Abstract
Hilar cholangiocarcinomas are often treated with liver resections. Hepatic dysfunction and infection are common postoperative complications. Although secondary bacterial peritonitis due to abdominal abscess or perforation is common, we report herein the first case of spontaneous bacterial peritonitis after hepatic resection. A 61-year-old male patient without underlying liver disease was diagnosed as having a Klatskin tumor, and a right trisectionectomy with caudate lobectomy was performed. From postoperative days 18-28, the patient gained 4.1 kg as ascites developed, and showed evidence of hepatic insufficiency with prolonged prothrombin time and jaundice. Computed tomography, performed at postoperative day 28 when fever had developed, showed only ascites without bowel perforation or abscess. When paracentesis was performed, the serum-ascites albumin gradient was 2.3 g/dL, indicating portal hypertension, and the ascites' polymorphonuclear cell count was 1,156/mm3. Since the clinical, laboratory, and image findings were compatible with spontaneous bacterial peritonitis, we started empirical antibiotics without additional intervention. Follow-up analysis of the ascites after 48 hours revealed that the polymorphonuclear cell count had decreased markedly to 108/mm3; the fever and leukocytosis had also improved. After 2 weeks of antibiotic treatment, the patient recovered well, and was discharged without any problem.
Keywords: Spontaneous bacterial peritonitis, Hepatic resection, Cholangiocarcinoma
INTRODUCTION
Increasing numbers of hilar cholangiocarcinomas are being treated with liver resections, and various types of hepatobiliary resections have been tried.1-3 Liver resection is still accompanied by a certain risk of postoperative complications and the overall incidence of complication is significantly increasing towards extended hepatic resection.4,5 Reduction of functioning liver mass following hepatic resection causes hepatic dysfunction. And increased out flow resistance due to the reduction in the capacity of hepatic vascular bed causes ascites, or rarely hepatic encephalopathy presenting portal hypertension. Along with hepatic dysfunction and portal hypertension, infection is also common condition necessitating prolonged treatment and hospital stay following liver resection.4,6-8
Although the secondary bacterial peritonitis due to abdominal abscess is a common consequence of infection after hepatectomy,4 the spontaneous bacterial peritonitis (SBP) after hepatic resection has not been reported yet in a patient without underlying chronic liver disease or liver cirrhosis. As we experienced the first case of SBP after hepatic resection in a patient without evidence of liver cirrhosis and the patient recovered well after the use of antibiotics, we report this case.
CASE REPORT
A 61-year old male patient was admitted Seoul National University Hospital with a complaint of poor oral intake and weight loss of 10 kg over the last one year. There was no other significant past medical history including diabetes mellitus, hypertension, thyroid disease, and he had been on no medication which can induce liver function abnormalities. He had smoked one pack of cigarettes a day for 35 years until the date of admission, but denied any alcohol consumption. His father died of colon cancer and his brother died of liver cirrhosis. His blood pressure was 113/68 mmHg with a regular pulse rate of 68 beats/min. The body temperature was 36.0℃. The height was 176.9 cm, and the body weight was 73.3 kg. When calculated based on these two values, body mass index was in the normal range of 23.4 kg/m2. On physical examination, the patient appeared to be chronic ill-looking and he had mildly icteric sclera, but other physical findings were normal.
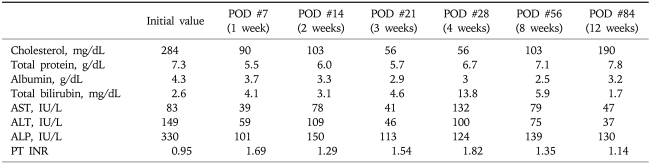
In the course of the evaluation, laboratory values were as follows: cholesterol 284 mg/dL, total protein 7.3 g/dL, albumin 4.3 g/dL, total bilirubin 2.6 mg/dL, direct bilirubin 2.0 mg/dL, aspartate aminotransferase (AST) 83 IU/L, alanine transaminase (ALT) 149 IU/L, alkaline phosphatase (ALP) 330 IU/L, and white blood cells (WBC) 4,900/mm3 (neutrophil 44.7%, lymphocyte 43.1%), hemoglobin 15.3 g/dL, platelet 283,000/mm3, prothrombin time INR 0.95. Tumor markers of carcinoembryonic antigen and cancer antigen 19-9 were 2.7 ng/mL and 4.3 U/mL, respectively. The hepatitis serologic profile revealed neither of hepatitis B virus surface antigen nor anti-hepatitis C virus antibody positive.
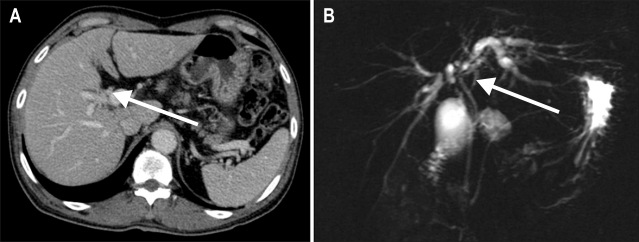
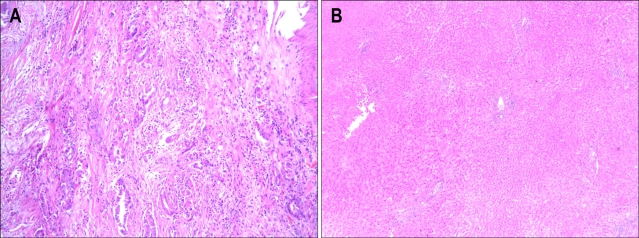
Abdominal ultrasound sonography (US) showed mildly dilated intrahepatic bile ducts, whereas, splenomegaly or ascites which suggests the presence of portal hypertension was not observed. Subsequent contrast-enhanced computed tomography (CT) (Fig. 1A) and magnetic resonance cholangiography (Fig. 1B) demonstrated hilar mass with partial obstruction of intrahepatic bile duct, so he was diagnosed as Klatskin tumor, Bisthmus type IIIa growth.2 As a curative purpose, right trisectionectomy with caudate lobectomy of the liver was done without acute complication. During the operation, vital sign was stable and estimated blood loss was 250 mL. Gross operative findings showed smooth surfaced liver with light greenish tint. Histological analysis of the tumor revealed adenocarcinoma (Fig. 2A) and that of non-tumorous portion showed only mild cholestasis without any evidence of regenerating nodules or fibrosis (Fig. 2B). The postoperative stage was stage IIIc (T3N1M0) Klatskin tumor according to the American Joint Committee on Cancer staging system.9
Fig. 1.
Imaging findings. (A) Computed tomography (CT) scan demonstrating a focally enhanced wall thickening of the hilar duct (arrow) progressing to the right second confluence and separating both intrahepatic bile ducts. There were no enlarged lymphatic nodes or vascular invasion. (B) Magnetic resonance cholangiography showing a Klatskin tumor with Bisthmus type IIIa growth, demonstrating more detailed bile duct anatomy than in the CT scan.
Fig. 2.
Microscopic appearance of the tumor and surrounding liver. (A) The pathologic findings of the tumorous portion of the liver show nuclear atypism, abnormal glandular structures, and dense cellular fibrous stroma (H&E stain, ×100). (B) The histology findings of the specimen from the nontumorous portion of the liver shows normal liver architecture without any evidence of cirrhotic nodules (H&E stain, ×40).
From the 2nd day after hepatic resection, the patient started diet and parenteral nutrition was discontinued. Glimepride and metformin was prescribed as diabetes mellitus was diagnosed during the evaluation of the Klatskin tumor. The amount of two abdominal Jakson-Pratt drainages on both sides of the abdomen was decreased from about 1,000 mL/day to less than 100 mL/day at postoperation 9 day. So, two abdominal drainages were removed at postoperation 10 day. There was no change in body weight and abdominal girth right after the removal of drainages, and patient recovered well. Table 1 shows the laboratory profile of serum biochemistry in this period.
Table 1.
Laboratory Findings of the Patient
POD, postoperation day; AST, aspartate aminotransferase; ALT, alanine transaminase; ALP, alkaline phosphatase; PT, prothrombin time.
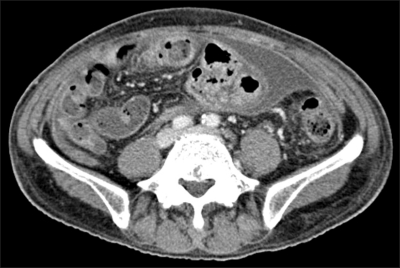
However, from the 18th day to the 28th day after hepatic resection, abdominal girth and body weight increased slowly from 89.5 cm to 94.5 cm and from 72.0 kg to 76.1 kg, respectively. The patient complained of abdominal distension, and shifting dullness was obvious on physical examination. In association with newly appeared ascites, laboratory findings showed the evidence of hepatic decompensation reflected by decreased serum albumin level 3.0 g/dL, increased total serum bilirubin level 13.8 mg/dL and alkaline phosphatase level 132 IU/L, and prolonged prothrombin time INR 1.82. Serum AST and ALT levels were 100 IU/L and 124 IU/L, respectively. CT scan, which was taken at the 28th day after operation when fever of 37.8℃ occurred, showed newly developed ascites but neither evidence of abscess formation, fistula, nor bowel perforation (Fig. 3). Ascitic fluid chemistry showed total protein 2.3 g/dL, albumin 0.7 g/dL, glucose 125 mg/dL, lactate dehydrogenase (LDH) 257 U/mL. As serum albumin was 3.0 g/dL at that time, serum-ascites albumin gradient (SAAG) was 2.3 g/dL. The cell count of ascitic fluid demonstrated leukocyte count 1,584/mm3 (polymorphonuclear cell [PMN] fraction, 73%; calculated PMN count, 1,156/mm3), and red blood cell count 3,312/mm3. Two consecutive cytology tests of ascites were negative for malignant cells. As clinical, laboratory and image findings were compatible with SBP, we started empirical antibiotics of cefotaxime 2 g every 8 hours. Follow-up analysis of ascites at 48 hours after the initiation of antibiotics treatment, showed markedly decreased PMN count of 108/mm3 in ascitic fluid. Total protein, glucose and LDH also decreased to the level of 1.6 g/dL, 55 mg/dL and 126 U/mL, respectively. After administration of cefotaxime for 3 days, fever decreased, and total WBC count of 17,220/mm3 (neutrophil 86.0%, lymphocyte 6.0%) in blood decreased to the level of 7,880/mm3 (neutrophil 77.1%, lymphocyte 16.2%). C-reactive protein also decreased from 8.37 mg/L to 4.35 mg/L. Four days later, ascites culture study reported the growth of methicillin resistant Staphylococcus aureus (MRSA) which has resistance to cefotaxime. However, we considered MRSA as an innocuous bystander or contaminant since clinical symptoms and laboratory findings were markedly improved by cefotaxime treatment alone. We, therefore, maintained cefotaxime for two weeks. When administration of cefotaxime for two weeks was completed, ascites completely disappeared and maintenance diuretics therapy was not any longer needed. Biochemical liver function tests and prothrombin time also gradually improved.
Fig. 3.
Postoperative CT scan showing peritoneal enhancement and ascites, implying peritonitis.
Finally, the patient recovered well, and was discharged without any residual problem at the 8th week after operation, in other word, four weeks after antibiotics treatment. Laboratory values were as follows: cholesterol 103 mg/dL, total protein 7.1 g/dL, albumin 2.5 g/dL, total bilirubin 5.9 mg/dL, alkaline phosphatase 139 IU/L, AST 79 IU/L, ALT 75 IU/L. When the patient visited the hospital, 12 weeks after hepatic resection, prothrombin time was INR 1.14, and values of liver function test were as follows: cholesterol 190 mg/dL, total protein 7.8 g/dL, albumin 3.2 g/dL, total bilirubin 1.7 mg/dL, alkaline phosphatase 130 IU/L, AST 47 IU/L, ALT 37 IU/L (Table 1).
DISCUSSION
SBP is characterized by the spontaneous infection of ascitic fluid in the absence of intraabdominal source of infection such as viscus perforation or intraabdominal abscess.10 Although SBP occurs most commonly in conjunction with cirrhosis of the liver and ascites, it is sometimes reported in patients with ascites from other causes such as nephrotic syndrome, systemic lupus erythematosus, or malignancy.11 In patients with liver cirrhosis, the presence of portal hypertension and splanchnic vasodilation contribute to the development of ascites through activation of renin-angiotensin-aldosterone system, and SBP is a common and severe complication of ascites. Although the pathogenesis of SBP has not been established definitively, it is believed to be caused by the translocation of bacteria from the intestinal lumen to lymph nodes. And subsequent hematogenous spread of organisms occurs as altered portal circulation result in a defect in the usual filtration function in patients with liver cirrhosis.12,13 Organisms multiply in ascites only when ascites provides a good medium for growth. The proteins of the complement cascade have been found in peritoneal fluid, with lower levels in cirrhotic patients than in patients with ascites of other etiologies. Because the opsonic and phagocytic properties of PMNs are diminished in patients with severe liver disease who have lower protein content, SBP is more common with advanced liver disease.12
Herein, we report a case of the development of SBP after extensive hepatic resection in a patient without underlying chronic liver disease or liver cirrhosis. To our best knowledge, it is the first case report. In this case, the patient was diagnosed as SBP by clinical, radiological and laboratory findings. To find the cause of postoperative ascites in this patient, we calculated SAAG, first, which turned out to be greater than 1.1 g/dL, because ascites is pathophysiologically classified according to the SAAG. SAAG greater than 1.1 g/dL in this patient indicated that his ascites developed secondary to portal hypertension with positive likelihood ratio of 4.6.13-15 As this patient had no underlying chronic liver disease, heart disease or nephrotic syndrome, and image findings (CT scan and US) revealed no outflow obstruction or vascular abnormality, we could suppose that ascites in this patient might be caused by newly developed portal hypertension after extensive hepatic resection.15 Ascites cell count of PMN >250/mm3 in this patient implied peritonitis. CT scan showed no evidence of the secondary peritonitis due to bowel perforation or abscess in the peritoneal cavity. Moreover, the patient successfully recovered from peritonitis with antibiotics alone without adjuvant surgical and radiological intervention. With 2 days use of antibiotics only, the ascites neutrophil count decreased dramatically from 1,156/mm3 to 108/mm3. The exponential decline in neutrophil count after antibiotics treatment alone, together with the clinical, laboratory, image findings collectively indicate that the peritonitis that the patient suffered was SBP associated with transient portal hypertension following extensive hepatic resection.10,16
Infection following liver resection is a very common complication, necessitating prolonged treatment and hospital stay.4,7,8,17,18 Liver is the largest reserve of fixed tissue macrophages in the body (Kupffer cells) and regulates the synthesis of hepatic proteins responsible for recognition and opsonization of pathogens. Significant loss of Kupffer cells and decreased synthesis of hepatic proteins involved in immune system following major liver resection are considered to be responsible for the impaired innate immune function. As a result, decreased liver function after liver resection renders the patient more susceptible to infection.18 The most prevalent postoperative infections were lung infection (11.9%) and abdominal abscess with or without biliary fistula (7.1%).7 However, any case of SBP has not been reported yet, especially in the patient without underlying liver cirrhosis or significant fibrosis.
In addition to the increased risk of infection, hepatic insufficiency and ascites can be followed in variable rates from 0.5% to 5.5% after extensive hepatic resection.4 Jarnagin et al.4 reported posthepatectomy hepatic insufficiency defined as prolonged hyperbilirubinemia unrelated to biliary obstruction or leakage, clinically apparent ascites, prolonged prothrombin time, and/or hepatic encephalopathy, in 5.5% of 1,803 patients. Nuzzo et al.19 also reported case series including 11 cases of transient hepatic insufficiency and 11 cases of moderate to severe hepatic decompensation after hepatic resection. We can infer the pathogenesis of post-hepatectomy hepatic insufficiency and ascites from a similar condition called small-for-size graft syndrome (SFSG), which indicates the occurrence of prolonged cholestasis and huge ascites after living donor liver transplantation with small graft. The occurrence of hepatic insufficiency and ascites in SFSG is caused by excessive portal inflow, small functional liver mass, and outflow congestion. Small functioning liver mass and portal hypertension is also the main cause of hepatic decompensation and ascites in patients with liver cirrhosis. So, several studies reported that hepatic insufficiency with ascites following liver resection is more common in patients with underlying liver cirrhosis.20-22 Although, some other factors such as age, graft hemodynamics, recipient model for end-stage liver disease score may be related to the SFSG, the main cause is reduction of functioning liver mass, which is the same in this case.23,24 To suppose small functioning liver mass as a major cause of hepatic insufficiency, other conditions affecting post-operative liver function abnormality should be excluded. And in this patient, we did not observe the clinical evidence of other conditions which can affect post-operative liver function abnormality such as total parenteral nutrition, ischemic event during operation, bile duct injury, etc.
We could diagnose this patient as SBP by clinical, laboratory, and image findings as we mentioned above. Strictly speaking, this patient fits the criteria of culture negative neutrocytic ascites, a variant form of SBP, since MRSA cultured from ascites was rather considered as contaminants than an infective organism. Culture negative neutrocytic ascites also has similar clinical, prognostic and therapeutic characteristics to those of SBP.25 Usually, SBP is treated with a third-generation cephalosporin, such as cefotaxime.10,26 This patient was also treated with cefotaxime alone, and recovered well, suggesting that our patient suffered from SBP, not from secondary bacterial peritonitis. In addition to the findings previously mentioned, total protein, LDH, and glucose levels in ascitic fluid also aid in the differentiation between SBP and secondary bacterial peritonitis, though high total protein and LDH level made it somewhat hard to differentiate spontaneous and secondary bacterial peritonitis in this case. However, these laboratory findings are not essential in differentiation of spontaneous and secondary bacterial peritonitis.27 Therefore, diagnosis of SBP in our patient was established.
SBP occurs frequently in the patients with liver cirrhosis at the rate of 10-30% according to the severity of liver cirrhosis.10 Since extensive hepatic resection result in transient hepatic decompensation and ascites due to portal hypertension, SBP might occur in these circumstances. However, the SBP following hepatic resection has not been reported yet, and it has not been concerned how frequently SBP occurs after liver resection. As patients frequently reveal postoperative ascites, and are more susceptible to infection after liver resection, it is rather strange that SBP has not been frequently observed yet after hepatic resection. Perhaps, one plausible explanation might be that many cases of SBP after hepatectomy were regarded as secondary bacterial peritonitis, and then treated with antibiotics, resulting in improvement. So, the incidence rate of SBP after hepatectomy might have been clinically underestimated. Another possible explanation is that SBP is actually rare in patients who underwent liver resection. In cases with underlying normal liver, remnant normal functioning liver parenchyma might regenerate soon enough to compensate for removed liver after hepatic resection within a couple of weeks, except for patients with underlying liver cirrhosis. However, there is no evidence supporting this hypothesis and it remains to be investigated why SBP after extensive hepatic resection has not been reported thus far in patients with the underlying normal liver.
On the basis of this first case report, we suggest that SBP should be included in the differential diagnosis of peritonitis encountered in patients after extensive hepatic resection, even in patients with the underlying normal liver.
References
- 1.Nagino M, Kamiya J, Arai T, Nishio H, Ebata T, Nimura Y. "Anatomic" right hepatic trisectionectomy (extended right hepatectomy) with caudate lobectomy for hilar cholangiocarcinoma. Ann Surg. 2006;243:28–32. doi: 10.1097/01.sla.0000193604.72436.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215:31–38. doi: 10.1097/00000658-199201000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyagawa S, Makuuchi M, Kawasaki S. Outcome of extended right hepatectomy after biliary drainage in hilar bile duct cancer. Arch Surg. 1995;130:759–763. doi: 10.1001/archsurg.1995.01430070081016. [DOI] [PubMed] [Google Scholar]
- 4.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shirabe K, Shimada M, Gion T, et al. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188:304–309. doi: 10.1016/s1072-7515(98)00301-9. [DOI] [PubMed] [Google Scholar]
- 6.Farges O, Belghiti J, Kianmanesh R, et al. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208–217. doi: 10.1097/01.SLA.0000048447.16651.7B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 8.Melendez J, Ferri E, Zwillman M, et al. Extended hepatic resection: a 6-year retrospective study of risk factors for perioperative mortality. J Am Coll Surg. 2001;192:47–53. doi: 10.1016/s1072-7515(00)00745-6. [DOI] [PubMed] [Google Scholar]
- 9.Greene FL, Page DL, Fleming ID, et al. AJCC cancer staging manual. 6th ed. New York: Springer-Verlag; 2002. [Google Scholar]
- 10.Gines P, Cardenas A, Arroyo V, Rodes J. Management of cirrhosis and ascites. N Engl J Med. 2004;350:1646–1654. doi: 10.1056/NEJMra035021. [DOI] [PubMed] [Google Scholar]
- 11.Bac DV, de Marie S, van Blankenstein M. Spontaneous bacterial peritonitis complicating malignancy-related ascites. Dig Dis Sci. 1996;41:131–132. doi: 10.1007/BF02208594. [DOI] [PubMed] [Google Scholar]
- 12.Such J, Runyon BA. Spontaneous bacterial peritonitis. Clin Infect Dis. 1998;27:669–674. doi: 10.1086/514940. [DOI] [PubMed] [Google Scholar]
- 13.Anthony SF, Eugene B, Dennis LK, et al. Harrison's Principles of internal medicine. 17th ed. Volume 1. New York: McGraw Hill; 1979. pp. 808–809. [Google Scholar]
- 14.Wong CL, Holroyd-Leduc J, Thorpe KE, Straus SE. Does this patient have bacterial peritonitis or portal hypertension? How do I perform a paracentesis and analyze the results? JAMA. 2008;299:1166–1178. doi: 10.1001/jama.299.10.1166. [DOI] [PubMed] [Google Scholar]
- 15.Chung RT, Iafrate AJ, Amrein PC, Sahani DV, Misdraji J. Case records of the Massachusetts General Hospital: case 15-2006. A 46-year-old woman with sudden onset of abdominal distention. N Engl J Med. 2006;354:2166–2175. doi: 10.1056/NEJMcpc069006. [DOI] [PubMed] [Google Scholar]
- 16.Runyon BA, Hoefs JC. Spontaneous vs secondary bacterial peritonitis: differentiation by response of ascitic fluid neutrophil count to antimicrobial therapy. Arch Intern Med. 1986;146:1563–1565. doi: 10.1001/archinte.146.8.1563. [DOI] [PubMed] [Google Scholar]
- 17.Wigmore SJ, Madhavan K, Currie EJ, Bartolo DC, Garden OJ. Does the subspecialty of the surgeon performing primary colonic resection influence the outcome of patients with hepatic metastases referred for resection? Ann Surg. 1999;230:759–765. doi: 10.1097/00000658-199912000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289–296. doi: 10.1136/gut.2004.046524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nuzzo G, Giuliante F, Giovannini I, Vellone M, De Cosmo G, Capelli G. Liver resections with or without pedicle clamping. Am J Surg. 2001;181:238–246. doi: 10.1016/s0002-9610(01)00555-4. [DOI] [PubMed] [Google Scholar]
- 20.Midorikawa Y, Kubota K, Takayama T, et al. A comparative study of postoperative complications after hepatectomy in patients with and without chronic liver disease. Surgery. 1999;126:484–491. [PubMed] [Google Scholar]
- 21.Cunningham JD, Fong Y, Shriver C, Melendez J, Marx WL, Blumgart LH. One hundred consecutive hepatic resections: blood loss, transfusion, and operative technique. Arch Surg. 1994;129:1050–1056. doi: 10.1001/archsurg.1994.01420340064011. [DOI] [PubMed] [Google Scholar]
- 22.Sitzmann JV, Greene PS. Perioperative predictors of morbidity following hepatic resection for neoplasm: a multivariate analysis of a single surgeon experience with 105 patients. Ann Surg. 1994;219:13–17. doi: 10.1097/00000658-199401000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imura S, Shimada M, Ikegami T, Morine Y, Kanemura H. Strategies for improving the outcomes of small-for-size grafts in adult-to-adult living-donor liver transplantation. J Hepatobiliary Pancreat Surg. 2008;15:102–110. doi: 10.1007/s00534-007-1297-3. [DOI] [PubMed] [Google Scholar]
- 24.Paik KY, Choi DW, Chung JC, Kang KT, Kim SB. Improved survival following right trisectionectomy with caudate lobectomy without operative mortality: surgical treatment for hilar cholangiocarcinoma. J Gastrointest Surg. 2008;12:1268–1274. doi: 10.1007/s11605-008-0503-1. [DOI] [PubMed] [Google Scholar]
- 25.Runyon BA, Hoefs JC. Culture-negative neutrocytic ascites: a variant of spontaneous bacterial peritonitis. Hepatology. 1984;4:1209–1211. doi: 10.1002/hep.1840040619. [DOI] [PubMed] [Google Scholar]
- 26.Rimola A, Garcia-Tsao G, Navasa M, et al. International Ascites Club. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. J Hepatol. 2000;32:142–153. doi: 10.1016/s0168-8278(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 27.Akriviadis EA, Runyon BA. Utility of an algorithm in differentiating spontaneous from secondary bacterial peritonitis. Gastroenterology. 1990;98:127–133. doi: 10.1016/0016-5085(90)91300-u. [DOI] [PubMed] [Google Scholar]