Abstract
Background/Aims
Halitosis is a symptom that bothers patients more socially than medically and its pathogenic mechanisms are unclear and treatment armamenterium is limited. Clinicians generally ignored active interventions. Since halitosis is closely associated with volatile sulfur compounds (VSCs), we used a Halimeter and gas chromatography to measure VSCs in patients with Helicobacter-pylori (H. pylori)-associated gastric diseases.
Methods
We categorized 72 patients with H. pylori infection into two groups based on their endoscopic findings: a non-erosive mucosal group (NE, n=24) and an erosive mucosal group (E, n=48). Halitosis was objectively assessed by applying either a Halimeter to breath air or gas chromatography to gastric juice. Simultaneously, the expression of VSC-generating enzyme was measured with reverse-transcriptase PCR using mRNA isolated from biopsy tissues.
Results
The levels of VSCs in exhaled breaths or aspirated gastric juices differed significantly between the NE and E groups (p<0.00001), suggesting that VSCs might reflect eroded epithelial damage induced by H. pylori infection. The expressions of cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) were broadly consistent with the degree of mucosal injury.
Conclusions
Erosive changes in esophagogastroduodenal mucosa were strongly correlated with increased VSC levels, suggesting that halitosis might result from H. pylori-associated erosive lesions.
Keywords: Hydrogen sulfide, Halitosis, Cystathionine beta-Synthase, Cystathionine gamma-Lyase
INTRODUCTION
Many patients come to the clinics due to the foul breath odor, halitosis, often accompanied with gastrointestinal troubles such as dyspepsia, indigestion or regurgitation, but little concerns or no clear treatment strategies were provided in clinic. Halitosis itself seems to be not so severe illness, but it could be very troublesome condition, especially in social relationships.1-3 Since studies about the etiology of halitosis were mostly concentrated on intra-oral problems such as dental caries, gingivitis, related with bacterial putrefactive activities, its treatment has been also limited to mouthwash, tongue cleansing, dental care, and some trials based antibiotics use. However, even after these interventions, treatments outcomes were not so satisfactory.4,5 This is why the patients with halitosis were not willing to open their complaints to medical doctors and instead decided to seek for the herb medicine or other alternative medicine rather than medical consultations. Therefore, we reached to the assumption that gastroenterologists should pay attention or invest thoughtful efforts toward the amelioration of halitosis because H. pylori infection and gastric lesion could be the principal etiology of halitosis.
Even though halitosis might originate from either intra-oral lesion or respiratory tract, gastrointestinal tract, or pharyngo-tonsillar problem and general systemic condition such as liver cirrhosis, chronic renal failure, and malignancy,1-4 a very plausible link exists between the bacterium Helicobacter pylori (H. pylori) and halitosis. In detail, some studies revealed that halitosis was quite prevalent in H. pylori positive group and others showed that halitosis was improved after the eradication therapy of H. pylori.6-10 H. pylori infection was responsible for volatile sulfur compounds (VSCs) production, known as causative constituents of halitosis.
VSCs, composed of hydrogen sulfide, methyl mercaptan, and dimethyl sulfide, have been known as the main factor of halitosis.11,12 It can be measured either by halimeter easily or gas chromatography from aspirated breath airs.13-15 Among these VSCs, endogenous hydrogen sulfide (H2S) is synthesized from most tissues in mammals,16,17 for which two enzymes are involved in this synthesis, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE).18-21
To prove the hypothesis that esophago-gastric mucosa might be the principal source for VSCs and diseased mucosa might be associated with halitosis, we measured the VSCs in either gastric juices or breath air and correlated the association between erosive mucosa and the levels of VSC.
MATERIALS AND METHODS
1. Patients
Among the patients who underwent esophagogastroduodenoscopy due to dyspepsia, 72 patients who showed positive Urea breath test (UBT) and halitosis symptom were enrolled for current study after receiving written consent. The current study was approved by Institutional Review Boards of Daejin Medical Center. They all checked VSCs concentrations by halimeter before endoscopy and 5 ml of the gastric juices were aspirated from fundus during endoscopy procedure after receiving another informed consent about collecting gastric juices during procedure. The patients with nonsteroidal anti-inflammatory drugs (NSAIDs) medications, proton pump inhibitor or H2 receptor antagonists, and other medications including antibiotics were excluded and the patients with systemic illness including diabetes mellitus, liver diseases, renal disease, and malignancy were also excluded. The total patients (n=72) were grouped into two based on their endoscopic findings; non-erosive mucosal lesions (NE group, n=24) and erosive mucosal lesions (E group, n=48). In detail, mild or moderate degree of superficial gastritis was nominated as NE group and gastric erosion, erosive gastro-esophageal reflux disease (eGERD), gastric ulcer, and duodenal ulcer were designated as E group. All the patients of GERD patients were the cases classified as LA classification A. Twenty four patients were grouped into the NE group and 48 patients into the E group. The mean age of the whole patients was 49.2 years (range 19-77 years), 29 males and 43 females and there was no significant difference in demographic data between groups. Although halimeter was measured in all enrolled patients, gas chromatographic evaluation of gastric juices was done in 31 patients and reverse transcriptase PCR and real time PCR for CBS/CSE expression were done in 31 patients due to acceptance of sampling.
2. Halimeter for halitosis measurement from breath
The portable sulfide monitor (Halimeter®, Model RH-17K: Interscan Co, Chatsworth, CA) has high sensitivity for VSCs. The monitor was adjusted to 0 on ambient air before measure it. Patients were asked to breathe through the nose, with the mouth closed, for 1 min. A straw attached to the halimeter was then inserted into the mouth and air withdrawn from the mouth for analysis. The results of VSC levels in breath air were recorded as parts per billion (ppb) of sulfide equivalents. Concentrations were determined in triplicate and a mean value was calculated. Measurement of VSCs was usually performed in the morning in fasting condition to avoid the influence from ingested foods.
3. Gas chromatography for gastric juice VSC concentrations
For separation and calibration of VSCs, a gas chromatography (Agilent 6890N, Agilent Technologies, Willington, DE) with a flame photometric detector specific for sulfur compounds was used. Since we obtained gastric juices for analysis, not breath air of the patients, we boiled the gastric juice at 60℃ for evaporating gas, among which 500 µ was entrapped into a syringe for gas chromatograpgy/flame photometric detection analysis. Five milliliters gastric juices were aspirated during the endoscope procedure using aspiration tube inserted onto biopsy channel (Olympus, Tokyo, Japan) and were stored at -70℃ deep freezer until the assay. After several times of trials at different temperature for the evaporating of gastric juices, we could success in finding that 60℃ were quite ideal for evaporating gastric juices for gas chromatography and headspace air injection, peak areas and retention times were recorded by CheStation software (3365 Chem Station revision A09, Agilent Technologies).
4. Real time and RT-PCR for cystathionine β-synthase and cystathionine γ-lyase
Two enzymes are involved in VSCs generation, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE). Real time PCR and reverse transcriptase PCR were performed using the following protocols; Total RNA was isolated from tissues of H. pylori positive patients using TRIzol reagent (Life Technologies, Milan, Italy), and 1.5 µg of each total RNA was transcribed to cDNA using M-MLV Reverse Transcriptase (Promega, Madison, WI) system for RT-PCR using Oligo-dt primer. Primers such as CSE (5'-AAG AAC CTA AAG CTA TTT ACT CTG-3' and 5'-TTG CTC CAT TTA ATT ACT CAG GAA-3'), CBS (5'-TGG ATG CAG GAT CAT TGG GGT G-3' and 5'-TCC CGG AGG ATC TCG ATG GTG-3'), and GAPDH (5'-AGG TCG GAG TCA ACG GAT TTG G-3' and 5'-ACA GTC TTC TGG GTG GCA GTG ATG-3') were used for PCR. The PCR reactions were carried out for 25-30 cycle of 94℃, 53.5℃ (CSE) or 60℃ (CBS, GAPDH) for 30 sec and 72℃ for 1min. For real time PCR assay, AccuPower Green Star qPCR premix (SYBR green RT PCR mixture, Bioneer, Cheonan, Korea) and cDNA or primers the same as reverse transcriptase PCR were used for real time PCR. The real time PCR reactions were performed for 45 cycles at 60℃ (CBS, GAPDH) for 30 sec and 72℃ for 1 min.
5. Statistics
VSCs concentrations from mouth breath and gastric juice were analyzed using statistical software (MINITAB, Inc, State College, Pennsylvania, PA). These data were compared with t-test, all p values of <0.05 were considered statistically significant.
RESULTS
1. Halimeter levels according to presence of any erosive changes in upper GI tract
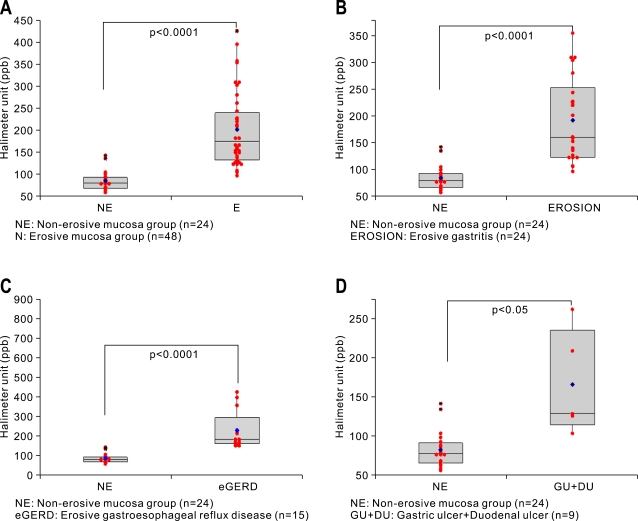
We divided the patients as NE and E group based on the presence of mucosal erosions or not after endoscopy examination. There was a statistically significant difference in VSCs levels measured with halimeter between NE group and E group (p<0.0001, Fig. 1A). Among 72 patients, 48 patients were diagnosed as either mild degree of superficial gastritis or erosive gastritis. The halimeter levels between chronic superficial gastritis (n=24) and erosive gastritis (n=24) were significantly different (p<0.0001, Fig. 1B). Statistically significant difference in halimeter levels were also observed between NE group (n=24) and erosive gastroesophageal reflux disease (n=15) (p<0.0001, Fig. 1C). However, the difference between NE group (n=24) and gastro-duodenal ulcer (n=9) was somewhat low, but showed statistical significance (p<0.05, Fig. 1D).
Fig. 1.
VSC concentrations in NE and E groups measured with a Halimeter (in ppb). (A) Comparison between NE and E. (B) Comparison between NE and E. (C) Comparison between NE and erosive gastroesophageal reflux disease. (D) Comparison between NE and gastroduodenal ulcer (Box and bar denote mean and SD values, respectively, and blue dot denotes the median value in each group).
2. VSC levels in gastric juice according to presence of any erosive changes in upper GI tract
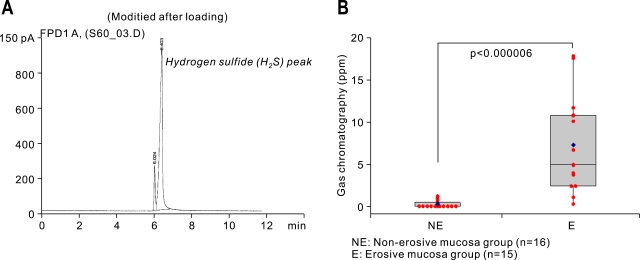
Since the amounts of VSC existing in gastric juice can be measured with gas chromatography, we designed the measuring method from aspirated gastric juices. Simply, the gastric juices were boiled with the instillation of 60℃, after which GC mass can detect the presence of VSC peak (Fig. 2A). The results of VSCs levels of gastric juice measured by gas chromatography were quite similar with those measured with halimeter (p<0.0001, Fig. 2B).
Fig. 2.
VSC concentrations in NE and E groups measured with gas chromatography (in ppm). (A) Gas chromatography with a flame photometric detector (Agilent 6890N, Willington, DE) used for measuring VSCs in aspirated gastric juices. The figure is a representative chromatograph showing the hydrogen sulfide (H2S) peak. (B) Comparison between changes in the NE and E groups (Box and bar denote mean and SD values, and blue dot denotes the median value in each group).
3. Levels of CB CBS and CSE mRNA measured by RT-PCR and real time PCR
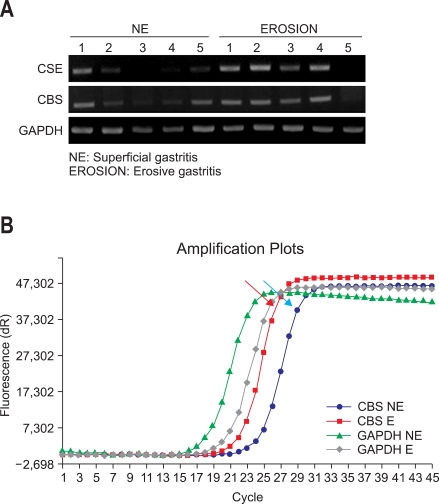
Since all the results from either halimeter or gas chromatography suggested that VSCs reflected the erosive changes in upper gastrointestinal tract, in order to put the clinical relevance of VSCs measurement, we isolated RNA from mucosal biopsy of 31 patients with informed consent and performed both reverse transcriptase PCR and real time PCR to quantify the gene, responsible for VSC synthesis, CBS and CSE. Average expressions of CSE and CBS were higher in E group than NE group, validating our previous findings that the higher levels of VSC were associated with erosive gastritis (Fig. 3A). Quantitative change was noted by real time PCR experiment, which is the result from pooled samples in each group (Fig. 3B) all confirming the results from RT-PCR.
Fig. 3.
Real-time PCR cystathionine β-synthase (CSE) and cystathionine β-lyase (CBS) enzyme activities. (A) Reverse-transcriptase PCR results. (B) Representative image from real-time PCR (Red arrow: real-time PCR of CBS expression in the E group; blue arrow: real-time PCR of CBS expression in the NE group).
DISCUSSION
For the first time, we discovered that VSCs were closely associated with erosive changes of upper gastrointestinal tract and halitosis might be the results of severely inflamed or eroded mucosa in accordance with increased VSCs. Combined with our another unpublished finding that H. pylori infection was responsible for the induction of CBS or CSE and CBS or CSE mRNA was quite in parallel with the degree of inflammatory mediators like IL-1, IL-8, and IL-6 (unpublished data), halitosis might be the one of manifestations suggestive of eroded and inflamed mucosa after H. pylori infection, and halimeter could be the one of biomarker tools suggesting erosive mucosal damages after H. pylori infection.
Though CSE and CBS enzyme exists in any tissue from central nervous system to cardiovascular smooth muscle, gastro-esophageal mucosa can also produce these enzymes and synthesize hydrogen sulfide.16,22
It is not so surprising that elevated hydrogen sulfide (H2S) levels were in patients with GERD since injured esophagus could produce much more H2S as ever. Therefore, as the extra-esophageal symptoms of GERD, hoarseness, chronic cough prevails, but we would like to add the halitosis could be another influencing symptom.23 Adler et al.24 investigated 46 patients with lingual dorsum hyperplasia and halitosis and found 40 out of 46(87%) to be H. pylori positive in the dorsum of the tongue and 93% of them with H. pylori in the stomach also. It is uncertain that H. pylori infection on the tongue caused GERD, but inflammation of oropharynx can be another source for bacterial putrefaction and enhanced synthesis of VSCs. We could also add the novel finding that enzyme expression responsible for VSCs generation was elevated in the erosive mucosal change group compared to non-erosive mucosal change group.
Since we included the sample associated with H. pylori infection, elevated H2S level might be solely due to the putrefactive action of H. pylori. However, there might be another possibility that H. pylori infection lead to higher chance of erosive changes. In accordance with the enzyme induction prerequisite for VSCs generation, higher levels of VSCs were produced in patients with erosive mucosal changes and ulcerative changes rather than inflamed mucosa, after which halitosis develops more frequently in patients with erosive mucosal changes than non-erosive mucosal changes. Therefore, our study could add the explanation of mechanism clearly that how H. pylori infection causes enhanced production of VSCs and halitosis might be the direct result of bacterial putrefaction or secondary event by enzyme induction for VSCs generation.
Conclusively, halitosis could an effective biomarker predicting that the gastric mucosa of affected patients might show erosive change beyond inflamed condition. In more extended point of view, some patients with troublesome halitosis could be benefited through either the eradication of H. pylori or healing the erosive mucosa. However, further extended scale of study will be required to draw more concrete conclusion.
ACKNOWLEDGEMENT
This study was supported with the grants from the Korea Ginseng Corporation.
References
- 1.Porter SR, Scully C. Oral malodour (halitosis) BMJ. 2006;333:632–635. doi: 10.1136/bmj.38954.631968.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tangerman A. Halitosis in medicine: a review. Int Dent J. 2002;52:201–206. doi: 10.1002/j.1875-595x.2002.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 3.Feller L, Blignaut E. Halitosis: a review. SADJ. 2005;60:17–19. [PubMed] [Google Scholar]
- 4.Donaldson AC, Riggio MP, Rolph HJ, Bagg J, Hodge PJ. Clinical examination of subjects with halitosis. Oral Dis. 2007;13:63–70. doi: 10.1111/j.1601-0825.2006.01248.x. [DOI] [PubMed] [Google Scholar]
- 5.Krespi YP, Shrime MG, Kacker A. The relationship between oral malodor and volatile sulfur compound-producing bacteria. Otolaryngol Head Neck Surg. 2006;135:671–676. doi: 10.1016/j.otohns.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 6.Tiommy E, Arber N, Moshkowitz M, Peled Y, Gilat T. Halitosis and Helicobacter pylori. A possible link? J Clin Gastroenterol. 1992;15:236–237. doi: 10.1097/00004836-199210000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Serin E, Gumurdulu Y, Kayaselcuk F, Ozer B, Yilmaz U, Boyacioglu S. Halitosis in patients with Helicobacter pylori-positive non-ulcer dyspepsia: an indication for eradication therapy? Eur J Intern Med. 2003;14:45–48. doi: 10.1016/s0953-6205(02)00206-6. [DOI] [PubMed] [Google Scholar]
- 8.Ierardi E, Amoruso A, La Notte T, et al. Halitosis and Helicobacter pylori: a possible relationship. Dig Dis Sci. 1998;43:2733–2737. doi: 10.1023/a:1026619831442. [DOI] [PubMed] [Google Scholar]
- 9.Hoshi K, Yamano Y, Mtsunaga A, Shimizu S, Kagawa J, Oguichi H. Gastrointestinal disease and halitosis: association of gastric Helicobacter pylori infection. Int Dent J. 2002;52:207–211. doi: 10.1002/j.1875-595x.2002.tb00926.x. [DOI] [PubMed] [Google Scholar]
- 10.Katsinelos P, Tziomalos K, Chatzimavroudis G, et al. Eradication therapy in Helicobacter pylori-positive patients with halitosis; long term outcome. Med Princ Pract. 2007;16:119–123. doi: 10.1159/000098364. [DOI] [PubMed] [Google Scholar]
- 11.Tonzetich J. Direct gas chromatographic analysis of sulphur compounds in mouth air in man. Arch Oral Biol. 1971;16:587–597. doi: 10.1016/0003-9969(71)90062-8. [DOI] [PubMed] [Google Scholar]
- 12.Richter VJ, Tonzetich J. The application of instrumental technique for the evaluation of odoriferous volatiles from saliva and breath. Arch Oral Biol. 1964;9:47–53. doi: 10.1016/0003-9969(64)90043-3. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg M, McCulloch CAG. Measurement of oral malodor: current methods and future prospects. J Periodontol. 1992;63:776–782. doi: 10.1902/jop.1992.63.9.776. [DOI] [PubMed] [Google Scholar]
- 14.Yaegaki K, Coil JM. Examination, classification, and treatment of halitosis; clinical perspectives. J Can Dent Assoc. 2000;66:257–261. [PubMed] [Google Scholar]
- 15.Rosenberg M, Septon I, Eli I, Bar-Ness R, Gelernter I, Brenner S, Gabbay J. Halitosis measurement by an industrial sulphide monitor. J Periodontol. 1991;62:487–489. doi: 10.1902/jop.1991.62.8.487. [DOI] [PubMed] [Google Scholar]
- 16.Lowicka E, Beltowsi J. Hydrogen sulfide (H2S)-the third gas of interest for pharmacologists. Pharmacol Rep. 2007;59:4–24. [PubMed] [Google Scholar]
- 17.Schicho R, Krueger D, Zeller F, et al. Hydrogen sulfide is a novel prosecretary neuromodulator in the guinea pig and human colon. Gastroenterology. 2006;131:1542–1552. doi: 10.1053/j.gastro.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 18.Bukovska G, Kery V, Kraus JP. Expression of human cystathionine beta-synthase in Escherichia coli: purification and characterization. Protein Expr Purif. 1994;5:442–448. doi: 10.1006/prep.1994.1063. [DOI] [PubMed] [Google Scholar]
- 19.Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 1982;206:267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamizhselvi R, Moore PK, Bhatia M. Hydrogen sulfide acts as a mediator of inflammation in acute pancreatitis: In vitro studies using isolated mouse pancreatic acinar cells. J Cell Mol Med. 2007;11:315–326. doi: 10.1111/j.1582-4934.2007.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mok YY, Atan MS, Yoke Ping C, et al. Role of hydrogen sulfide in hemorrhagic shock in the rat: protective effect of inhibitors of hydrogen sulphide biosynthesis. Br J Pharmacol. 2004;143:881–889. doi: 10.1038/sj.bjp.0706014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiorucci S, Antonelli E, Distrutti E, et al. Inhibition of hydrogen sulfide generation contributes to gastric injury caused by nonsteroidal anti-inflammatory drugs. Gastroenterology. 2005;129:1210–1224. doi: 10.1053/j.gastro.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 23.Moshikowitz M, Horowitz N, Leshno M, Halpern Z. Halitosis and gastroesophageal reflux disease: a possible association. Oral Dis. 2007;13:581–585. doi: 10.1111/j.1601-0825.2006.01341.x. [DOI] [PubMed] [Google Scholar]
- 24.Adler I, Denninghoff VC, Alvarez MI, Avagnina A, Yoshida R, Elsner B. Helicobacter pylori associated with glossitis and halitosis. Helicobacter. 2005;10:312–317. doi: 10.1111/j.1523-5378.2005.00322.x. [DOI] [PubMed] [Google Scholar]