Abstract
Background/Aims
Revaprazan, a novel acid-pump antagonist, and proton-pump inhibitors (PPIs) have pH-independent effects on ulcer healing. The addition of a PPI promotes the cell restitution rate as well as vessel regeneration and maturation for ulcer repair. Revaprazan is known to protect the mucosa by increasing the prostaglandin concentration.
Methods
We reviewed the medical records of patients who underwent endoscopic submucosal dissection (ESD) for gastric neoplasia at Yeungnam University Hospital between January 2008 and May 2009. We conducted a matched case-control study to compare the healing rates effected by revaprazan and rabeprazole.
Results
Each group consisted of 30 patients. The baseline characteristics did not differ significantly between the two groups. Stage S1 disease was observed in 97% and 100% of patients after 8 weeks of treatment in the revaprazan and rabeprazole groups, respectively. In the revaprazan group, only one patient had stage H2 disease: a 54-year-old man with a 5.5-cm lesion after ESD of the ulcer, type IIa early gastric cancer, and adenocarcinoma. No serious adverse effects occurred during the treatment period in either group.
Conclusions
The safety and efficacy profiles of revaprazan and rabeprazole are similar for the treatment of ESD-induced ulcers.
Keywords: Revaprazan, Rabeprazole, Proton pump inhibitors, Acid pump antagonists, Endoscopic submucosal dissection
INTRODUCTION
Proton pump inhibitors (PPIs), which are specific for H+/K+-ATPase, impede the function of the proton pump responsible for the terminal step in gastric acid secretion; they are currently the most widely used drugs for acid-related diseases including peptic ulcer disease, gastroesophageal reflux disease and Zollinger-Ellison syndrome.1 Reversible acid pump antagonists (APAs), another class of proton pump inhibitors, act by K+-competitive and reversible binding to the gastric proton pump.2
APAs and PPIs have pH-independent effects on ulcer healing. The addition of PPIs to treatment promotes the cell restitution rate as well as vessel regeneration and maturation during wound healing.3 Rabeprazole demonstrates the significant protection of the gastric-mucosa of PPIs by increasing the prostaglandin E2 production and reducing the leukotriene B4 production.4 Revaprazan, a new drug, protects the mucosa by inhibiting secretion of pepsinogen and increasing the prostaglandin concentration.5 In previous study that compared revaprazan with a PPI for the treatment of gastric ulcers, the ulcer healing rates did not differ.
After the introduction of endoscopic submucosal dissection (ESD) in the late 1990s, ESD has become widely used as an alternative to surgical resection in patients with early-stage gastric cancer or for adenomas.6 However, EST causes artificial ulcers. It is unknown whether the healing of ESD induced ulcers differ from peptic ulcers with regard to healing rate and the factors that affect healing. Furthermore, the efficacy of APA for ESD induced ulcers has not been previously studied. Therefore, the aim of this study was to compare the efficacy of revaprazan with rabeprazole for treatment of ESD-induced ulcers.
MATERIALS AND METHODS
1. Endoscopic submucosal dissection
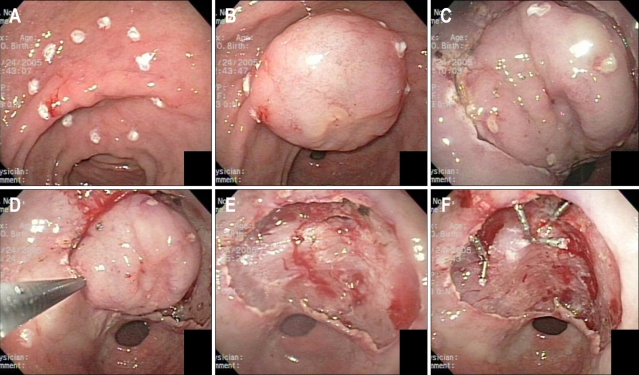
All ESD procedures were performed by a single endoscopist. ESD was performed by the endoscopic submucosal dissection method using a flex knife for en bloc resection (Fig. 1). If bleeding or visible vessels were found during the ESD, thermal coagulation or endoscopic hemoclipping was performed as required (Fig. 1).
Fig. 1.
After initial incision of the mucosa with the needle knife to allow insertion of tip of insulation-tipped diathermic (IT) knife, complete incision is made by IT knife circumferentially. (D) En bloc dissection of the submucosal tissue was performed. (E) EGC was resected completely. (F) Hemostasis was achieved with hemoclips and cauterization.
2. Study design
We reviewed the medical records of patients that underwent ESD for gastric neoplasm at Yeungnam University hospital from January 2008 to May 2009. Demographic and clinical characteristics, including age and gender, were investigated. Location, size, and histopathological type of lesions were also investigated. Location was divided by antrum, proximal body, mid-body, distal body, angle and pre-pylorus. Histopathological types included adenomas (with low grade dysplasia, moderate dysplasia and severe dysplasia) and adenocarcinomas (well differentiated, moderated and poorly differentiated). Patients were evaluated for Helicobacter pylori infection by rapid urease test (CLO test) and endoscopic biopsy specimens by Giemsa staining.
Loading dose of pantoprazole 80 mg was given intravenously 2 hours before the ESD, and 8 mg/hr of intravenous pantoprazole was given continuously for the first 48 hours as maintenance. Starting three days after the ESD, oral acid suppressing agents were administered for 8 weeks. In the revaprazan group, revaprazan 200 mg was administered orally for 8 weeks, and in the rabeprazole group, rabeprazole 20 mg was given orally for the same period.
Bleeding encountered during the ESD was defined as immediate, and bleeding after the ESD as delayed. After the ESD, the patients were observed for their vital signs and complete blood counts. Delayed bleeding was suspected in patients with one or more of the following bleeding signs: fresh hematemesis, hematochezia, instability of vital signs (tachycardia or hypotension), or a reduction of hemoglobin by more than 2 g/dL after the ESD. When delayed bleeding was suspected, immediate endoscopy was performed.
Follow-up endoscopy was performed the next day, 56 days and 5-months after the ESD. The ulcer size was measured the day after the ESD. Ulcer stages were assessed using a six-stage system as proposed by Sakita and Fukutomi (Table 1) at 56 days after the ESD.7
Table 1.
Gastric Ulcer Stages Using a Six-Stage System
English version of ulcer stages as classified by Sakita and Fukutomi.7
3. Patients
This study included 193 patients that underwent ESD for a gastric neoplasm between January 2008 to May 2009. Among them, 54 patients had received revaprazan therapy and 139 patients were treated with rabeprazole. The indications for ESD were as follows: gastric adenoma and early gastric adenocarcinoma (well or moderately differentiated; no ulceration; and no lymph node involvement or metastasis by CT). Patients were excluded if they had a previous history of upper gastrointestinal surgery or vagotomy; known hypersensitivity to revaprazan or rabeprazole; current use of aspirin, non-steroidal anti-inflammatory drugs, corticosteroids, PPIs, prostaglandins, or muco-protecting agents, or had surgery for vertical margin- positive adenocarcinoma in the resection specimen. The patients that had delayed bleeding or gastric perforation the day after the ESD and required rapid treatment with IV PPIs (duration of taking acid suppressing agents influences gastric ulcer healing)8 were also excluded.
4. Case control study
All matching were performed with the Statistical Analysis Systems software package (Release 9.1.3; SAS Institute, Cary, NC, USA). The matching variables were age, gender, histology and initial ulcer size. The patients were matched on gender and histology by exact match, and matched on age intervals (plus or minus 5 years). The matched patients were divided into three categories based on initial ulcer size according to the medical literature:9 ≤30 mm, between 31 mm and 40 mm, and >40 mm. Then, thirty patients were randomly selected for each group using SAS program.
5. Statistical analysis
Continuous data are summarized as the mean (95% confidence interval [CI]). The Student t-test was used to compare the mean values of continuous variables. The Pearson's chi-square test with Yates' correction for continuity and the Fisher's exact test were used as appropriate for comparison of the categorical variables. The analysis was performed with the statistical software package (SPSS 17.0 version for Windows; SPSS, Chicago, IL, USA). A p value less than 0.05 was accepted as statistically significant.
RESULTS
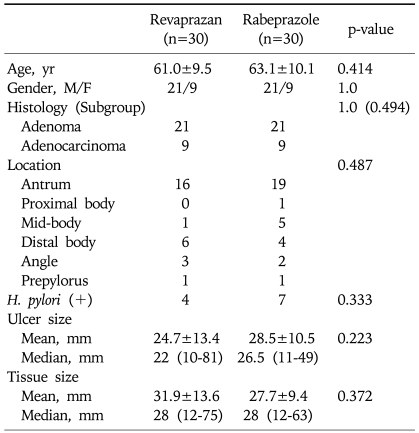
Data regarding the clinical and endoscopic features of the patients are outlined in Table 2. There were no significant differences between the two groups with respect to age, gender, ulcer size, location of ulcer, tissue size, histopathology (included histopathology of subgroup) and positive H. pylori.
Table 2.
Baseline Characteristics of Patients
Continuous data are expressed as mean±standard deviation and median (minimum-maximum).
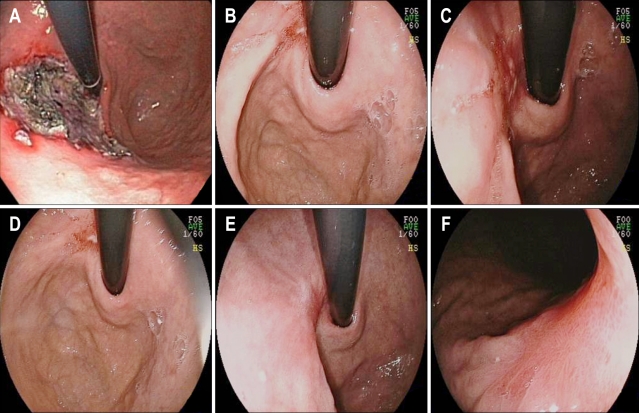
Ninety seven percent and 100% of the patients had S1 stage disease after eight weeks of treatment in the revaprazan and rabeprazole group, respectively. In the revaprazan group, only one patient had H2 stage disease (Fig. 2); a 54-year-old man with a 5.5 cm post ESD ulcer on the lesser curvature of the proximal body the day after the ESD, type IIa EGCA and well differentiated adenocarcinoma. The endoscopic findings revealed a 0.5 cm ulcer almost completely covered with regenerating epithelium, eight weeks after the ESD and a red scar with rough epithelialization without mucosal breaks at five months after the ESD (Fig. 2).
Fig. 2.
A patient with stage H2 disease 8 weeks after endoscopic submucosal dissection (ESD). (A) At the lesser-curvature side of the proximal body, an ulcer of about 5.5 cm with several reddish spots was noted 1 day post-ESD. (B-D) An ulcer of about 0.5 cm that was almost completely covered with regenerating epithelium was noted at 8 weeks post-ESD. (E, F) A red scar with rough epithelialization without mucosal breaks was noted at 5 months post-ESD.
During follow-up, no significant side effects were associated with the medications in either treatment group. There were no cases of delayed gastric perforation or bleeding after discharge.
DISCUSSION
The use of endoscopic mucosal resection (EMR) procedures for early gastric cancer has been increasing. This is a preferred procedure because the patient quality of life after an EMR is superior to that of patients after a surgical gastrectomy. After the introduction of the endoscopic submucosal dissection (ESD), in the late 1990s, EMR has become more technically feasible for all tumors, regardless of size. Currently, ESD is most widely used as an alternative to surgical resection in patients with early-stage gastric cancer or adenomas.6
ESD induces the development of ulcers. It is unclear whether these ESD induced ulcers differ from peptic ulcers with regard to the healing rate or the factors that influence healing. EMR-induced ulcers have been reported to heal faster and to recur less often than non-iatrogenic gastric ulcers.10 Increased healing has been associated with preserved contractility of the proper muscle layer, which is usually damaged in patients with peptic ulcers,11 and the increased blood supply at the margin of EMR-induced ulcers resulting from increased levels of nitric oxide, epidermal growth factor and endogenous prostaglandins.12,13 The healing process of ESD-induced ulcers may be similar to EMR-induced ulcers. A recent study comparing the healing rate of EMR-induced ulcers with regard to the duration of treatment with omeprazole suggested that one week of treatment may be sufficient.14 In addition, an observational study showed that gastric ulcers caused by ESD procedures healed within eight weeks regardless of the size and location.11 However, Oh et al.9 reported that the degree of healing of ESD-induced ulcers was dependent on the initial ulcer size, indicating that the duration of treatment with PPI should take into consideration the initial size of the ulcer.
Gastric H+/K+-ATPase is a proton pump located at the apical membrane of the parietal cells; it transports H+ into the canaliculus of parietal cells in exchange for K+. Substituted benzimidazoles, such as omeprazole, lansoprazole and rabeprazole, inactivate the H+/K+-ATPase by covalent binding to the sulfohydryl group of H+/K+-ATPase, resulting in the long lasting inhibition of gastric acid secretion.15 PPIs also have pH-independent effects on ulcer healing. PPIs attenuate leukocyte-endothelial cell adhesion by inhibiting the expression of cellular adhesion molecules.16 PPIs have been reported to directly inhibit inflammation by suppressing neutrophil activity17,18 as well as by relaxing vascular smooth muscle.19 Thus, PPIs have multiple functions during gastric mucosal repair. In recent studies, PPIs were shown to promote the healing of injured gastric mucosal cells following injury by enhancing cell proliferation and migration;20 rabeprazole increased the expression of CXCR4 mRNA. Thus, it promoted vessel regeneration and maturation, facilitating ulcer healing.3
Revaprazan,5,6-dimethyl-2-(4-fluorophenylamino)-4-(1-methyl-1,2,3,4-tetrahydroisoquinoline-2-yl) pyrimidine hydrochloride, is a novel APA that is currently approved by Korean Food and Drug Administration (KFDA) as a new drug for the treatment of gastric diseases including peptic ulcer disease.21-23 This APA has a reversible mode of action against H+/K+-ATPase, which is distinct from PPIs that perform irreversible inhibition. The drug causes inhibition of gastric acid secretion rapidly and effectively without sustained hypergastrinemia. This inhibitor is competitive with the luminal K+ ions, and has a highly selective affinity for H+/K+-ATPase, and low affinity for Na+/K+-ATPase.24 Interestingly, Revaprazan, like pantoprazole, plays a regulatory role in MAPK ERK1/2 signaling. MAPK is an executive critical enzyme that is active during inflammatory response signaling as well as cell proliferation. In addition, revaprazan has been shown to provide cyto-protection of the gastric epithelium.5 In the present study, the ulcer healing effect of revaprazan for ESD-induced ulcers was similar to that of rabeprazole.
PPIs are generally considered to be extremely safe with little or no risk of serious adverse events. However, concerns continue to be expressed about existing and potential adverse effects of these drugs, especially with long-term use. In animal studies, in the K+ channel mutated mouse model, in beta subunit gastric H+/K+-ATPase deficient mice, and in the alpha subunit of the gastric H+/K+-ATPase deficient mice achlorhydria and vacuolization of the gastric parietal cells were described.25,26 Since the beta subunit of the gastric H+/K+-ATPase and gastrin double deficient mice did not exhibit such hyperplasia,27 it is speculated that such vacuolization might be attributed to secondary hypergastrinemia.28 Driman et al.29 reported enlarged parietal cells with luminal bulges of cytoplasm, expanded tubulovesicles, and poorly developed secretory canaliculi after omeprazole treatment for longer than 12 months in a human study. Bajaj et al.30 reported that PPI use during just more than 1 week was associated with a higher risk of SBP. These results suggest that PPI usage leads to hypergastrinemia,28 and hypergastrinemia leads to impaired intestinal permeability in humans.30 However, APA had a weaker effect on the serum gastrin concentration and antral gastrin content than PPIs.5,31 Therefore, APA would be beneficial for clinical use.
This is the first study to demonstrate the efficacy of APA for the treatment of ESD-induced ulcers. In conclusion, Revaprazan showed a similar safety and efficacy profile to rabeprazole for the treatment of ESD-induced ulcers. Further prospective studies are needed to explore the pathophysiology of this novel acid pump antagonist and proton pump inhibitors for the treatment of ESD-induced ulcers.
References
- 1.Sachs G. Proton pump inhibitors and acid-related diseases. Pharmacotherapy. 1997;17:22–37. [PubMed] [Google Scholar]
- 2.Pope AJ, Boehm MK, Leach C, Ife RJ, Keeling D, Parsons ME. Properties of the reversible K(+)-competitive inhibitor of the gastric (H+/K+)-ATPase, SK&F 97574. I. In vitro activity. Biochem Pharmacol. 1995;50:1543–1549. doi: 10.1016/0006-2952(95)02020-9. [DOI] [PubMed] [Google Scholar]
- 3.Akimoto M, Hashimoto H, Shigemoto M, Maeda A, Yamashita K. Effects of antisecretory agents on angiogenesis during healing of gastric ulcers. J Gastroenterol. 2005;40:685–689. doi: 10.1007/s00535-005-1611-2. [DOI] [PubMed] [Google Scholar]
- 4.Okazaki M, Shimizu I, Ishikawa M, et al. Gastric mucosal levels of prostaglandins and leukotrienes in patients with gastric ulcer after treatment with rabeprazole in comparison to treatment with ranitidine. J Med Invest. 2007;54:83–90. doi: 10.2152/jmi.54.83. [DOI] [PubMed] [Google Scholar]
- 5.Yeo M, Kwak MS, Kim DK, et al. The novel acid pump antagonists for anti-secretory actions with their peculiar applications beyond acid suppression. J Clin Biochem Nutr. 2006;38:1–8. [Google Scholar]
- 6.Ono H. Endoscopic submucosal dissection for early gastric cancer. Chin J Dig Dis. 2005;6:119–121. doi: 10.1111/j.1443-9573.2005.00206.x. [DOI] [PubMed] [Google Scholar]
- 7.Sakita T, Fukutomi H. Endoscopic diagnosis. In: Yoshitoshi Y, editor. Ulcer of stomach and duodenum. Tokyo: Nankodo; 1971. pp. 198–208. [Google Scholar]
- 8.Howden CW, Jones DB, Peace KE, Burget DW, Hunt RH. The treatment of gastric ulcer with antisecretory drugs: relationship of pharmacological effect to healing rates. Dig Dis Sci. 1988;33:619–624. doi: 10.1007/BF01798367. [DOI] [PubMed] [Google Scholar]
- 9.Oh TH, Jung HY, Choi KD, et al. Degree of healing and healing-associated factors of endoscopic submucosal dissection-induced ulcers after pantoprazole therapy for 4 weeks. Dig Dis Sci. 2009;54:1494–1499. doi: 10.1007/s10620-008-0506-5. [DOI] [PubMed] [Google Scholar]
- 10.Hashimoto T, Adachi K. Changes in gastric mucosal blood flow during healing of EMR-induced ulcer: comparison with peptic ulcer. Dig Endosc. 1997;9:127–131. [Google Scholar]
- 11.Kakushima N, Yahagi N, Fujishiro M, et al. The healing process of gastric artificial ulcers after endoscopic submucosal dissection. Dig Endosc. 2004;16:327–331. [Google Scholar]
- 12.Panes J, Casadevall M, Pique JM, Bosch J, Whittle BJ, Teres J. Effects of acute normovolemic anemia on gastric mucosal blood flow in rats: role of nitric oxide. Gastroenterology. 1992;103:407–413. doi: 10.1016/0016-5085(92)90828-m. [DOI] [PubMed] [Google Scholar]
- 13.Tarnawski A, Stachura J, Durbin T, Sarfeh IJ, Gergely H. Increased expression of epidermal growth factor receptor during gastric ulcer healing in rats. Gastroenterology. 1992;102:695–698. doi: 10.1016/0016-5085(92)90123-g. [DOI] [PubMed] [Google Scholar]
- 14.Lee SY, Kim JJ, Lee JH, et al. Healing rate of EMR-induced ulcer in relation to the duration of treatment with omeprazole. Gastrointest Endosc. 2004;60:213–217. doi: 10.1016/s0016-5107(04)01683-9. [DOI] [PubMed] [Google Scholar]
- 15.Sachs G, Shin JM, Briving C, Wallmark B, Hersey S. The pharmacology of the gastric acid pump: the H+,K+ ATPase. Annu Rev Pharmacol Toxicol. 1995;35:277–305. doi: 10.1146/annurev.pa.35.040195.001425. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida N, Yoshikawa T, Tanaka Y, et al. A new mechanism for anti-inflammatory actions of proton pump inhibitors: inhibitory effects on neutrophil-endothelial cell interactions. Aliment Pharmacol Ther. 2000;14(Suppl 1):74–81. doi: 10.1046/j.1365-2036.2000.014s1074.x. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki M, Mori M, Miura S, et al. Omeprazole attenuates oxygen-derived free radical production from human neutrophils. Free Radic Biol Med. 1996;21:727–731. doi: 10.1016/0891-5849(96)00180-3. [DOI] [PubMed] [Google Scholar]
- 18.Wandall JH. Effects of omeprazole on neutrophil chemotaxis, super oxide production, degranulation, and translocation of cytochrome b-245. Gut. 1992;33:617–621. doi: 10.1136/gut.33.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCabe RD, Young DB. Evidence of a K(+)-H(+)-ATPase in vascular smooth muscle cells. Am J Physiol. 1992;262:H1955–H1958. doi: 10.1152/ajpheart.1992.262.6.H1955. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki H, Masaoka T, Minegishi Y, Motosugi Y, Miura S, Ishii H. Lansoprazole promotes gastric mucosal cell proliferation and migration by activating p44/p42 mitogen-activated protein kinase. Wound Repair Regen. 2004;12:93–99. doi: 10.1111/j.1067-1927.2004.012116.x. [DOI] [PubMed] [Google Scholar]
- 21.Chang R, Chung IS, Park SH, et al. Phase III clinical trial of Revaprazan (Revanex(R)) for gastric ulcer. Korean J Gastrointest Endosc. 2007;34:312–319. [Google Scholar]
- 22.Choi MG, Park SH, Kim SK, et al. Phase III clinical trial of Revaprazan (Revanex(R)) for gastritis. Korean J Gastrointest Endosc. 2006;33:212–219. [Google Scholar]
- 23.Chung IS, Choi MG, Park SH, et al. Revaprazan (Revanex(R)), a novel acid pump antagonist, for duodenal ulcer: results of a double-blind, randomized, parallel, multi-center phase III clinical trial. Korean J Gastrointest Endosc. 2005;31:17–24. [Google Scholar]
- 24.Yu KS, Bae KS, Shon JH, et al. Pharmacokinetic and pharmacodynamic evaluation of a novel proton pump inhibitor, YH1885, in healthy volunteers. J Clin Pharmacol. 2004;44:73–82. doi: 10.1177/0091270003261321. [DOI] [PubMed] [Google Scholar]
- 25.Elso CM, Lu X, Culiat CT, et al. Heightened susceptibility to chronic gastritis, hyperplasia and metaplasia in Kcnq1 mutant mice. Hum Mol Genet. 2004;13:2813–2821. doi: 10.1093/hmg/ddh307. [DOI] [PubMed] [Google Scholar]
- 26.Roepke TK, Anantharam A, Kirchhoff P, et al. The KCNE2 potassium channel ancillary subunit is essential for gastric acid secretion. J Biol Chem. 2006;281:23740–23747. doi: 10.1074/jbc.M604155200. [DOI] [PubMed] [Google Scholar]
- 27.Franic TV, Judd LM, Robinson D, et al. Regulation of gastric epithelial cell development revealed in H(+)/K(+)-ATPase beta-subunit- and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1502–G1511. doi: 10.1152/ajpgi.2001.281.6.G1502. [DOI] [PubMed] [Google Scholar]
- 28.Masaoka T, Suzuki H, Hibi T. Gastric epithelial cell modality and proton pump inhibitor. J Clin Biochem Nutr. 2008;42:191–196. doi: 10.3164/jcbn.2008028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Driman DK, Wright C, Tougas G, Riddell RH. Omeprazole produces parietal cell hypertrophy and hyperplasia in humans. Dig Dis Sci. 1996;41:2039–2047. doi: 10.1007/BF02093608. [DOI] [PubMed] [Google Scholar]
- 30.Bajaj JS, Zadvornova Y, Heuman DM, et al. Association of proton pump inhibitor therapy with spontaneous bacterial peritonitis in cirrhotic patients with ascites. Am J Gastroenterol. 2009;104:1130–1134. doi: 10.1038/ajg.2009.80. [DOI] [PubMed] [Google Scholar]
- 31.Ito K, Kinoshita K, Tomizawa A, et al. The effect of subchronic administration of 7-(4-fluorobenzyloxy)-2,3-dimethyl-1-{[(1S,2S)-2-methylcyclopropyl]methyl}-1H-pyrrolo[2,3-d] pyridazine (CS-526), a novel acid pump antagonist, on gastric acid secretion and gastrin levels in rats. J Pharmacol Exp Ther. 2008;326:163–170. doi: 10.1124/jpet.108.137299. [DOI] [PubMed] [Google Scholar]