific PCR
Methylation-specific PCR (MSP) was performed on the bisulfite-modified DNA templates obtained from the human gastric carcinoma tissues to study the methylation status of the neoplastic and the nonneoplastic gastric mucosa. To examine the promoter methylation patterns, DNAs were extracted using TRI-reagent (Molecular Research Center, Inc, Cincinnati, USA), and the genomic DNAs were treated with sodium bisulfite as described previously.18 In brief, 2 µg of the genomic DNAs were denatured by treatment with 2 M NaOH and modified with 3 M sodium bisulfite for 16 hours. DNA samples were purified with a DNA purification kit (Intron®, Sung-nam, Kyungki-do, Korea), treated with 3 M NaOH, precipitated with ethanol, and resuspended in 20 uL water. Two-microliter aliquots were used as templates for the PCR. Pure DNA samples were subjected to PCR using the specific primer sequences for the methylated and unmethylated forms of RUNX3 (Accession no AL023096). The primer sets used for detecting the methylated and the unmethylated DNAs respectively were Rx3-M(F)(5'-TTACGAGGGGCGGTCGTACGCGGG-3') and Rx3-M(R) (5'-AAAACGACCGACGCGAACGCCTCC-3') (64970-65189; 220 bp), and Rx3-U(F)(5'-TTATGAGGGGTGGTTGTATGTGGG-3') and Rx3-U(R)(5'-AAAACAACCAACACAAACACCTCC-3') (64970-65189; 220 bp).7 The positive controls for the methylated and the unmethylated positive cell lines were HT-29 and MKN-45, respectively. Distilled water was used as the negative control.
3. PCR amplification
The amplification reaction was carried out in a 10-µL PCR mixture containing 2 µL of the DNA template and 0.1 µL of TaKaRa hotstart Taq polymerase, 0.2 mM of deoxynucleotide triphosphates, 0.5 µM of each primer (sense and antisense), 1 µL of the primer 10× PCR buffer, and 5.6 µL of distilled water. The PCR mixture was amplified using GeneAmp PCR system 9600 (Perkin-Elmer, Wellesley, MA, USA). The PCR conditions were as follows: 95℃ for 1 minute, 40 cycles of denaturation at 95℃ for 30 seconds, annealing at 66℃ (methylated) or 63℃ (unmethylated) for 30 seconds, and finally 30 seconds extension at 72℃. A final 5-minute extension at 72℃ completed each PCR. The PCR products were loaded onto a 2% agarose gel containing ethidium bromide and visualized under ultraviolet (UV) transillumination. The detection of distinct visible bands of the amplicon with methylation-specific primers was considered a positive result.
4. Statistical analysis
Statistical analysis was performed using Student's t-test, χ2-test or two-tailed Fisher's exact test. Continuous variables were determined as the mean±standard deviation. As for the analysis of the prognosis of the patients, survival was calculated from the endoscopic diagnosis until death or the date of the last follow-up. Survival was analyzed by the Kaplan-Meier method, and differences in the distribution were evaluated using the log-rank test. Data analyses were performed using the SPSS for Windows (V. 13.0, SPSS, Chicago, IL, USA), and p<0.05 was considered as significant.
RESULTS
1. Demographic and clinicopathological characteristics of patients with gastric carcinoma
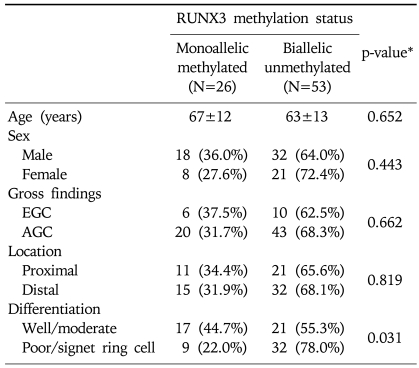
The patients included 50 males and 29 females with a mean age of 64±13 years. The gross findings were 16 cases (20.3%) with early gastric carcinoma (EGC) and 63 (79.7%) with advanced gastric carcinoma (AGC). The locations of tumors were classified as follows: antrum, angle, body, cardia, and fundus. All pathological diagnoses of the gastric carcinoma were adenocarcinoma except for one case (1.3%) of the adenosquamous cell carcinoma. Histological differentiations were divided into well, moderate, and poorly differentiated/signet ring cell types (Table 1).
Table 1.
Clinicopathological Characteristics of 79 Patients with Gastric Carcinoma
*EGC, early gastric cancer.
†AGC, advanced gastric cancer.
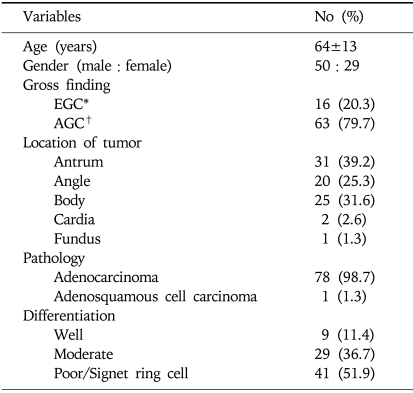
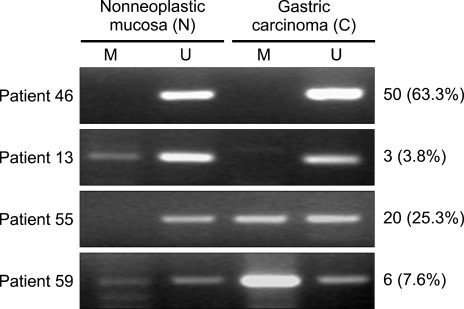
The expression of RUNX3 methylation of the gastric carcinoma specimens and the corresponding nonneoplastic mucosa were detected by MSP. The corresponding nonneoplastic mucosa included 61 cases (77.2%) of atrophic gastritis, 10 cases (12.6%) of metaplastic gastritis, 7 cases (8.9%) of chronic superficial gastritis, and 1 case (1.3%) of erosive gastritis. Representative images of the MSP analysis demonstrated the presence of the methylated and unmethylated PCR products (Fig. 1). The methylated group showed only a monoallelic methylation pattern (one band for methylated product) (Fig. 2). Among the 79 patients, RUNX3-specific methylation was identified in 32.9% (26/79) of the gastric carcinoma specimens and in 11.4% (9/79) of the nonneoplastic mucosa (p=0.053) (Fig. 3).
Fig. 1.
Representative images from methylation-specific polymerase chain reaction (PCR) analysis of the RUNX3 promoter region. M, methylated PCR product; U, unmethylated PCR products. This figure shows 50 cases (63.3%) of biallelic unmethylated nonneoplastic mucosa (N) and gastric carcinoma (C) specimens (patient 46), 3 cases (3.8%) of monoallelic methylation of N and biallelic unmethylation of C (patient 13), 20 cases (25.3%) of biallelic unmethylation of N and monoallelic methylation of C (patient 55), and 6 cases (7.6%) of monoallelic methylation of N and monoallelic methylation of C (patient 59).
Fig. 2.
Schematic of the RUNX3 promoter methylation status in the 79 patients. Gray boxes, monoallelic methylation (one band each for the methylated and unmethylated products) as detected by methylation-specific PCR; white boxes, biallelic unmethylated samples (one band for the unmethylated product).
Fig. 3.
Comparison of RUNX3 methylation between N and C specimens. RUNX3 hypermethylation was detected in 26 of 79 (32.9%) C specimens showing monoallelic methylation. Nine of 79 (11.4%) the N cases were monoallelic methylated.
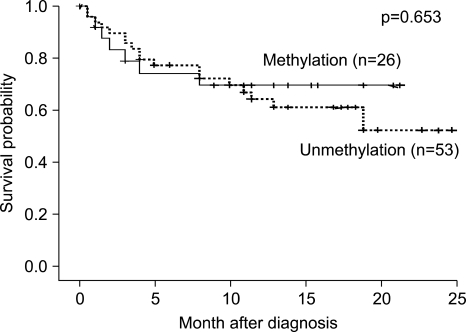
Comparison of the RUNX3 methylation status of the gastric carcinoma specimens with the clinicopathological features of the 79 patients revealed no correlation with age, gender, the gross findings of early gastric cancer versus advanced gastric cancer, and the location of the tumor between the RUNX3 monoallelic methylated and the biallelic unmethylated groups (Table 2). However, the monoallelic methylated gastric carcinoma specimens predominantly consisted of well and moderately differentiated carcinoma (44.7%) compared to the poorly differentiated or signet ring cell type (22.0%) (p=0.031); the survival of the methylated group was not significantly different from that of the unmethylated group during the 25 months of follow-up (p=0.653) (Fig. 4).
Table 2.
Association between the RUNX3 Methylation Status of Gastric Carcinoma Specimens and the Clinicopathological Features of 79 Patients
Proximal: body, cardia, fundus, Distal: antrum, angle.
*p-values were based on the χ2 test.
Fig. 4.
Kaplan-Meier plots of the overall survival of the C patients, whose methylation status did not differ significantly between the two groups during the 25-month follow-up (p=0.653).
2. Clinicopathological characteristics of patients with gastric carcinoma who underwent gastrectomy
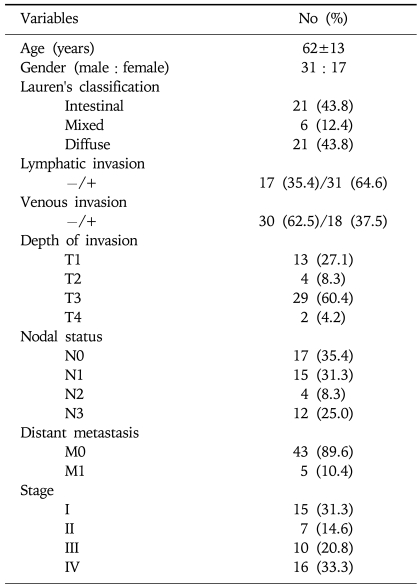
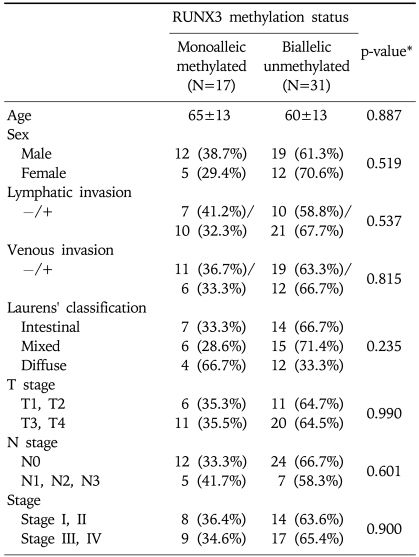
Among the patients studied, only 48 (60.8%) underwent gastrectomy. Thirty-one patients were excluded from the surgery due to 15 cases (48.4%) of inoperable advanced stage IV disease, 1 case (3.2%) of surgery declined, and 15 cases (48.4%) with inadequate information after transfer from another hospital. According to Lauren's classification, the histological types included the intestinal type, the mixed type, and the diffuse type. Among the 48 cancer cases, 31 (64.6%) showed lymphatic invasion. The blood vessels of 18 cases (37.5%) were affected. Depth of invasion (T1-4), nodal status (N0-3), distant metastasis (M0-1), TNM stage (I-IV) were showed, respectively (Table 3). There was no correlation between the two groups in age, gender, lymphatic invasion, vascular invasion, Lauren's classification, T grade, N grade, and tumor stage (Table 4).
Table 3.
Clinicopathological Characteristics of 48 Patients with Gastric Adenocarcinoma who Underwent Gastrectomy
Table 4.
Association between the RUNX3 Methylation Status and the Clinicopathological Features of 48 Gastric Carcinoma Patients who Underwent Gastrectomy
*p-value were based on the χ2 test.
DISCUSSION
CpG island hypermethylation is an important mechanism known to inactivate the tumor suppressor genes in gastric neoplasia,19 and is found in virtually all cancer tissue types.11 However, the role of RUNX3 as a possible gastric tumor suppressor is yet controversial.7,12,14-16 Moreover, the results of present study are not consistent with those of the previous reports in that the positive MSP expression of the RUNX3 gene was found only in 32.9% of the patients, which is relatively lower than those of the previous reports (45-69%),7,8 including the Korean-specific data (56-64%).11,12 RUNX3-specific methylation was more frequently identified in the gastric carcinoma specimens (32.9%) than in the nonneoplastic mucosa (11.4%), although marginally significant (p=0.053). A possible explanation for the absence of statistical significance between the two groups (methylated vs. unmethylated) is the nature of the tissue samples; nonneoplastic mucosa samples were obtained from the same gastric cancer patients and were not always under strict histological control. It is likely that if the comparison of the results were done with the histological normal mucosa, a statistically significant difference could have been observed. Even though MSP is a very sensitive technique, it cannot be quantitatively measured,18 leading to subjective interpretation of the faint bands that sometimes appear on the MSP. This may be another explanation for the discrepancies in the results of different studies. A recent report showed that microarray-based methylation assay is a promising method for the quantitative assessment of gene methylation;20 most of the samples with microarray >10% tested methylation-positive by the conventional MSP.
In the present study, the correlation between the clinicopathological features of the gastric carcinoma patients and RUNX3 aberrant monoallelic methylation was higher in the well and moderately differentiated samples (p=0.031) than in the poorly differentiated or signet ring carcinomas samples. This finding is similar to the somatic mutations of the APC gene detected in more than 50% of the well-differentiated gastric cancers, but not in the poorly differentiated type.21 As with the APC gene in colon cancer, RUNX3 is probably a gene mutated early during the development of gastric carcinoma.22 However, we could not conclude that such correlation actually mean that the methylation of RUNX3 is one of the carcinogenic mechanisms. It has the possibility of by-stander effect. Recent studies have demonstrated that DNA hypermethylation begins early during the progression into gastric cancer, and tends to accumulate during the multistep progression of the gastric carcinogenesis.23 However, this result on the gastric carcinogenesis is not consistent with those of the previous studies on the colorectal cancers,24,25 which demonstrated a significant difference in the histology associated with the number of methylated genes in the poorly differentiated colorectal cancers compared to the other differentiated types, suggesting that the poorly differentiated colorectal cancers preferentially exhibit gene methylation.25 There have been few reports on the expression of RUNX3 expression. Through immunohistochemistry and Western blot analysis, RUNX3 protein expression was shown to be correlated with the tumor differentiation and the Lauren's classification, but no relationship with the TNM stage was demonstrated.26 Therefore, the correlation between the differentiation of gastric carcinoma and RUNX3 expression requires further evaluation. Although previous reports showed that RUNX3 expression is predominantly found in the intestinal type rather than the diffuse type,26-28 data of the present study indicated no definite difference between the intestinal and the diffuse types.
Unexpectedly, the present data showed that the RUNX3 gene in the gastric cancers does not have biallelic methylation, but rather monoallelic methylation. Monoallelic methylation of the APC promoter was reported to be altered in normal gastric mucosa associated with the neoplastic lesions.29 Similar observations were made on the tumor suppressor gene GPx3, with respect to the promoter hypermethylation in Barrett's esophagus.30 These findings suggest that monoallelic methylation is common and the methylation of one allele may indicate the start of an early stages of tumorigenesis. Further study by Western blotting, or immunohistochemical staining is needed to determine whether the monoallelic methylation is associated with the partial loss of RUNX3 expression at the mRNA level.
The prognosis data showed that during the short-term follow-up period (mean 10 months: 1-25 months), there were no survival differences between the RUNX3 methylated and unmethylated groups. However, long-term follow-up data have shown that the loss of RUNX3 expression correlates with a poor prognosis in the gastric cancer patients.26 Based on the Cox proportional hazard model, RUNX3 expression was reported to be an independent predictor of a better survival.26 In contrast, another recent report showed that the median survival time of patients, who had tumors with a negative RUNX3 protein expression, was longer than that of the RUNX3-positive patients.25 These conflicting results might be caused by the long-term prognosis of patients with gastric cancer is influenced by not only RUNX3 but also other factors such as patient's performance, tumor stage, ethnic differences, and the performance of gastrectomy. More patients undergoing gastrectomy need to be studied to elucidate the methylation status and the possibly associated clinicopathological characteristics.
In summary, we have demonstrated that methylation of the RUNX3 gene was detected more frequently in the gastric carcinoma than in the nonneoplastic mucosa, suggesting the involvement of methylation of the tumor suppressor gene RUNX3 in the pathogenesis of gastric carcinoma.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006;20:633–649. doi: 10.1016/j.bpg.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 3.2005 Morbidity rate of cancer. Korea National Statistical Office. http://www.nso.go.kr.
- 4.Fuchs CS, Mayer RJ. Gastric carcinoma. New Engl J Med. 1995;333:32–41. doi: 10.1056/NEJM199507063330107. [DOI] [PubMed] [Google Scholar]
- 5.Chan AO, Luk JM, Hui WM, Lam SK. Molecular biology of gastric carcinoma: from laboratory and bedside. J Gastroenterol Hepatol. 1999;14:1150–1160. doi: 10.1046/j.1440-1746.1999.02000.x. [DOI] [PubMed] [Google Scholar]
- 6.Rhyu MG, Park WS, Jung YJ, Choi SW, Meltzer SJ. Allelic deletions of MCC/APC and p53 are frequent late events in human gastric carcinogenesis. Gastroenterology. 1994;106:1584–1588. doi: 10.1016/0016-5085(94)90414-6. [DOI] [PubMed] [Google Scholar]
- 7.Li QL, Ito K, Sakakura C, et al. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113–124. doi: 10.1016/s0092-8674(02)00690-6. [DOI] [PubMed] [Google Scholar]
- 8.Sakakura C, Hagiwara A, Miyagawa K, et al. Frequent downregulation of the runt domain transcription factors RUNX1, RUNX3 and their cofactor CBFB in gastric cancer. Int J Cancer. 2005;113:221–228. doi: 10.1002/ijc.20551. [DOI] [PubMed] [Google Scholar]
- 9.Bae SC, Choi JK. Tumor suppressor activity of RUNX3. Oncogene. 2004;24:4336–4340. doi: 10.1038/sj.onc.1207286. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalgo ML, Jones PA. Mutagenic and epigenetic effects of DNA methylation. Mutat Res. 1997;386:107–118. doi: 10.1016/s1383-5742(96)00047-6. [DOI] [PubMed] [Google Scholar]
- 11.Park SY, Kim BH, Kim JH, et al. Methylation profiles of CpG island loci in major types of human cancers. J Korean Med Sci. 2007;22:311–317. doi: 10.3346/jkms.2007.22.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim TY, Lee HJ, Hwang KS, et al. Methylation of RUNX3 in various types of human cancers and premalignant stages of gastric carcinoma. Lab Invest. 2004;84:479–484. doi: 10.1038/labinvest.3700060. [DOI] [PubMed] [Google Scholar]
- 13.So K, Tamura G, Honda T, et al. Multiple tumor suppressor genes are increasingly methylated with age in non-neoplastic gastric epithelia. Cancer Sci. 2006;97:1155–1158. doi: 10.1111/j.1349-7006.2006.00302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osaki M, Moriayma M, Adachi K, et al. Expression of RUNX3 protein in human gastric mucosa, intestinal metaplasia and carcinoma. Eur J Clin Invest. 2004;34:605–612. doi: 10.1111/j.1365-2362.2004.01401.x. [DOI] [PubMed] [Google Scholar]
- 15.Oshimo Y, Oue N, Mitani Y, et al. Frequent loss of RUNX3 expression by promoter hypermethylation in gastric carcinoma. Pathobiology. 2004;71:137–143. doi: 10.1159/000076468. [DOI] [PubMed] [Google Scholar]
- 16.Friedrich MJ, Rad R, Langer R, et al. Lack of RUNX3 regulation in human gastric cancer. J Pathol. 2006;210:141–146. doi: 10.1002/path.2042. [DOI] [PubMed] [Google Scholar]
- 17.Lauren P. Two histological main types of gastric carcinoma, diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 18.Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821–9829. doi: 10.1073/pnas.93.18.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rashid A, Issa JP. CpG island methylation in gastroenterologic neoplasia: a maturing field. Gastroenterology. 2004;127:1578–1588. doi: 10.1053/j.gastro.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 20.So K, Tamura G, Honda T, et al. Quantitative assessment of RUNX3 methylation in neoplastic and non-neoplastic gastric epithelia using a DNA microarray. Pathol Int. 2006;56:571–575. doi: 10.1111/j.1440-1827.2006.02010.x. [DOI] [PubMed] [Google Scholar]
- 21.Nakatsuru S, Yanagisawa A, Ichii S, et al. Somatic mutation of the APC gene in gastric cancer: frequent mutations in very well differentiated adenocarcinoma and signet-ring cell carcinoma. Hum Mol Genet. 1992;1:559–563. doi: 10.1093/hmg/1.8.559. [DOI] [PubMed] [Google Scholar]
- 22.Carvalho R, Milne AN, Polak M, Corver WE, Offerhaus GJ, Weterman MA. Exclusion of RUNX3 as a tumor-suppressor gene in early-onset gastric carcinomas. Oncogene. 2005;24:8252–8258. doi: 10.1038/sj.onc.1208963. [DOI] [PubMed] [Google Scholar]
- 23.Imamura Y, Hibi K, Koike M, et al. RUNX3 promoter region in specifically methylated in poorly-differentiated colorectal cancer. Anticancer Res. 2005;25:2627–2630. [PubMed] [Google Scholar]
- 24.Hibi K, Nakao A. Highly-methylated colorectal cancers show poorly-differentiated phenotype. Anticancer Res. 2005;26:4263–4266. [PubMed] [Google Scholar]
- 25.Wu BW, Zhang J, Fei XF, Zhu ZG, Cao WX. Reappraisal of the impacts of human Runt-related transcriptional factor gene 3 expression on differentiation and prognosis of gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2007;10:165–168. [PubMed] [Google Scholar]
- 26.Wei D, Gong W, Oh SC, et al. Loss of RUNX3 expression significantly affects the clinical outcome of gastric cancer patients and its restoration causes drastic suppression of tumor growth and metastasis. Cancer Res. 2005;65:4809–4816. doi: 10.1158/0008-5472.CAN-04-3741. [DOI] [PubMed] [Google Scholar]
- 27.Fukamachi H, Ito K, Ito Y. Runx3-/- gastric epithelial cells differentiate into intestinal type cells. Biochem Biophys Res Commun. 2004;321:58–64. doi: 10.1016/j.bbrc.2004.06.099. [DOI] [PubMed] [Google Scholar]
- 28.Fukamachi H. RUNX3 controls growth and differentiation of gastric epithelial cells in mammals. Dev Growth Differ. 2006;48:1–13. doi: 10.1111/j.1440-169X.2006.00832.x. [DOI] [PubMed] [Google Scholar]
- 29.Clement G, Bosman FT, Fontolliet C, Benhattar J. Monoallelic methylation of the APC promoter is altered in normal gastric mucosa associated with neoplastic lesions. Cancer Res. 2004;64:6867–6873. doi: 10.1158/0008-5472.CAN-03-2503. [DOI] [PubMed] [Google Scholar]
- 30.Lee OJ, Schneider-Stock R, McChesney PA, et al. Hypermethylation and loss of expression of glutathione peroxidase-3 in Barrett's tumorigenesis. Neoplasia. 2005;7:854–861. doi: 10.1593/neo.05328. [DOI] [PMC free article] [PubMed] [Google Scholar]