Abstract
Background/Aims
Obesity is associated with the risk of colorectal cancer. However, there is a lack of information about the relationship between obesity and colorectal adenoma. We investigated whether general and abdominal obesity are risk factors for colorectal adenoma.
Methods
Subjects who received health check-ups, including colonoscopy, from April 2006 to September 2007 in Chung-Ang University Hospital were included (n=1,316). The frequency and characteristics of colorectal adenomas were analyzed according to demographic features, past history, blood tests, body mass index, and components of metabolic syndrome. Abdominal obesity was defined as a waist circumference of ≥80 cm in women and ≥90 cm in men.
Results
The sex ratio of the subjects was 1.9:1 (male:female) and their age was 47.7±10.0 years (mean±SD). In univariate analysis, abdominal obesity was significantly associated with the frequency of colorectal adenoma (26.5% "yes" vs 16.9% "no"; p<0.001). The frequency of colorectal adenoma was significantly higher among males, older patients, current smokers, and subjects with fasting hyperglycemia (≥100 mg/dL) or fatty liver (p<0.05). Multivariate analysis identified that male sex (odds ratio [OR], 1.5; 95% confidence interval [CI], 1.0-2.2), old age (age ≥60 years; OR, 6.7; 95% CI, 3.5-12.5), and abdominal obesity (OR, 1.5; 95% CI, 1.0-2.2) were independent risk factors for colorectal adenoma (p<0.05). The frequency of multiple adenomas (more than two sites) was also significantly higher in subjects with abdominal obesity. However, the effect of abdominal obesity on the development of colorectal adenoma decreased in elderly people.
Conclusions
Abdominal obesity is an independent risk factor for colorectal adenoma and its multiplicity, especially in younger people in South Korea.
Keywords: Abdominal obesity, Colorectal adenoma
INTRODUCTION
Colorectal cancer (CRC) is one of the most prevalent cancers in developed nations and has increased rapidly in South Korea. For the prevention of colorectal cancer, the importance of colorectal adenoma identification and removal is abundantly clear.1,2 Comprehensive strategies that individualize screening based on risk stratification are used; for example, higher intensity regimens for those with family or personal history of colorectal adenomas or carcinoma.3 Screening effectiveness requires that at-risk groups may change as new risk factors are identified.
Over the past decade, it has been increasingly recognized that obesity is not only associated with the risk of cardiovascular and metabolic disease, but there are also previously unsuspected associations with gastrointestinal disease.4 Obesity and its related conditions (such as diabetes mellitus and hypertriglyceridemia) are related to increased CRC risk.5-7 Although a connection between obesity and colonic adenomas has long been postulated,8,9 further data is needed. Therefore, we investigated whether general or abdominal obesity and the components of metabolic syndrome are risk factors for colorectal adenomas.
MATERIALS AND METHODS
1. Subjects
We studied a consecutive series of subjects who received routine health examinations and underwent colonoscopy at the Health Promotion Center, Chung-Ang University Hospital in Seoul, South Korea, from April, 2006, through September, 2007. On the day of colonoscopy, anthropometric measurements were taken in each subject, and each answered a self administered questionnaire on demographics, socioeconomic and behavioral features, and medical history, and underwent a physical examination and blood tests. Smokers were defined as those who had ever smoked cigarettes daily for at least 1 year. Alcohol drinkers were defined as those having drunk alcoholic beverages at least once a week for at least 1 year. As for physical activity, subjects were asked about the frequency of participation in recreational exercises and sports on average in the past year using closed-ended question (≥1/week and <1/week).
We screened a total of 1,820 patients. We excluded subjects with missing anthropometric measurements (n=35), incomplete colonoscopies (n=22), a colonic examination within the previous 5 years (n=240), a history of colectomy (n=6) or colorectal polypectomy (n=125), a history of cancer (n=53), and those in whom cancer was detected during this study (n=23). There were no subjects who had history of chronic colorectal disease including inflammatory bowel disease, or were detected during colonoscopy. Thus, the study population consisted of 1,316 subjects. The study protocol was approved by the Institutional Review Board of Chung-Ang University Hospital.
2. Anthropometric measurements and laboratory tests
Anthropometric measurements were made by well-trained examiners on individuals wearing light clothing and without shoes. Height was measured to the nearest 0.1 cm and weight to the nearest 0.1 kg using Inbody 3.0 (Biospace, Seoul, Korea); Body mass index (BMI) was calculated by dividing weight (kg) by height squared (m2). Waist circumference was measured at the end of normal expiration to the nearest 0.1 cm, at the narrowest point between the lower border of the rib cage and the iliac crest. Blood pressure was measured using standard mercury sphygmomanometers in a sitting position after 10 minutes of rest, and mean systolic and diastolic values from two measurements were recorded. Following an 8-hour fast, venous blood was obtained from each subject, and fasting plasma glucose, total cholesterol, triglycerides (TG), high-density lipoprotein cholesterol (HDL-C) and low density lipoprotein cholesterol (LDL-C) were measured using an autoanalyzer (TBA-200FR; Toshiba, Tokyo, Japan).
Subjects were considered hypertensive if their systolic and/or diastolic pressure were ≥130/85 mm (a component of metabolic syndrome) or if they were receiving blood pressure lowering drugs; diabetes mellitus (DM) was classified as fasting glucose ≥126 mg/dL or if subjects were receiving hypoglycemic medication, where fasting hyperglycemia in non-diabetics is classified as fasting glucose ≥100 mg/dL.
3. Definition of obesity and components of metabolic syndrome
General obesity was classified as BMI ≥25 kg/m2 and abdominal obesity as waist circumference ≥80 cm in women and 90 cm in men using the World Health Organization (WHO) Asian obesity guidelines.10 Components of metabolic syndrome were determined using the International Diabetes Federation definition:11
Abdominal obesity defined as waist circumference ≥90 cm for men and ≥80 cm for women for Asia-Pacific people.10
Triglyceride level ≥150 mg/dL (1.7 mmol/L), or specific treatment for this lipid abnormality.
HDL cholesterol <40 mg/dL (1.03 mmol/L) in males and <50 mg/dL (1.29 mmol/L) in females, or specific treatment for this lipid abnormality.
Systolic BP ≥130 mm Hg or diastolic BP ≥85 mm Hg, or treatment of previously diagnosed hypertension.
Fasting plasma glucose ≥100 mg/dL (5.6 mmol/L), or previously diagnosed type 2 diabetes.
4. Colonoscopy
Following bowel preparation with 4 liters of polyethylene glycol-electrolyte oral lavage solution (Taejoon Pharm, Seoul, Korea), colonoscopy was performed on each subject by one of three experienced gastroenterologists using a video colonoscope (CF260L; Olympus, Tokyo, Japan). The entire colon was examined from the rectum to the cecum and all visualized lesions were biopsied and histologically assessed by experienced pathologists.
Location, size, number, and colonoscopic appearance of colorectal adenomas detected were recorded. The location of the colorectal adenoma was divided into the proximal colon, including the cecum, ascending colon, and transverse colon (at least one location for multiple adenomas), and the distal colon, including the splenic flexure, descending colon, sigmoid colon, and rectum. The size of each adenoma was estimated using open-biopsy forceps, 8 mm in diameter, and was classified into <5, 5 to 9, and ≥10 mm. The largest size was used for multiple adenomas. The number of adenomas was classified into 1 or ≥2 sites. Colonoscopic appearance was classified into sessile or pedunculated according to the presence or absence of a stalk. Advanced colorectal adenoma was defined as ≥10 mm in estimated diameter, containing >25% villous features, and/or high-grade dysplasia. Histologically confirmed colorectal adenoma was classified into tubular, tubulovillous, villous, and serrated adenoma. Hyperplastic polyps were excluded from the analysis.
5. Statistical analysis
The frequency and characteristics of colorectal adenomas were analyzed in relation to demographic features, medical history, obesity, the components of metabolic syndrome, and blood markers. SPSS version 13.0 (SPSS, Chicago, IL, USA) was used for the statistical analysis, and p<0.05 was accepted as significant; all tests were 2-sided. Categorical data were compared using Pearson's chi-squared test or Fisher's exact test. Multivariate logistic regression analysis was used to estimate the crude and adjusted strength of association between the colorectal adenoma and various factors. Each odds ratio (OR) is presented together with its 95% confidence interval (CI).
RESULTS
1. Subject characteristics
The sex ratio was 1.9:1 (864 men and 452 women) and the mean age was 47.7±10.0 years. The frequency of colorectal adenoma and advanced adenoma were 20.0% (263/1,316) and 2.7% (35/1,316), respectively. The frequency of general obesity and abdominal obesity were 30.9% (406/1,316), and 32.1% (423/1,316), respectively.
2. Risk factors for colorectal adenoma
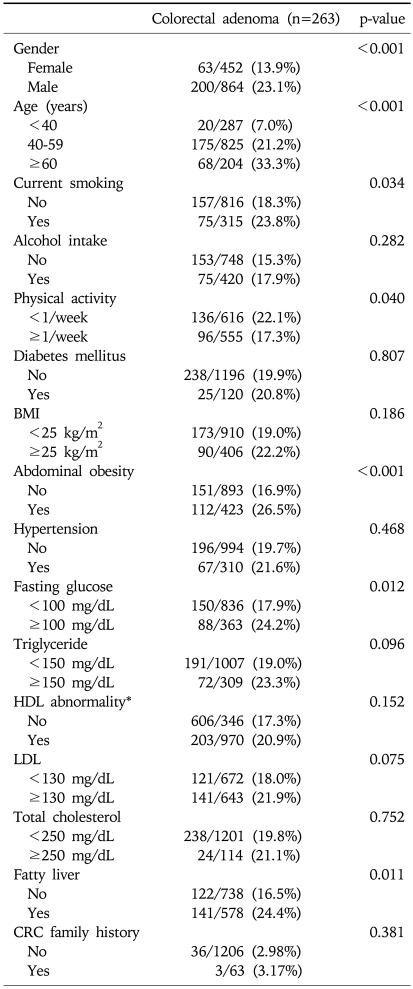
In univariate analysis, abdominal obesity was significantly associated with the frequency of colorectal adenoma (26.5% vs 16.9%, p<0.001). The frequency of colorectal adenoma was significantly higher in males, older patients, current smokers, physical activity (≥1/week), and subjects with fasting hyperglycemia (≥100 mg/dL) or fatty liver on abdominal ultrasonography (p<0.05) (Table 1).
Table 1.
Factors Related to the Presence of Colorectal Adenoma
BMI, body mass index; CRC, colorectal cancer.
*HDL abnormality: <40 mg/dL in male, <50 mg/dL in female.
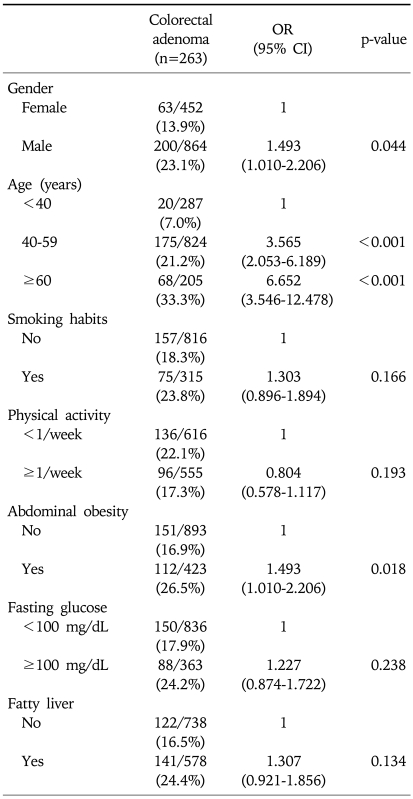
Multivariate regression analysis identified that male sex (OR 1.5; 95% CI, 1.0-2.2), old age (age≥60) (OR 6.7; 95% CI, 3.5-12.5), and abdominal obesity (OR 1.5; 95% CI, 1.0-2.2) were independent risk factors for colorectal adenoma (p<0.05) (Table 2), but current smoking, physical inactivity, fasting hyperglycemia, and fatty liver were not. Although male sex was an independent risk factor for colorectal adenoma, but the frequency was not different in subjects above 55 years old according to gender (34.3% in male vs 24.4% in female, p=0.062) and those were 34.8% in male and 30.6% in female above 60 years old (p=0.534). Also, the frequency of colorectal adenoma was not different according to abdominal obesity in subjects above 55 years old (35.4% yes vs 26.6% no, p=0.089) and those were 36.7% and 30.5% in subjects above 60 years old with abdominal obesity and without (p=0.377).
Table 2.
Multivariate Analysis for Factors Related to the Presence of Colorectal Adenoma
OR, odds ratio; CI, confidence interval.
3. Characteristics of colorectal adenoma according to abdominal obesity
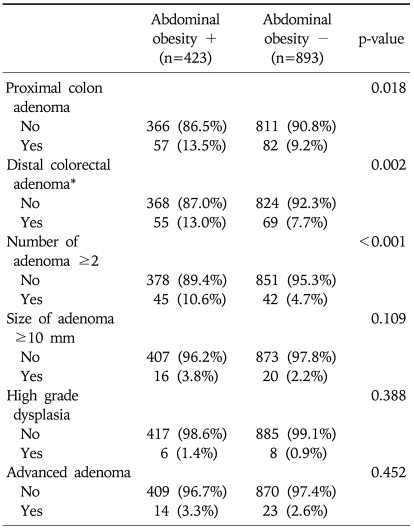
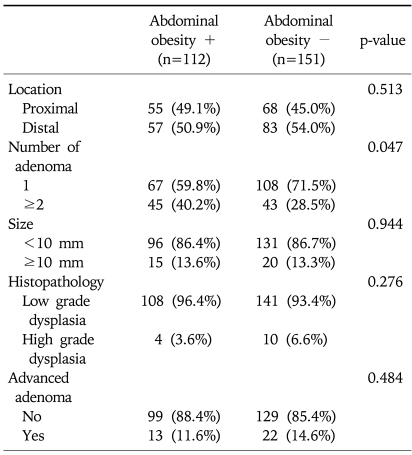
The characteristics of colorectal adenoma in relation to abdominal obesity in total subjects were shown in Table 3. The frequency of proximal or distal colon adenoma and multiple adenomas (≥2 sites) was significantly higher in subjects with abdominal obesity than the subjects without (p<0.001). And we studied the characteristics of colorectal adenoma in relation to abdominal obesity among subjects with colorectal adenoma. The analysis conducted according to location, number, size and histopathology of colorectal adenoma and being a case of advanced adenoma in the subjects with colorectal adenoma were shown in Table 4. The frequency of multiple adenomas (≥2 sites) was significantly higher in subjects with abdominal obesity (40.2% vs 28.5%, p=0.047), but other characteristics such as proximal adenoma, polyp size, histopathology and advanced adenoma were not.
Table 3.
Characteristics of Colorectal Adenoma in Relation to Abdominal Obesity in All Subjects
OR, odds ratio; CI, confidence interval.
*Subjects who had only distal colorectal adenoma without proximal adenoma.
Table 4.
Characteristics of Colorectal Adenoma in Relation to Abdominal Obesity among Subjects with Colorectal Adenoma
DISCUSSION
Colorectal adenoma is a precancerous lesion of colon cancer. According to the adenoma-carcinoma sequence hypothesis, more than 95% of colon cancer originates from colorectal adenoma.12,13 Consequently, primary prevention of colon cancer requires understanding the risk factors for colorectal adenoma. Dietary habits, especially a high fat and/or low fiber diet, alcohol consumption, cigarette smoking and low physical activity may play an important etiological role in CRC.14-16 Obesity and its related conditions can also increase CRC risk.5-7 However, little work has been done on the relationship between abdominal obesity and colorectal adenomas and its characteristics.
Our study showed that general obesity was not related to colorectal adenoma, but abdominal obesity was an independent risk factor for colorectal adenoma and the frequency of multiple adenoma (≥2 sites). Colorectal adenoma was more common in the elderly and in men, as in previous studies.17,18 However, we could observe that gender difference in the frequency of colorectal adenoma was disappeared as getting older. As estrogen has known to be a preventive factor of colorectal cancer,19,20 we can suspect that estrogen or progesteron depletion in elderly women is related to the increase of colorectal adenoma. Also, the influence of abdominal obesity on the frequency of colorectal adenoma decreased in subjects above 55 years old. There is no clear explanation for this result, but it can be thought that since the aging process is more potent factor for developing colorectal adenoma, it masks the effect of abdominal obesity. These data need to be validated by further study.
Abdominal obesity increases CRC risk more than BMI, a marker of general obesity, even independent of BMI. In a Japanese report, a high waist-to-hip ratio was associated with an increased risk of colon adenoma in men.21 In addition, Giovannucci et al. reported that waist circumference and waist-to-hip ratio were strong risk factors of large colon adenoma,17 and another study showed similar findings.22 Recently, some reports showed that distribution of adenomatous polyps shift to proximal colon23 and increased BMI was associated with increased incidence of proximal colon cancer but not distal colon cancer.7 However, in our data, the frequency of distal colorectal adenoma as well as proximal adenoma was significantly higher in subjects with abdominal obesity. Abdominal obesity was related only with the multiplicity in the characteristics of colorectal adenoma. Several mechanisms can explain why abdominal obesity is a CRC risk factor. Abdominal obesity reflects visceral fat deposition, which is associated with insulin resistance and higher circulating levels of insulin growth factor-1 (IGF-1). IGF-1 stimulates the proliferation of the colonic epithelium, and high affinity IGF-1 receptors were present in human colon cancer cell lines and in resected human colon cancer tissues. Insulin and IGF-1 are major determinants of both proliferation and apoptosis; thus, they may influence carcinogenesis by increasing cell proliferation and reducing apoptosis.24-27 Furthermore, the increased production of pro-inflammatory cytokines and decreased production of anti-inflammatory adiponectin in adipocytes may be relevant to the growth of adenomas.28
Metabolic syndrome, the cluster of metabolic abnormalities linked to insulin resistance, raises the risk of cardiovascular disease and several carcinomas. According to the recent studies, metabolic syndrome is significantly associated with colorectal adenoma as well.18,29 Serum insulin levels, impaired glucose tolerance (IGT), and type 2 DM are associated with increased risk of colorectal adenomas.10,30,31 However, we did not find differences in the frequency of colorectal adenoma with metabolic syndrome (International Diabetes Federation definition11) or IGT. These differences could result from incomplete medication history or recall bias on the patient-completed questionnaire. Further studies on populations with more accurate medical history may be warranted.
This study has several limitations. First, our study was not a cohort study but a cross-sectional study that precluded a determination of causality between obesity and colorectal adenoma. Second, although we showed that abdominal obesity was associated with colorectal adenoma, we did not estimate accurate visceral adipose tissue in the abdomen using abdominal CT scanning or the mechanism by which abdominal obesity could influence the development of colorectal adenoma. Further studies directly investigating serum insulin, IGF-1, or adipose tissue-derived peptide hormone, adiponectin may reveal more clearly the underlying mechanism by which visceral obesity contributes to the development of colorectal adenoma. Third, there might be a selection bias in the study population. Subjects in this study were recruited from individuals who visited the hospital for regular health examinations and underwent colonoscopy, and thus, they were more concerned about their health and could be of a higher socioeconomic status than the general population, with better control over glucose or hypertension.
In conclusion, our data suggest that the frequency of colorectal adenoma was significantly associated with abdominal obesity as well as age and gender. Moreover, individuals with abdominal obesity are more likely to have multiple adenomas. However, the effect of abdominal obesity on colorectal adenoma development decreases in elderly people. Further studies are needed to clarify the role of abdominal obesity in the development of colorectal adenoma.
References
- 1.Jackman RJ, Mayo CW. The adenoma-carcinoma sequence in cancer of the colon. Surg Gynecol Obstet. 1951;93:327–330. [PubMed] [Google Scholar]
- 2.Rider JA, Kirsner JB, Moeller HC, Palmer WL. Polyps of the colon and rectum; their incidence and relationship to carcinoma. Am J Med. 1954;16:555–564. doi: 10.1016/0002-9343(54)90372-1. [DOI] [PubMed] [Google Scholar]
- 3.Ramsey SD, Yoon P, Moonesinghe R, Khoury MJ. Population-based study of the prevalence of family history of cancer: implications for cancer screening and prevention. Genet Med. 2006;8:571–575. doi: 10.1097/01.gim.0000237867.34011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frezza EE, Wachtel MS, Chiriva-Internati M. Influence of obesity on the risk of developing colon cancer. Gut. 2006;55:285–291. doi: 10.1136/gut.2005.073163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu FB, Manson JE, Liu S, et al. Prospective study of adult onset diabetes mellitus (type 2) and risk of colorectal cancer in women. J Natl Cancer Inst. 1999;91:542–547. doi: 10.1093/jnci/91.6.542. [DOI] [PubMed] [Google Scholar]
- 6.Yamada K, Araki S, Tamura M, et al. Relation of serum total cholesterol, serum triglycerides and fasting plasma glucose to colorectal carcinoma in situ. Int J Epidemiol. 1998;27:794–798. doi: 10.1093/ije/27.5.794. [DOI] [PubMed] [Google Scholar]
- 7.Lin J, Zhang SM, Cook NR, Rexrode KM, Lee IM, Buring JE. Body mass index and risk of colorectal cancer in women (United States) Cancer Causes Control. 2004;15:581–589. doi: 10.1023/B:CACO.0000036168.23351.f1. [DOI] [PubMed] [Google Scholar]
- 8.Todoroki I, Kono S, Shinchi K, et al. Relationship of cigarette smoking, alcohol use, and dietary habits with sigmoid colon adenomas. Ann Epidemiol. 1995;5:478–483. doi: 10.1016/1047-2797(95)00064-x. [DOI] [PubMed] [Google Scholar]
- 9.Lupton JR, Turner ND. Potential protective mechanisms of wheat bran fiber. Am J Med. 1999;106:24S–27S. doi: 10.1016/s0002-9343(98)00343-x. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida I, Suzuki A, Vallee M, et al. Serum insulin levels and the prevalence of adenomatous and hyperplastic polyps in the proximal colon. Clin Gastroenterol Hepatol. 2006;4:1225–1231. doi: 10.1016/j.cgh.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome: a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 12.Waye JD. Colon polyps: problems, promises, prospects. Am J Gastroenterol. 1986;81:101–103. [PubMed] [Google Scholar]
- 13.Hill MJ, Morson BC, Bussey HJ. Aetiology of adenoma: carcinoma sequence in large bowel. Lancet. 1978;1:245–247. doi: 10.1016/s0140-6736(78)90487-7. [DOI] [PubMed] [Google Scholar]
- 14.Nilsen TI, Vatten LJ. Prospective study of colorectal cancer risk and physical activity, diabetes, blood glucose and BMI: exploring the hyperinsulinaemia hypothesis. Br J Cancer. 2001;84:417–422. doi: 10.1054/bjoc.2000.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho E, Smith-Warner SA, Ritz J, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohort studies. Ann Intern Med. 2004;140:603–613. doi: 10.7326/0003-4819-140-8-200404200-00007. [DOI] [PubMed] [Google Scholar]
- 16.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300:2765–2778. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 17.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Lim YJ, Kim YH, et al. Is metabolic syndrome a risk factor for colorectal adenoma? Cancer Epidemiol Biomarkers Prev. 2007;16:1543–1546. doi: 10.1158/1055-9965.EPI-07-0199. [DOI] [PubMed] [Google Scholar]
- 19.Clendenen TV, Koenig KL, Shore RE, Levitz M, Arslan AA, Zeleniuch-Jacquotte A. Postmenopausal levels of endogenous sex hormones and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:275–281. doi: 10.1158/1055-9965.EPI-08-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunter MJ, Hoover DR, Yu H, et al. Insulin, insulin-like growth factor-I, endogenous estradiol, and risk of colorectal cancer in postmenopausal women. Cancer Res. 2008;68:329–337. doi: 10.1158/0008-5472.CAN-07-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kono S, Handa K, Hayabuchi H, et al. Obesity, weight gain and risk of colon adenomas in Japanese men. Jpn J Cancer Res. 1999;90:805–811. doi: 10.1111/j.1349-7006.1999.tb00819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morimoto LM, Newcomb PA, Ulrich CM, Bostick RM, Lais CJ, Potter JD. Risk factors for hyperplastic and adenomatous polyps: evidence for malignant potential? Cancer Epidemiol Biomarkers Prev. 2002;11:1012–1018. [PubMed] [Google Scholar]
- 23.Park SY, Kim BC, Shin SJ, Lee SK, Kim TI, Kim WH. Proximal shift in the distribution of adenomatous polyps in Korea over the past ten years. Hepatogastroenterology. 2009;56:677–681. [PubMed] [Google Scholar]
- 24.Watkins LF, Lewis LR, Levine AE. Characterization of the synergistic effect of insulin and transferrin and the regulation of their receptors on a human colon carcinoma cell line. Int J Cancer. 1990;45:372–375. doi: 10.1002/ijc.2910450227. [DOI] [PubMed] [Google Scholar]
- 25.Koenuma M, Yamori T, Tsuruo T. Insulin and insulin-like growth factor 1 stimulate proliferation of metastatic variants of colon carcinoma 26. Jpn J Cancer Res. 1989;80:51–58. doi: 10.1111/j.1349-7006.1989.tb02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaaks R, Toniolo P, Akhmedkhanov A, et al. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst. 2000;92:1592–1600. doi: 10.1093/jnci/92.19.1592. [DOI] [PubMed] [Google Scholar]
- 27.Schoen RE, Weissfeld JL, Kuller LH, et al. Insulin-like growth factor-I and insulin are associated with the presence and advancement of adenomatous polyps. Gastroenterology. 2005;129:464–475. doi: 10.1016/j.gastro.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 28.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 29.Morita T, Tabata S, Mineshita M, Mizoue T, Moore MA, Kono S. The metabolic syndrome is associated with increased risk of colorectal adenoma development: the Self-Defense Forces health study. Asian Pac J Cancer Prev. 2005;6:485–489. [PubMed] [Google Scholar]
- 30.Marugame T, Lee K, Eguchi H, Oda T, Shinchi K, Kono S. Relation of impaired glucose tolerance and diabetes mellitus to colorectal adenomas in Japan. Cancer Causes Control. 2002;13:917–921. doi: 10.1023/a:1021967301138. [DOI] [PubMed] [Google Scholar]
- 31.Nishii T, Kono S, Abe H, et al. Glucose intolerance, plasma insulin levels, and colon adenomas in Japanese men. Jpn J Cancer Res. 2001;92:836–840. doi: 10.1111/j.1349-7006.2001.tb01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]