Abstract
Cerulein pancreatitis is similar to human edematous pancreatitis, manifesting with dysregulation of digestive enzyme production and cytoplasmic vacuolization, the death of acinar cells, edema formation, and infiltration of inflammatory cells into the pancreas. Reactive oxygen species are involved in nuclear factor-κB activation, cytokine expression, apoptosis and pathogenesis of pancreatitis. There is recent evidence that cerulein activates NADPH oxidase, which is a major source of reactive oxygen species during inflammation and apoptosis in pancreatic acinar cells. In addition, the Janus kinase/signal transducer and activator of transcription pathway has been suggested as being involved in inflammatory signaling in the pancreas. This review discusses the involvement of oxidative stress in inflammation and apoptosis in pancreatic acinar cells stimulated with cerulein as an in vitro model of pancreatitis.
Keywords: Cerulein, Pancreatitis, Inflammation, Apoptosis
INTRODUCTION
Acute pancreatitis is a multifactorial disease associated with the release of digestive enzymes to the pancreatic interstitium and to the systemic circulation with increased cytokine production and release, which can ultimately lead to deleterious local and systemic effects.1-3 Evidence suggests that pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6 act as mediators of the local and systemic manifestations of acute pancreatitis.4-6 Activated pancreatic macrophages release IL-1β and TNF-α in response to local tissue damage. These cytokines act locally to aggravate acute pancreatitis and systemically with IL-6 to increase capillary permeability and promote leukocyte adherence and extravasation to cause multiple organ failure.4-11
Among several animal models of experimental pancreatitis that exhibit the biochemical, morphological, and pathophysiological similarities to various aspects of human pancreatitis, cerulein pancreatitis was shown to be one of the best-characterized and widely used experimental models.12,13 Doses of a cholecystokinin (CCK) analog cerulein beyond those that cause the maximum pancreatic secretion of amylase and lipase14,15 result in pancreatitis, which is characterized by a dysregulation of the production and secretion of digestive enzymes, particularly, the inhibition of pancreatic secretion and an elevation in their serum levels, cytoplasmic vacuolization and the death of acinar cells, edema formation, and an infiltration of inflammatory cells into the pancreas.12,13,16
Oxidative stress is regarded as a major pathogenic factor in acute pancreatitis.17 In human acute pancreatitis, the increased levels of lipid peroxide in the bile or pancreatic tissue and subnormal levels of antioxidant vitamins in the blood were reported.18-20 Cerulein produces large amounts of reactive oxygen species (ROS) activates oxidant-sensitive nuclear transcription factor NF-κB and thus induces cytokine expression in freshly isolated pancreatic acinar cells without inflammatory cells in vitro.21
Apoptosis linked to oxidative stress has been shown in the pancreas of the patients with acute pancreatitis. Supraphysiologic concentrations of cerulein induce apoptosis in the rat pancreatic acinar AR42J cells.22 High concentration of cerulein promoted the expression of proapoptotic gene bax and p53 and DNA fragmentation in AR42J cells, which was mediated by intracellular Ca++.23,24
Janus kinase/signal transducers and activators of transcription (Jak/Stat) signaling pathways have been reported to be involved not only in the immune response of numerous cytokines but also in the actions of primarily non-immune mediators such as growth factors and hormones. The Jak/Stat pathway is well known to be activated by the family of cytokine receptors and to mediate a wide variety of biological effects, such as immune response, differentiation, cell survival, proliferation, or oncogenesis.25 Recently, it was reported that cerulein induced Jak2/Stat3 activation in pancreatic acinar cells.26
Therefore, the investigation of the relationship among oxidative stress, inflammation and apoptosis of pancreatic acinar cells stimulated with cerulein may provide clues for determining the pathophysiologic mechanism of pancreatitis.
CERULEIN AND RECEPTOR
Cerulein, a CCK analogue (pGlu-Gln-[Met3]-CCK8, sulphated), acts on two distinct CCK receptor subtypes, namely CCK1 (previously named CCKA) and CCK2 (previously named CCKB) receptors.27 CCK receptors are G-protein-coupled receptors initiating transient Ca++ oscillations by activating phospholipase C and induction of inositol triphosphate (IP3)-dependent Ca++ release from endoplasmic reticulum in pancreatic acinar cells.28,29 While CCK1 receptors are highly discriminating with respect to the presence of a sulphate moiety on the tyrosine residue in the sequence of CCK and peptide analogues including cerulein, CCK2 receptors (CCK2R) bind sulphated and non-sulphated analogues with much less difference in affinity.30,31 CCK1R mediates for example the secretion of pancreatic digestive enzymes and may also be involved in the regulation of satiety and feeding behavior, while CCK2R stimulate gastric acid production.32 Increase in blood flow induced by sulphated CCK-8 analogue and nonsulphated gastrin-17 in the gastric mucosa of rats have been demonstrated to be due to the activation of CCK2R.33 CCK1R has a role in the exocrine effects of cerulein such as amylase secretion. The CCK receptors (CCKR) are seven-helix membrane-spanning receptor principally coupled to G proteins.34 Initially, this type of receptor was implicated in the secretory effects of digestive peptide hormones. However, CCK2R is now recognized to mediate the mitogenic and anti-apoptotic effects of gastrin on gastrointestinal and pancreatic cells. In a pancreatic tumor cell line expressing the endogenous CCK2R, the proliferative effects of the CCK2R have been shown to be induced by the activation of the Jak2/Stat3 pathway by this receptor.35
ROS, NF-κB, AND CYTOKINE
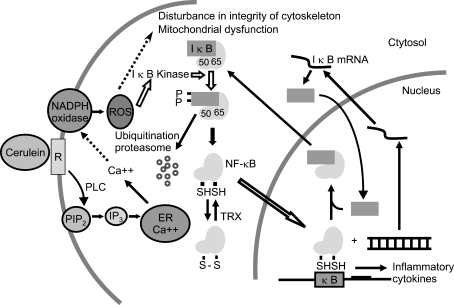
ROS are the important mediators in the initiation and development of pancreatitis.36-38 Cerulein induced NF-κB activation and cytokine expression, which may be mediated by ROS that are produced by NADPH oxidase in pancreatic acinar cells. ROS generated from cerulein is the main contributor to cytokine production in acinar cells by directly activating the oxidant-sensitive transcription factor, NF-κB (Fig. 1). ROS production may disturb the integrity of cytoskeleton39,40 and induce mitochondrial dysfunction of the cells.41,42 This hypothesis was supported by our previous proteomic analysis of cerulein-stimulated pancreatic acinar cells.43,44 The expression of cytoskeletal protein, tubulin beta chain and mitochondrial ATP synthase (beta chain precursor and subunit D) was induced by cerulein in pancreatic acinar cells. In vivo studies suggest that the intrapancreatic IL-1β, IL-6 and TNF-α are rapidly and coordinately increased during the early stages of acute pancreatitis.45,46 In the experimental pancreatitis in vivo, the inflammatory cytokines are produced from the infiltrating inflammatory cells. However, our previous studies showed the production of inflammatory cytokines in pancreatic acinar cells by stimulation with secretagogue21 and PMA-primed neutrophils.47
Fig. 1.
Proposed mechanism of how cerulein induces nuclear factor (NF)-κB activation and cytokine expression in pancreatic acinar cells. Cerulein binds the CCK receptor (R), which is a G-protein-coupled receptor. Ligand-receptor binding initiates transient Ca++ oscillations by activating phospholipase C (PLC) and the induction of inositol 1,4,5-trisphosphate (IP3)-dependent Ca++ release from the endoplasmic reticulum (ER) in pancreatic acinar cells. Ca++ can activate NADPH oxidase, which produces reactive oxygen species (ROS) that induce the activation of IκB kinase, which in turn phosphorylates IκB in the cytosol. IκB is an inhibitory subunit bound to NF-κB, a p65/p50 heterodimer in the cytosol. Phosphorylated IκB is ubiquitinated and degraded in a proteasome-dependent manner. NF-κB translocates to the nucleus and regulates cytokine expression. NF-κB is recycled after binding to IκB in the nucleus and transported to the cytosol. Therefore, ROS generated from cerulein appears to be the main contributor to cytokine production in acinar cells by directly activating the oxidant-sensitive transcription factor, NF-κB. ROS can disturb the integrity of the cytoskeleton and induce mitochondrial dysfunction in the cells. In the cytosol, the disulfide form of NF-κB is reduced to NF-κB by theoredoxin (TRX). PIP2, phosphatidylinositol 4, 5-bisphosphate; NF-κB subunit p50; 65, NF-κB subunit p65.
NF-κB belongs to the Rel family of transcription factors that regulate the activation of the cellular stress-related and early-response genes such as cytokines, growth factors, adhesion molecules, and acute-phase proteins.48,49 The human Rel proteins include NF-κB subunit p50 (p50), p52, Rel (c-Rel), Rel A (p65) and Rel B. The classic form of the activated NF-κB is a heterodimer consisting one p50 and one p65 subunit. The induction of a nuclear translocation of a p50/p65 NF-κB heterodimer and a p50 NF-κB homodimer was previously identified in pancreatic acinar cells exposed to stimulated neutrophils.47
Rebamipide is an anti-ulcer agent that scavenges ROS50 and inhibits the production of superoxide in the activated neutrophils.51 Rebamipide treatment at a concentration that efficiently inhibited NF-κB activation, suppressed IL-6 expression induced by cerulein. These results suggest that antioxidant might inhibit the expressions of the inflammatory cytokines by suppressing NF-κB activation and/or the NF-κB interaction with the upstream regulatory binding site of the cytokines such as IL-6. ROS production is mediated by NADPH oxidase, which induces inflammation of pancreatic acinar cells during pancreatitis. Therefore, inhibition of NF-κB activation and cytokine expression by scavenging ROS and inhibition of NADPH oxidase might alleviate the inflammatory response in pancreatic acinar cells.
ROS, Ca++ AND NADPH OXIDASE
Once produced, ROS can act as a molecular trigger of pancreatitis. They can attack the biological membranes directly and trigger the accumulation of neutrophil and their adherence to the capillary wall.52,53 Therefore, it is likely that ROS play a central role in perpetuating the pancreatic inflammation and the development of extrapancreatic complication.54 Previously we demonstrated that oxidative stress induced upregulation of inflammatory cytokines in pancreatic acinar cells.21
There are increasing evidences that a major source of ROS during inflammation is NADPH oxidase in leukemic cells and HL-60 cells.55-57 In phagocytic cells, the NADPH oxidase is composed of the membrane-bound subunits gp91phox and p22phox and the cytosolic subunits p67phox and p47phox. Upon activation of the enzyme, a complex of the cytosolic subunits translocates to the membrane and facilitates NADPH-dependent formation of superoxide (O2-), which in turn gives rise to the production of other secondary ROS (H2O2). Recently, it was suggested that the source of ROS is the NADPH oxidase in the stimulated neutrophils in cerulein pancreatitis in vivo.58 Our previous study showed that cerulein produces large amounts of ROS, activates oxidant-sensitive nuclear transcription factor NF-κB and thus induces cytokine expression in freshly isolated pancreatic acinar cells without inflammatory cells in vitro.21 Since ROS are mainly produced in the activated inflammatory cells during inflammation in vivo, relatively small amounts of ROS which may be produced in pancreatic acinar cells could be considered as negligible production. However, since ROS can act as chemoattractants for inflammatory cells,52,53 inhibition of ROS production in pancreatic acinar cells might be the first step to prevent the inflammatory process by inflammatory cells by inhibiting the infiltration of inflammatory cells into pancreas.
NADPH oxidase subunits Nox1, p22phox, p47phox, p67phox are expressed in pancreatic acinar AR42J cells. Cerulein stimulated NADPH oxidase activity, and induced the translocation of cytosolic subunits p47phox and p67phox to the membrane in AR42J cells.24 Ca++ may be involved in the translocation of p47phox and p67phox to the membrane since a Ca++ chelator BAPTA-AM, completely blocked the translocation of cytosolic subunits of NADPH oxidase to the membrane.24 Since cerulein-evoked Ca++ oscillation was not affected by an inhibitor of NADPH oxidase diphenyleneiodonium (DPI), DPI may directly inhibit the translocation of cytosolic subunits of NADPH oxidase to the membrane without disturbing Ca++ influx caused by cerulein in pancreatic acinar cells. DPI changes the redox state of cells not only by modulation of the level of ROS production, but also by induction of the efflux of glutathione to extracellular medium via specific transport channel in T24 cells.59 Further study should be performed on the inhibitory mechanism of DPI on the activation of NADPH oxidase in pancreatic acinar cells.
CYTOKINE AND JAK/STAT PATHWAY
Although Stat proteins were discovered during analysis of interferon signaling, recent studies have revealed that Stat signaling can account for various cellular responses to several cytokines, growth factors, and hormones.60,61 The Jak/Stat pathway is well known to be activated by the family of cytokine receptors and to mediate a wide variety of biological effects, such as immune response, differentiation, cell survival, proliferation, or oncogenesis.25 The mechanism of Jak activation is well known for the cytokine receptors. Ligand binding induces oligomerization of the receptors' subunits, constitutively associated with Jaks, and a transphosphorylation of the tyrosine kinases. Activated Jaks in turn phosphorylate the receptor that recruits the Stat protein.25
Cerulein mediates the secretory and inflammatory events through CCK receptors. Therefore, cerulein-induced Jak2/Stat3 activation might be mediated by CCK receptor(s) even though we could not conclude which type of receptor is involved in this signaling. AG 490 has been known to inhibit the activation of Jak262-67 and Stat368-72 in various cells and tissues In cerulein pancreatitis model, AG 490 effectively inhibited Jak2 phosphorylation. Likewise, Stat3 phosphorylation was blocked by AG 490. Presumably it may be caused by inactivation of Jak2, which would be required to catalyze the event of Stat phosphorylation. These data provide a strong evidence to indicate that cerulein activates Stat3 through the activation of Jak2. Interestingly, AG490 inhibited phosphorylation of Jak2 and Stat3 and rapidly reduced the expression of IL-1β, regulators of immune responses for acute pancreatitis. AG490 may negatively regulate inflammation in the episodes of acute pancreatitis.
Further study should be performed either by using the cells transfected with dominant negative gene of Stat3 or by examining at the promoter level by using cytokine such as IL-1β promoter reporter construct to determine the transcriptional regulation of cytokines by Stat3. In addition, the possible involvement of Jak3 in signaling for cytokine expression in pancreatic acinar cells should be determined since recent studies showed that AG 490 achieved inhibition of Jak3 and its downstream substrates Stat5a/b in vivo.73,74
ROS AND APOPTOSIS
Apoptosis linked to oxidative stress has been shown in the pancreas of the patients with acute pancreatitis. Supraphysiologic concentrations of cerulein induce apoptosis in the rat pancreatic acinar AR42J cells.22 High concentration of cerulein promoted the expression of proapoptotic gene bax and p53 and DNA fragmentation in AR42J cells, which was mediated by intracellular Ca++.23,24
There is a possible relationship between cerulein-induced activation of NADPH oxidase and apoptosis because ROS produced during pancreatitis are important mediators of apoptosis.75,76 In cerulein-induced pancreatitis, a high degree of ROS production and apoptosis were observed in pancreatic acinar cells.77,78 Cerulein induced increases in apoptotic indices including the expression of apoptosis-inducing factor (AIF), DNA fragmentation, TUNEL staining, and caspase-3 activity in AR42J cells.79 Apoptotic cell death is contributed by the activation of caspase-3 and the accumulation of p53, a known signaling molecule that acts upstream of caspase-3.80 p53 is induced in cerulein-stimulated pancreatic acinar cells.23 The signaling pathways leading to caspase activation during apoptosis involves the release of cytochrome c and other apoptogenic factors from injured mitochondria. AIF is conserved mitochondrial protein that is released into the cytoplasm and nucleus during apoptotic stimuli, inducing chromatin condensation and DNA fragmentation, in human and rodent cells. AIF induces apoptotic changes in purified nuclei, even in the presence of caspase inhibitors,81,82 suggesting the mediator function of AIF for apoptosis in a caspase- independent fashion. Caspase-3 activation and AIF expression were induced by cerulein in pancreatic acinar AR42J cells, which suggests that cerulein affects mitochondria to cause the transcriptional activation and regulation of caspase-3 and thus induces the expression of apoptotic gene such as AIF.
Cerulein-induced up-regulation of AIF with an increase in DNA fragmentation, TUNEL staining, and caspase-3 activity were inhibited in the cells treated with NADPH oxidase inhibitor DPI.79 The study suggests that the activation of NADPH oxidase and an increase in ROS production mediate apoptotic cell death in caspase-dependent and caspase-independent manner in pancreatic acinar cells stimulated with cerulein.
CONCLUSION
ROS production in pancreatic acinar cells during pancreatitis may be mediated by NADPH oxidase, which induces cytokine expression and apoptosis of pancreatic acinar cells. ROS mediates NF-κB activation and Jak/Stat pathway, which regulates inflammatory gene and apoptotic gene expression in pancreatic acinar cells. Thus, the activation of NADPH oxidase, NF-κB and Jak2/Stat3 seems to be the molecular mechanisms underlying the pathogenesis of acute pancreatitis. Inhibition of NADPH oxidase, NF--κB and Jak/Stat may alleviate inflammation and apoptosis of pancreatic acinar cells during pancreatitis by suppressing the expression of inflammatory cytokines and apoptosis-associated gene and caspase-3 activity. Therefore, NADPH oxidase, NF-κB and Jak/Stat may serve as the potential therapeutic targets in the development of new drugs in the treatment of acute pancreatitis.
ACKNOWLEDGEMENT
This study was supported by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea Government (MOST) (R11-2007-040-01002-0). The author is grateful to the Brain Korea 21 Project, College of Human Ecology, Yonsei University.
References
- 1.Kingsnorth A. Role of cytokines and their inhibitors in acute pancreatitis. Gut. 1997;40:1–4. doi: 10.1136/gut.40.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Formela LJ, Galloway SW, Kingsnorth AN. Inflammatory mediators in acute pancreatitis. Br J Surg. 1995;82:6–13. doi: 10.1002/bjs.1800820105. [DOI] [PubMed] [Google Scholar]
- 3.Frossard JL, Hadengue A, Pastor CM. New serum markers for the detection of severe acute pancreatitis in humans. Am J Respir Crit Care Med. 2001;164:162–170. doi: 10.1164/ajrccm.164.1.2008026. [DOI] [PubMed] [Google Scholar]
- 4.Viedma JA, Perez-Mateo M, Dominguez JE, Carballo F. Role of interleukin-6 in acute pancreatitis. Comparison with C-reactive protein and phospholipase A. Gut. 1992;33:1264–1267. doi: 10.1136/gut.33.9.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heath DI, Cruickshank A, Gudgeon M, et al. Role of interleukin-6 in mediating the acute phase protein response and potential as an early means of severity assessment in acute pancreatitis. Gut. 1993;34:41–45. doi: 10.1136/gut.34.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sameshima H, Ikei S, Mori K, et al. The role of tumor necrosis factor-alpha in the aggravation of cerulein-induced pancreatitis in rats. Int J Pancreatol. 1993;14:107–115. doi: 10.1007/BF02786116. [DOI] [PubMed] [Google Scholar]
- 7.McKay CJ, Gallagher G, Brooks B, Imrie CW, Baxter JN. Increased monocyte cytokine production in association with systemic complications in acute pancreatitis. Br J Surg. 1996;83:919–923. doi: 10.1002/bjs.1800830712. [DOI] [PubMed] [Google Scholar]
- 8.Tracey KJ, Cerami A. Tumor necrosis factor and regulation of metabolism in infection: role of systemic versus tissue levels. Proc Soc Exp Biol Med. 1992;200:233–239. doi: 10.3181/00379727-200-43426. [DOI] [PubMed] [Google Scholar]
- 9.Zamir O, Hasselgren PO, Kunkel SL, et al. Evidence that tumor necrosis factor participates in the regulation of muscle proteolysis during sepsis. Arch Surg. 1992;127:170–174. doi: 10.1001/archsurg.1992.01420020052008. [DOI] [PubMed] [Google Scholar]
- 10.Schirmer WJ, Schirmer JM, Fry DE. Recombinant human tumor necrosis factor produces hemodynamic changes characteristic of sepsis and endotoxemia. Arch Surg. 1989;124:445–448. doi: 10.1001/archsurg.1989.01410040055012. [DOI] [PubMed] [Google Scholar]
- 11.Kishimoto T, Taga T, Akira S. Cytokine signal transduction. Cell. 1994;76:253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 12.Gorelick FS, Adler G, Kerin HF. Cerulein-induced pancreatitis. In: Go VW, DiMagno EP, Gardner JD, Lebenthal E, Reber HA, Scheele GA, editors. The pancreas: biology, pathobiology, and disease. 2nd ed. New York: Raven Press; 1993. pp. 501–526. [Google Scholar]
- 13.Willemer S, Elsasser HP, Adler G. Hormone-induced pancreatitis. Eur Surg Res. 1992;24(S1):29–39. doi: 10.1159/000129237. [DOI] [PubMed] [Google Scholar]
- 14.Jensen RT, Wank SA, Rowley WH, Sato S, Gardner JD. Interaction of CCK with pancreatic acinar cells. Trends Pharmacol Sci. 1989;10:418–423. doi: 10.1016/0165-6147(89)90192-2. [DOI] [PubMed] [Google Scholar]
- 15.Sato S, Stark HA, Martinez J, et al. Receptor occupation, calcium mobilization, and amylase release in pancreatic acini: effect of CCK-JMV-180. Am J Physiol. 1989;257(Gastrointest Liver Physiol 20):G202–G209. doi: 10.1152/ajpgi.1989.257.2.G202. [DOI] [PubMed] [Google Scholar]
- 16.Lerch MM, Adler G. Experiemental animal models of acute pancreatitis. Int J Pancreatol. 1994;15:159–170. [PubMed] [Google Scholar]
- 17.Schoenberg MH, Buchler M, Gaspar M, et al. Oxygen free radicals in acute pancreatitis of the rat. Gut. 1990;31:1138–1143. doi: 10.1136/gut.31.10.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park BK, Chung JB, Lee JH, et al. Role of oxygen free radicals in patients with acute pancreatitis. World J Gastroenterol. 2003;9:2266–2269. doi: 10.3748/wjg.v9.i10.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott P, Bruce C, Schofield D. Vitamin C status in patients with acute pancreatitis. Br J Surg. 1993;80:750–754. doi: 10.1002/bjs.1800800632. [DOI] [PubMed] [Google Scholar]
- 20.Schenberg MH, Birk D, Berger HG. Oxidative stress in acute and chronic pancreatitis. Am J Clin Nutr. 1995;62:1306S–1314S. doi: 10.1093/ajcn/62.6.1306S. [DOI] [PubMed] [Google Scholar]
- 21.Yu JH, Lim JW, Namkung W, Kim H, Kim KH. Suppression of cerulein-induced cytokine expression by antioxidants in pancreatic acinar cells. Lab Invest. 2002;10:1359–1368. doi: 10.1097/01.lab.0000032377.09626.c7. [DOI] [PubMed] [Google Scholar]
- 22.Santa N, Klonowski-Stumpe H, Han B, et al. Supraphysiologic concentrations of cerulein induce apoptosis in the rat pancreatic acinar cell line AR42-J. Pancreas. 1999;19:76–82. doi: 10.1097/00006676-199907000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Yu JH, Kim H, Kim KH. Calcium - dependent apoptotic gene expression in cerulein - treated AR42J cells. Ann N Y Acad Sci. 2003;1010:66–69. doi: 10.1196/annals.1299.009. [DOI] [PubMed] [Google Scholar]
- 24.Yu JH, Lim JW, Kim KH, Morio T, Kim H. NADPH oxidase and apoptosis in cerulein - stimulated pancreatic acinar AR42J cells. Free Radic Biol Med. 2005;39:590–602. doi: 10.1016/j.freeradbiomed.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci. 2004;117:1281–1283. doi: 10.1242/jcs.00963. [DOI] [PubMed] [Google Scholar]
- 26.Yu JH, Kim KH, Kim H. Suppression of IL-1beta expression by the Jak 2 inhibitor AG490 in cerulein-stimulated pancreatic acinar cells. Biochem Pharmacol. 2006;72:1555–1562. doi: 10.1016/j.bcp.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Alexander SDH, Mathie A, Peters JA. Nomenclature supplement. Trends Pharmacol Sci. 2001;22:S1–S145. [Google Scholar]
- 28.Archer-Lahlou E, Escrieut C, Clerc P, et al. Molecular mechanism underlying partial and full agonism mediated by the human cholecystokinin-1 receptor. J Biol Chem. 2005;280:10664–10674. doi: 10.1074/jbc.M409451200. [DOI] [PubMed] [Google Scholar]
- 29.Arnould M, Tassa A, Ferrand A, et al. The G-protein-coupled CCK2 receptor associates with phospholipase Cgamma1. FEBS Lett. 2004;568:89–93. doi: 10.1016/j.febslet.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Fujimoto M, Igano K, Watanabe K, et al. Effects of cerulein - related peptides on CCK receptor bindings in brain and pancreas. Biochem Pharmacol. 1985;34:1103–1107. doi: 10.1016/0006-2952(85)90616-1. [DOI] [PubMed] [Google Scholar]
- 31.Huang SC, Yu DH, Wank SA, et al. Importance of sulfation of gastrin or cholecystokinin (CCK) on affinity for gastrin and CCK receptors. Peptides. 1989;10:785–789. doi: 10.1016/0196-9781(89)90114-9. [DOI] [PubMed] [Google Scholar]
- 32.Noble F, Wank SA, Crawley JN, et al. XXI. Structure, distribution, and functions of cholecystokinin receptors. Pharmacol Rev. 1999;51:745–781. [PubMed] [Google Scholar]
- 33.Heinemann A, Jocic M, Holzer-Petsche U, et al. Mediation by CCKB receptors of the CCK - evoked hyperaemia in rat gastric mucosa. Br J Pharmacol. 1995;116:2274–2278. doi: 10.1111/j.1476-5381.1995.tb15064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gales C, Kowalski-Chauvel A, Dufour MN, et al. Mutation of Asn-391 within the conserved NPXXY motif of the cholecystokinin B receptor abolishes Gq protein activation without affecting its association with the receptor. J Biol Chem. 2000;275:17321–17327. doi: 10.1074/jbc.M909801199. [DOI] [PubMed] [Google Scholar]
- 35.Ferrand A, Kowaski-Chauvel A, Bertrand C, et al. A novel mechanism for JAK2 activation by a G protein-coupled receptor, the CCK2R. J Biol Chem. 2005;280:10710–10715. doi: 10.1074/jbc.M413309200. [DOI] [PubMed] [Google Scholar]
- 36.Okumura N, Sakakibara A, Hayakawa T. Pancreatic endocrine function in experimental pancreatolithiasis in dogs. Am J Gastroenterol. 1982;77:392–396. [PubMed] [Google Scholar]
- 37.Aho HJ, Nevalainen TJ, Havia VT. Human acute pancreatitis. A light and electron microscopic study. Acta Pathol Microbiol Immunol Scand Section A. 1982;90:367–373. [PubMed] [Google Scholar]
- 38.Uys CJ, Bank S, Marks IN. The pathology of chronic pancreatitis in Cape Town. Digestion. 1973;9:454–468. doi: 10.1159/000197474. [DOI] [PubMed] [Google Scholar]
- 39.Snook JH, Li J, Helmke BP, Guilford WH. Peroxynitrite inhibits myofibrillar protein function in an in vitro assay of motility. Free Radic Biol Med. 2008;44:14–23. doi: 10.1016/j.freeradbiomed.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crimi E, Ignarro LJ, Napoli C. Microcirculation and oxidative stress. Free Radic Res. 2007;41:1364–1375. doi: 10.1080/10715760701732830. [DOI] [PubMed] [Google Scholar]
- 41.Cassina P, Cassina A, Pehar M, et al. Mitochondrial dysfunction in SOD1G93A-bearing astrocytes promotes motor neuron degeneration: prevention by mitochondrial-targeted antioxidants. J Neurosci. 2008;28:4115–4122. doi: 10.1523/JNEUROSCI.5308-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren XY, Li YN, Qi JS, Niu T. Peroxynitrite-induced protein nitration contributes to liver mitochondrial damage in diabetic rats. J Diabetes Complications. 2008 doi: 10.1016/j.jdiacomp.2007.06.013. in press. [DOI] [PubMed] [Google Scholar]
- 43.Yu JH, Yun SY, Lim JW, Kim H, Kim KH. Proteome analysis of rat pancreatic acinar cells; Implication for cerulean-induced pancreatitis. Proteomics. 2003;3:2446–2453. doi: 10.1002/pmic.200300545. [DOI] [PubMed] [Google Scholar]
- 44.Yu JH, Yun SY, Lim JW, Kim H, Kim KH. Mass spectrometry and tandem mass spectrometry analysis of rat mitochondrial ATP synthase: up-regulation in pancreatic acinar cells treated with cerulein. Proteomics. 2003;3:2437–2445. doi: 10.1002/pmic.200300585. [DOI] [PubMed] [Google Scholar]
- 45.Heath DL, Cruickshank DH, Gudgeon M. Role of interleukin-6 in mediating the acute phase protein response and potential as an early means of severity assessment in acute pancreatitis. Gut. 1993;66:41–45. doi: 10.1136/gut.34.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Norman J, Franz M, Riker A. Rapid elevation of systemic cytokines during acute pancreatitis and their origination within the pancreas. Surg Forum. 1994;45:148–150. [Google Scholar]
- 47.Seo JY, Kim H, Seo JT, Kim KH. Oxidative stress-induced cytokine production in isolated rat pancreatic acinar cells: Effects of small molecule antioxidants. Pharmacology. 2002;64:63–70. doi: 10.1159/000056152. [DOI] [PubMed] [Google Scholar]
- 48.Barnes PJ, Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 49.Wulczyn FG, Krappmann D, Scheidereit C. The NF-κB/Rel and IκB gene families: mediators of immune response and inflammation. J Mol Med. 1996;74:749–769. doi: 10.1007/s001090050078. [DOI] [PubMed] [Google Scholar]
- 50.Naito Y, Yoshikawa T, Tanigawa T, et al. Hydroxyl radical scavenging by rebamipide and related compounds: Electron paramagnetic resonance study. Free Radic Biol Med. 1995;18:117–123. doi: 10.1016/0891-5849(94)00110-6. [DOI] [PubMed] [Google Scholar]
- 51.Ogino K, Hobara T, Ishiyama H, et al. Antiulcer mechanism of action of rebamipide, a novel antiulcer compound, a diethyl dithiocarbamate-induced antral gastric ulcers in rats. Eur J Pharmacol. 1992;212:9–13. doi: 10.1016/0014-2999(92)90065-c. [DOI] [PubMed] [Google Scholar]
- 52.Petrone WF, English DK, Wong K. Free radicals and inflammation: superoxide-dependent activation of a neutrophil chemotactic factor in plasma. Proc Natl Acad Sci U S A. 1980;77:1159–1163. doi: 10.1073/pnas.77.2.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bjork J, Arfors KE. Oxygen free radicals and leukotriene B4 induced increase in vascular leakage is mediated by polymorphonuclear leukocytes. Agents Actions Suppl. 1984;11:63–73. [PubMed] [Google Scholar]
- 54.Guice KS, Oldham KT, Caty MG, Johnson KJ, Ward PA. Neutrophil-dependent, oxygen-radical mediated lung injury associated with acute pancreatitis. Ann Surg. 1989;210:740–747. doi: 10.1097/00000658-198912000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hiraoka W, Vazquez N, Nieves-Neira W, Chanock SJ, Pommier Y. Role of oxygen radicals generated by NADPH oxidase in apoptosis induced in human leukemia cells. J Clin Invest. 1998;102:1961–1968. doi: 10.1172/JCI3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 57.Arroyo A, Modriansky M, Serinkan FB, et al. NADPH oxidase-dependent oxidation and externalization of phosphatidylserine during apoptosis in Me2SO-differentiated HL-60 cells. Role in phagocytic clearance. J Biol Chem. 2002;277:49965–49975. doi: 10.1074/jbc.M204513200. [DOI] [PubMed] [Google Scholar]
- 58.Gukovskaya AS, Vaquero E, Zaninovic V, et al. Neutrophils and NADPH oxidase mediate intrapancreatic trypsin activation in murine experimental acute pancreatitis. Gastroenterology. 2002;122:974–984. doi: 10.1053/gast.2002.32409. [DOI] [PubMed] [Google Scholar]
- 59.Pullar JM, Hampton MB. Diphenyleneiodonium triggers the efflux of glutathione from cultured cells. J Biol Chem. 2002;277:19402–19407. doi: 10.1074/jbc.M111053200. [DOI] [PubMed] [Google Scholar]
- 60.Fu XY, Kessler DS, Veals SA, Levy DE, Darnelle JE. ISGF3, the transcriptional activator induced by interferon alpha, consists of multiple interacting polypeptide chains. Proc Natl Acad Sci USA. 1990;87:8555–8559. doi: 10.1073/pnas.87.21.8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kessler DS, Veaks SA, Fu XY, Leavy DE. Interferon-alpha regulates nuclear translocation and DNA-binding affinity of ISGF3, a multimeric transcriptional activator. Genes Dev. 1990;4:1753–1765. doi: 10.1101/gad.4.10.1753. [DOI] [PubMed] [Google Scholar]
- 62.Gazit A, Yaish P, Gilon C, Levitzki A. Synthesis and biological activity of protein tyrosine kinase inhibitors. J Med Chem. 1989;32:2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- 63.Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- 64.Meydan N, Grunberger T, Dadi H, et al. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 1996;379:645–648. doi: 10.1038/379645a0. [DOI] [PubMed] [Google Scholar]
- 65.Simon HU, Yousefi S, Dibbert B, Levischaffer F, Blaser K. Antiapoptitic signals of granulocyte-macrophage colony stimulating factors are transduced via JAK2 tyrosine kinase in eosinophils. Eur J Immunol. 1997;27:3536–3539. doi: 10.1002/eji.1830271256. [DOI] [PubMed] [Google Scholar]
- 66.Marrero MB, Schieffer B, Li B, et al. Role of Janus kinase signal transducer and activator of transcription and mitogenactivated protein kinases cascades in angiotensin II- and platelet-derived growth factor-induced vascular smooth muscle cell proliferation. J Biol Chem. 1997;272:24684–24690. doi: 10.1074/jbc.272.39.24684. [DOI] [PubMed] [Google Scholar]
- 67.McWhinney CD, Hunt RA, Conrad KM, Dostal DE, Baker KM. The type I angiotensin II receptor couples to Stat1 and Stat3 activation through JAK2 kinase in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 1997;29:2513–2524. doi: 10.1006/jmcc.1997.0489. [DOI] [PubMed] [Google Scholar]
- 68.Nielsen M, Kaltoft M, Nordahl M, et al. Constitutive activation of a slowly migrating isoform of Stat3 in mycosis fungoides: tyrphostin AG-490 inhibits Stat3 activation and growth of mycosis fungoides tumor cell lines. Proc Natl Acad Sci USA. 1997;94:6764–6769. doi: 10.1073/pnas.94.13.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Toyonaga T, Nakano K, Nagano M, et al. Blockade of constitutively activated Janus kinase/signal transducer and activator of transcription-3 pathway inhibits growth of human pancreatic cancer. Cancer Lett. 2003;201:107–116. doi: 10.1016/s0304-3835(03)00482-8. [DOI] [PubMed] [Google Scholar]
- 70.Rahaman SO, Harbor PC, Chernova O, et al. Inhibition of constitutively active Stat3 suppresses proliferation and induces apoptosis in glioblastoma multiforme cells. Oncogene. 2002;21:8404–8413. doi: 10.1038/sj.onc.1206047. [DOI] [PubMed] [Google Scholar]
- 71.Burke WM, Jin X, Lin HJ, et al. Inhibition of constitutively active Stat3 suppresses growth of human ovarian and breast cancer cells. Oncogene. 2001;20:7925–7934. doi: 10.1038/sj.onc.1204990. [DOI] [PubMed] [Google Scholar]
- 72.Burdelya L, Catlett-Falcone R, Levitzki A, et al. Combination therapy with AG-490 and interleukin 12 achieves greater antitumor effects than either agent alone. Mol Cancer Ther. 2002;1:893–899. [PubMed] [Google Scholar]
- 73.Higuchi T, Shiraishi T, Shirakusa T, et al. Prevention of acute lung allograft rejection in rat by the Janus kinase 3 inhibitor, tyrphostin AG490. J Heart Lung Transplant. 2005;24:1557–1564. doi: 10.1016/j.healun.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 74.Behbod F, Erwin-Cohen RA, Wang M-E, et al. Concomitant inhibition of Janus kinase 3 and calcineurin-dependent signaling pathways synergistically prolongs the survival of rat heart allografts. J Immunol. 2001;166:3724–3732. doi: 10.4049/jimmunol.166.6.3724. [DOI] [PubMed] [Google Scholar]
- 75.Kaiser AM, Saluja AK, Sengupta A, Saluja MP, Steer ML. Relationship between severity, necrosis, and apoptosis in five models of experimental acute pancreatitis. Am J Physiol. 1995;269:C1295–C1304. doi: 10.1152/ajpcell.1995.269.5.C1295. [DOI] [PubMed] [Google Scholar]
- 76.Sandoval D, Gukovskaya A, Reavey P, et al. The role of neutrophils and platelet-activating factor in mediating experimental pancreatitis. Gastroenterology. 1996;111:1081–1091. doi: 10.1016/s0016-5085(96)70077-x. [DOI] [PubMed] [Google Scholar]
- 77.Gukovskaya AS, Gukovsky I, Zaninovic V, et al. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J Clin Invest. 1997;100:1853–1862. doi: 10.1172/JCI119714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kimura K, Shimosegawa T, Sasano H, et al. Endogenous glucocorticoids decrease the acinar cell sensitivity to apoptosis during cerulein pancreatitis in rats. Gastroenterology. 1998;114:372–381. doi: 10.1016/s0016-5085(98)70490-1. [DOI] [PubMed] [Google Scholar]
- 79.Yu JH, Lim JW, Kim KH, Morio T, Kim H. NADPH oxidase and apoptosis in cerulein-stimulated pancreatic acinar AR42J cells. Free Radic Biol Med. 2005;39:590–602. doi: 10.1016/j.freeradbiomed.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 80.Kim S-J, Ju J-W, Oh C-D, et al. ERK-1/2 and p38 kinase oppositely regulate nitric oxide-induced apoptosis of chondrocytes in association with p53, caspase-3, and differentiation status. J Biol Chem. 2002;277:1332–1339. doi: 10.1074/jbc.M107231200. [DOI] [PubMed] [Google Scholar]
- 81.Cande C, Cecconi F, Dessen P, Kroemer G. Apoptosis-inducing factor (AIF): key to the conserved caspase-independent pathways of cell death? J Cell Sci. 2002;115:4727–4734. doi: 10.1242/jcs.00210. [DOI] [PubMed] [Google Scholar]
- 82.Susin SA, Lorenzo HK, Zamzami N, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]