Abstract
Antiviral treatment of hepatitis B is one of the most rapidly evolving fields in current medicine. Guidelines for the management of chronic hepatitis B (CH-B) have been proposed and revised by many academic societies and groups. Recommendations for nucleoside or nucleotide analogue (NUC) therapy from representative current guidelines are compared herein with each other and with previous guidelines. Several differences among individual recommendations may reflect regional and temporal differences as well as differences in the available data upon which the guidelines are based. Nevertheless, these guidelines share a common principle regarding NUC treatment for CH-B: long-term viral suppression by the drugs with potent antiviral activity and low rate of development of drug resistance to prevent disease progression. A review of the past and current guidelines for the management of CH-B would be useful for evaluating the current status of management of the disease and to identify better solutions for improving the outcome of patients with CH-B.
Keywords: Chronic hepatitis B, Liver cirrhosis, Viral suppression, Management, Guideline
INTRODUCTION
Hepatic B virus (HBV) infection is a serious health problem involving about 350 million people worldwide.1 Persistent HBV infection can lead to liver cirrhosis (LC), hepatic decompensation, and hepatocellular carcimoma (HCC) in a significant portion of the patients. Since the introduction of lamivudine, many nucleoside or nucleotide analogues (NUCs) have been developed to reduce or delay disease progression in HBV-infected patients. Increasing knowledge gained from a large numbers of studies on the effects and the limitations of these antiviral drugs has contributed greatly to the rapid progress in hepatitis B management.
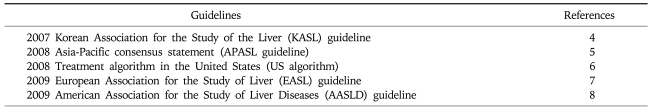
Among the early guidelines for the management of Chronic hepatitis B (CH-B), 2000 NIH conference on the management of hepatitis B, 2000 Asia-Pacific consensus statement, and 2000 American Association for the Study of Liver Diseases guidelines are representative.1-3 Since then, many national and international societies for the study of liver diseases have revised their own guidelines to improve the recommended approaches in the diagnosis, treatment, and prevention of chronic HBV infection.4-8 While these guidelines share many principles and practical approaches, some disagreements are inevitable according to the time and the place that the guidelines were made. For example, there are considerable regional and temporal differences in economic status, reimbursement policy, and availability of NUCs. Natural history and the response rate to antiviral agent might be different according to the prevalent viral genotypes and common modes of infection prevalent in the particular geographical area. In addition, the earlier recommendations have been revised according to the more up-to-date information from recent studies. In this article, we compared the recommendations on hepatitis B treatment from the five representative current guidelines to evaluate the current status and to identify unresolved issues and find the better solution in the management of chronic hepatitis B (Table 1).
Table 1.
Current Guidelines for Management of Chronic Hepatitis B
Most guidelines deal with many aspects of hepatitis B management including prevention, diagnosis, monitoring, and treatment. However, this review will focus on the hepatitis B treatment using NUCs.
INDICATION OF ANTIVIRAL TREATMENT
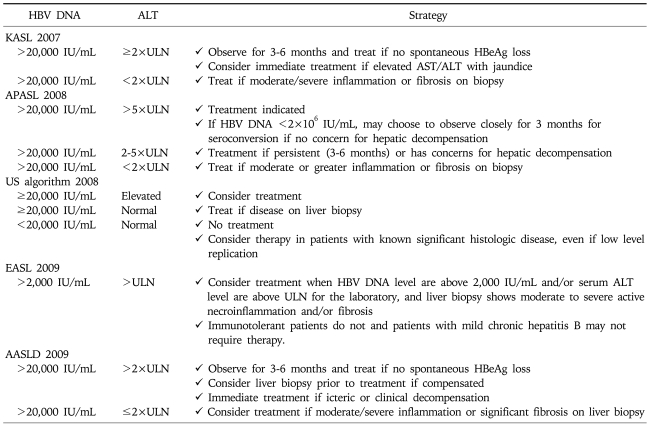
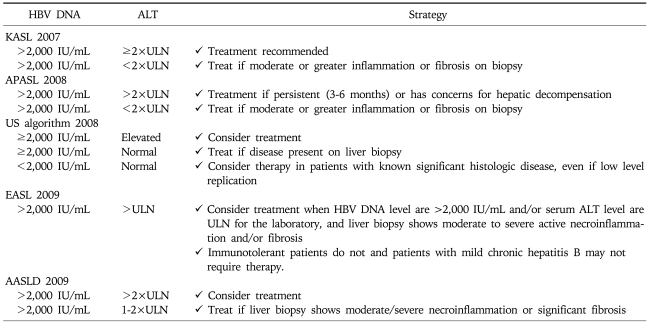
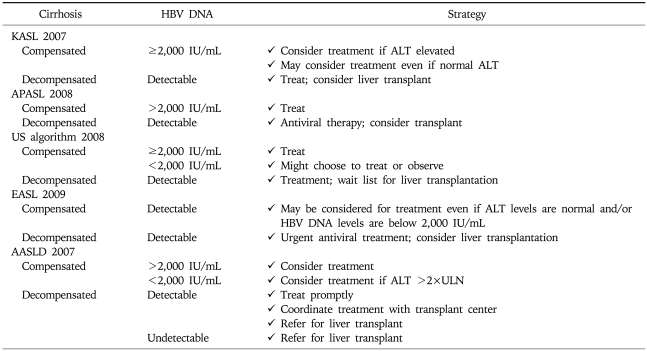
Chronic hepatitis B patients with active viral replication and significant inflammation and fibrosis are the proper target for antiviral treatment. Early guidelines generally agreed that antiviral treatment could be recommended for chronic hepatitis B patients (especially those without LC) with serum HBV DNA level above 105 copies/mL (20,000 IU/mL) and serum alanine aminotransferase (ALT) level greater than two times the normal.1-3 However, most current guidelines suggest that the indication of antiviral treatment should be expanded to the patients with lower serum HBV DNA level and/or lower serum ALT level. Treatment indication is recommended according to the disease categories; HBeAg-positive CH-B (Table 2), HBeAg-negative CH-B (Table 3), and LC (Table 4).
Table 2.
Treatment Indication for HBeAg-Positive Chronic Hepatitis B by Current Guidelines
KASL, Korean Association for the Study of the Liver; APASL, Asia-Pacific consensus statement; US algorithm, treatment algorithm in the United States; EASL, European Association for the Study of Liver; AASLD, American Association for the Study of Liver Diseases.
Table 3.
Treatment Indication for HBeAg-Negative Chronic Hepatitis B by Current Guidelines
KASL, Korean Association for the Study of the Liver; APASL, Asia-Pacific consensus statement; US algorithm, treatment algorithm in the United States; EASL, European Association for the Study of Liver; AASLD, American Association for the Study of Liver Diseases.
Table 4.
Treatment Indication for Cirrhosis by Current Guidelines
KASL, Korean Association for the Study of the Liver; APASL, Asia-Pacific consensus statement; US algorithm, treatment algorithm in the United States; EASL, European Association for the Study of Liver; AASLD, American Association for the Study of Liver Diseases.
Serum HBV DNA level is a marker of viral replication and efficacy of antiviral treatment in individuals with chronic hepatitis B. Progression to cirrhosis in HBV-infected persons is reported to be correlated strongly with the level of circulating virus.9 However, HBV DNA level of 105 copies/mL was arbitrarily chosen by early guidelines as the cut-off level for indication of antiviral treatment. Some patients with lower serum HBV DNA level (300-105 copies/mL), especially those with HBeAg negative hepatitis and/or cirrhosis, frequently show progression of liver disease and may need treatment.3,8,10 A population-based prospective cohort study of 3,582 untreated hepatitis B-infected patients in Taiwan (a mean follow-up time of 11 years) showed that the cumulative incidence of cirrhosis increased with the HBV-DNA level and ranged from 4.5% to 36.2% for patients with a hepatitis B viral load of less than 300 copies/mL and 106 copies/mL or more, respectively (p<0.001).9 Another study from Taiwan involving almost the same cohort showed that the incidence of HCC increased with serum HBV DNA level at the time of study entry in a dose-dependent manner, ranging from 108/100,000 person-years for an HBV DNA level <300 copies/mL to 1,152/100,000 person-years for an HBV DNA ≥1 million copies/mL.11
Serum ALT has been used as a convenient surrogate marker for liver injury and an increased serum ALT level was indicated as a risk factor for disease progression in CH-B.9 Serum ALT level greater than two times normal was suggested as indication of antiviral treatment for CH-B by the early guidelines, especially in CH-B patients without cirrhosis.1-3 However, an increased risk for developing LC and HCC has been documented in patients with mildly increased serum ALT and even in those with serum ALT level of upper normal range. According to a Chinese study with mean follow-up period of 46.8 months in 3,233 CH-B patients, the patients with ALT levels of 0.5-1 times the upper limit of normal (ULN) and 1-2× ULN had an increased risk for the development of complications compared with the patients with ALT levels <0.5× ULN (p<0.0001 for both).12 A prospective cohort study from Korea involving 94,533 men and 47,522 women with eight years' follow-up demonstrated that the adjusted relative risks for AST concentration of 20-29 IU/L and 30-39 IU/L were 2.5 and 8.0 in men and 3.3 and 18.2 in women, respectively, compared with the concentration <20 IU/L.13 Another study suggested the updated upper limits (for men, 30 U/L; for women, 19 U/L) and showed their superior sensitivity in identifying the high risk patients with liver disease.14
According to the recommendation from current guidelines, the definite indications for the treatment of CH-B are serum HBV DNA level ≥105 copies/mL and serum ALT≥2×ULN, especially in HBeAg positive patients without LC. Prompt antiviral treatment is necessary for decompenstated cirrhosis with any detectable HBV DNA, irrespective of serum ALT level.4-8,15 Most guidelines generally agreed that the treatment for patients with lower serum HBV DNA level and/or mildly increased or upper normal serum ALT could be recommended in HBeAg negative CH-B or compensated LC.4-8
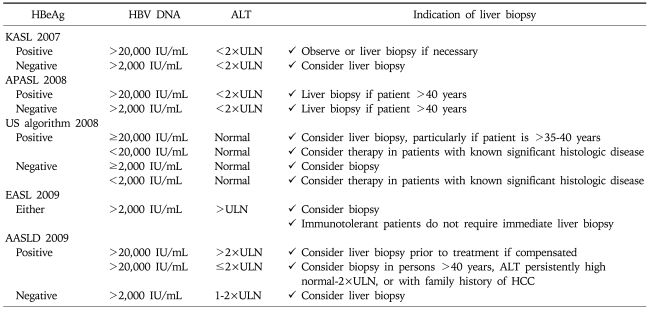
INDICATION OF LIVER BIOPSY
Liver biopsy has three major roles: diagnosis, assessment of prognosis (disease staging), and/or assist in making therapeutic management decisions.16 A retrospective review of CH-B patients revealed that 37% of patients with persistently normal ALT had significant fibrosis or inflammation. Subgroup analysis showed the majority with fibrosis belonged to the high normal ALT group and that only a minority who were young and immune tolerant had significant findings on biopsy.17 In CH-B, liver biopsy is most useful for patients who do not meet definite criteria for treatment but still has possible risk for significant disease.8 Age of the patient, serum HBV DNA level, serum ALT level, or family history of HCC should be taken into consideration before deciding whether to do biopsy or not (Table 5).
Table 5.
Indication of Liver Biopsy by Current Guidelines
KASL, Korean Association for the Study of the Liver; APASL, Asia-Pacific consensus statement; US algorithm, treatment algorithm in the United States; EASL, European Association for the Study of Liver; AASLD, American Association for the Study of Liver Diseases.
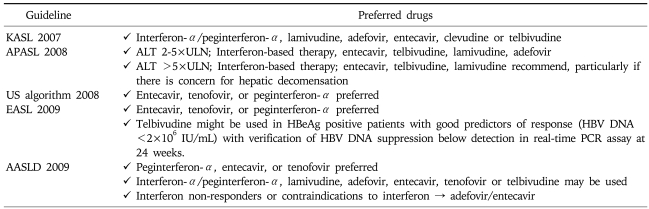
RECOMMENDED NUCS AS ININTIAL THERAPY
NUCs including lamivudine, adefovir, entecavir, tenofovir or telbivudine may be used as a first line monotherapy. Clevudine is a recently introduced antiviral agent showing potent and sustained viral suppression and low rate of resistance and was licensed as a first line agent in South Korea (Table 6).18,19
Table 6.
Recommended Drugs for Initial Therapy of Chronic Hepatitis B by Current Guidelines
KASL, Korean Association for the Study of the Liver; APASL, Asia-Pacific consensus statement; US algorithm, treatment algorithm in the United States; EASL, European Association for the Study of Liver; AASLD, American Association for the Study of Liver Diseases.
NUCs with potent viral suppression and high genetic barrier to resistance would be ideal drugs to achieve sustained viral suppression. Adefovir is not an ideal option due to weak antiviral activity and high rate of resistance after 48 weeks. Lamivudine and telbivudine are not preferred due to weak antiviral potency and frequent drug resistance.8 Hence, entecavir or tenofovir is preferred, if available in the region.6-8,20-22 Entecavir is a potent antiviral agent showing potent viral suppression and low rate of resistance and is one of the preferred first line agents in NUCs-naïve patients.21,22 Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare (1.2%) through 5 years of therapy.23 Tenofovir disoproxil fumarate was recently approved for the treatment of CH-B in Europe and the United States. While tenofovir is structurally similar to adefovir, tenofovir at a daily dose of 300 mg had superior antiviral efficacy with a similar safety profile as compared with adefovir dipivoxil at a daily dose of 10 mg both in patients who had previously received lamivudine and in those who had not.8,20
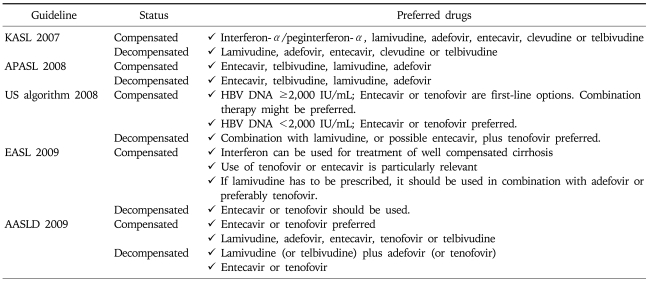
For compensated LC, potent NUCs with low rate of resistance are preferred. Other NUCs may be used in these patients. For decompensated LC, lamivudine is the most extensively evaluated drug showing considerable effect. However, frequent development of resistance is a significant limitation. Entecavir, tenofovir, or combination therapy (i.e., lamivudine plus adefovir) is preferred, although sufficient data on their role is not yet available (Table 7).
Table 7.
Recommended Drugs for Initial Therapy of Cirrhosis by Current Guidelines
KASL, Korean Association for the Study of the Liver; APASL, Asia-Pacific consensus statement; US algorithm, treatment algorithm in the United States; EASL, European Association for the Study of Liver; AASLD, American Association for the Study of Liver Diseases.
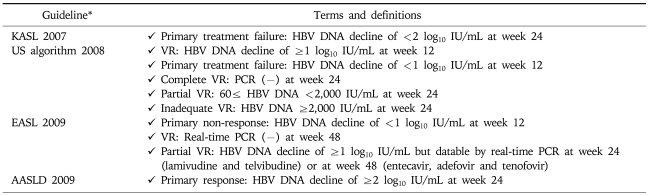
ON-TREATMENT MONITORING DURING NUCS TREATMENT
Delayed HBV DNA suppression was suggested to predispose the emergence of genotypic mutations that reduce the effectiveness of a specific drug.24 Hence, the roadmap concept was introduced by 2007 international workshop.25 Evaluation of early virologic responses and subsequent modification of antiviral treatment (switching to or adding more potent drug without cross-resistance) has been suggested to lead to better outcomes including a reduced risk of viral resistance and high probability of viral suppression. This idea of on-treatment monitoring strategies to identify outcomes of therapy has been adapted by some guidelines (Table 8).
Table 8.
On-treatment Monitoring of Serum HBV DNA Levels during NUCs Therapy by Current Guidelines
KASL, Korean Association for the Study of the Liver; US algorithm, treatment algorithm in the United States; EASL, European Association for the Study of Liver; AASLD, American Association for the Study of Liver Diseases; VR: virologic response.
*Not described in APASL 2008.
RECOMMENDED DURATION OF NUCS TREATMENT
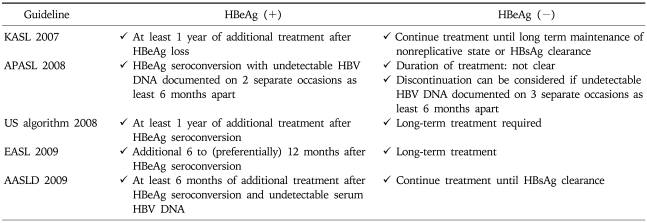
According to 2009 EASL guideline, the ideal end-point of therapy is a sustained HBsAg loss with or without seroconversion to anti-HBs; however, it is difficult to achieve.7 Durable HBe seroconversion in HBeAg-positive patients is a satisfactory end-point. Consolidation therapy of 6 to 12 months is commonly recommended after HBe seroconversion.4-8 Sustained maintenance of undetectable HBV DNA level on treatment with NUCs is the next most desirable end-point in all others including those with HBeAg negative or LC.7
For HBeAg positive CH-B patients, most guideline agreed that NUCs can be stopped after continuing therapy for additional 6-12 months after HBeAg loss or seroconversion to anti-HBe. In contrast, the duration of the treatment has not been defined for HBeAg negative CH-B patients. Long-term treatment or treatment until HBsAg clearance is recommended by most guidelines. Notably, only 2008 APASL guideline recommend that discontinuation of NUCs may be considered if undetectable HBV DNA is documented on 3 separate occasions at least 6 months apart (Table 9).5
Table 9.
Recommended Duration of NUCs Therapy for Chronic Hepatitis B by Current Guidelines
KASL, Korean Association for the Study of the Liver; APASL, Asia-Pacific consensus statement; US algorithm, treatment algorithm in the United States; EASL, European Association for the Study of Liver; AASLD, American Association for the Study of Liver Diseases.
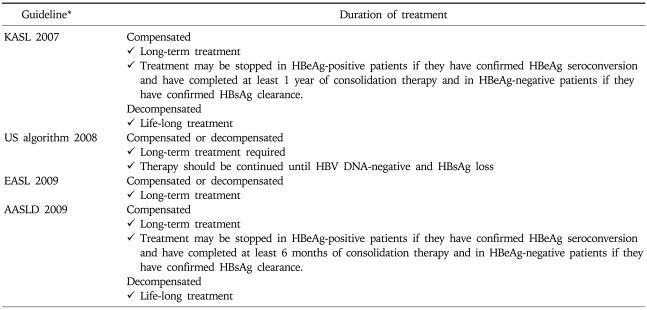
Long-term or life-long treatment is recommended for LC by most guidelines. Both 2007 KASL guideline and 2008 APASL guideline recommend that in patients with compensated LC, the treatment may be stopped in HBeAg-positive patients if they have confirmed HBeAg seroconversion and have completed at least 1 year of consolidation therapy; and in HBeAg-negative patients if they have confirmed HBsAg clearance.4,5 Life-long treatment is warranted for decompensated LC (Table 10).4,8
Table 10.
Recommended Duration of NUCs Therapy for Cirrhosis by Current Guidelines
KASL, Korean Association for the Study of the Liver; US algorithm, treatment algorithm in the United States; EASL, European Association for the Study of Liver; AASLD, American Association for the Study of Liver Diseases.
*Not described in APASL 2008.
MANAGEMENT OF ANTIVIRAL RESISTANCE
In case of the drug resistance, a rescue therapy with the most effective antiviral effect and the minimal risk to induce multiple drug-resistant strains of HBV would be an ideal option.7 Adding-on a second drug without cross-resistance is preferred but switching to more potent drug might be considered based on cross-resistance profile. Susceptibility profile of HBV mutants and a history of prior exposure and/or resistance to other NUCs should be considered in optimizing the rescue therapy.
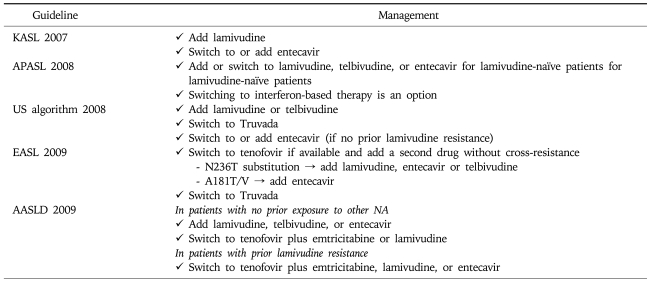
Currently, add-on adefovir therapy is the most widely accepted strategy in case of lamivudine resistance. An early study on small number of cases indicated that both adefovir dipivoxil alone and adefovir in combination with ongoing lamivudine therapy may provide similar effective antiviral therapy up to week 16 in patients with lamivudine-resistant HBV.26 However, subsequent studies with long-term follow up demonstrated the superiority of add-on adefovir therapy. Under prolonged adefovir-lamivudine therapy, patients with lamivudine-resistant hepatitis B were unlikely to develop genotypic resistance to adefovir and had durable prevention of virologic and clinical breakthrough. The 1-, 2-, 3-, and 4-year cumulative rates of de novo rtA181T were reported to be 1%, 2%, 4%, and 4%, respectively.27 However, virologic and biochemical breakthroughs due to development of adefovir resistance occurred in 21% of patients on adefovir monotherapy 15 to 18 months from the start of the treatment (p=0.0174).28
Entecavir has been suggested as an effective agent for NUCs-naïve patients and lamivudine-refractory patients.21,22,29 While entecavir shows profound viral suppression and low rate of resistance in NUCs-naïve patients, it is not an ideal option for patient with lamivudine-resistance.21,22,30 Preexisting mutations of HBV in patients with lamivudine resistance would reduce barrier to entecavir resistance. A 5-year cumulative probability of genotypic entecavir resistance and genotypic entecavir resistance associated with breakthrough were reported to be 51% and 43%, respectively.23
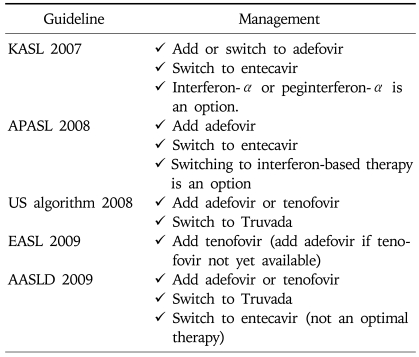
Tenofovir may be an effective alternative for the treatment of patients with lamivudine-resistant HBV infection.31 A study comparing adefovir and tenofovir in patients with lamivudine resistance showed that only 44% of these patients had HBV DNA levels <105 copies/mL in contrast to 100% of the tenofovir-treated patients at week 48 (p=0.001). No evidence of phenotypic viral resistance was demonstrated in the tenofovir-treated patients in the long term period up to 130 weeks (Table 11).31
Table 11.
Recommended Management of Lamivudine Resistance by Current Guidelines
KASL, Korean Association for the Study of the Liver; APASL, Asia-Pacific consensus statement; US algorithm, treatment algorithm in the United States; EASL, European Association for the Study of Liver; AASLD, American Association for the Study of Liver Diseases.
In principle, adefovir resistance could be managed by switching to or adding on NUCs without cross-resistance. In clinical practice, the rescue therapy for adefovir resistance should be individualized according to the level of susceptibility of HBV variants and prior exposure history to other NUCs (especially, lamivudine). Adefovir-refractory patients with N236T mutation are sensitive to lamivudine, telbivudine, entecavir, and tenofovir; however, those with A181T/V mutation shows reduced susceptibility to lamivudine while maintaining the sensitivity to other drugs. In patients with prior lamivudine resistance including those receiving sequential monotherapy, adding-on lamivudine lead to early coming-back of lamivudine-resistant virus (Table 12).32
Table 12.
Recommended Management of Adefovir Resistance by Current Guidelines
KASL, Korean Association for the Study of the Liver; APASL, Asia-Pacific consensus statement; US algorithm, treatment algorithm in the United States; EASL, European Association for the Study of Liver; AASLD, American Association for the Study of Liver Diseases.
Entecavir resistance results from HBV reverse transcriptase substitutions at positions T184, S202, or M250, which emerge in the presence of lamivudine resistance substitutions M204I/V +/- L180M. Lamivudine resistance mutations preexisting in the lamivudine-refractory patients reduce a barrier to entecavir resistance.23 Switching to or adding a second drug without cross resistance is recommended (Table 13).
Table 13.
Recommended Management of Entecavir Resistance by Current Guidelines
KASL, Korean Association for the Study of the Liver; US algorithm, treatment algorithm in the United States; EASL, European Association for the Study of Liver; AASLD, American Association for the Study of Liver Diseases.
*Not described in APASL 2008.
Since telbivudine and clevudine show similar mutation patterns with lamivudine, the resistance to these drugs could be managed by using a similar management strategy used in lamivudine resistance.8
CONCLUSION
Durable long-term viral suppression by a drug or drugs with potent viral suppression and high genetic barrier to resistance is an ultimate goal of antiviral treatment in CH-B, leading to prevention of disease progression.6-8 Current guidelines generally share this common principle, despite some differences in the details of the recommendations. Treatment could be and should be individualized according to many factors; host factors such as mode of infection, disease status and immunity, viral factors such as genotypes, prior antiviral treatment, mutation, and susceptibility level, drug factors such local availability, cost, and reimbursement policy, and so on. Moreover, it can not be overemphasized that the current guidelines are not a fixed law and will be changed as new drugs and new data become available.
References
- 1.Lok AS, Heathcote EJ, Hoofnagle JH. Management of hepatitis B: 2000: summary of a workshop. Gastroenterology. 2001;120:1828–1853. doi: 10.1053/gast.2001.24839. [DOI] [PubMed] [Google Scholar]
- 2.Consensus statements on the prevention and management of hepatitis B and hepatitis C in the Asia-Pacific region: Core Working Party for Asia-Pacific Consensus on Hepatitis B and C. J Gastroenterol Hepatol. 2000;15:825–841. doi: 10.1046/j.1440-1746.2000.02324.x. [DOI] [PubMed] [Google Scholar]
- 3.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2001;34:1225–1241. doi: 10.1053/jhep.2001.29401. [DOI] [PubMed] [Google Scholar]
- 4.Lee KS, Kim DJ. Management of chronic hepatitis B. Korean J Hepatol. 2007;13:447–488. doi: 10.3350/kjhep.2007.13.4.447. [DOI] [PubMed] [Google Scholar]
- 5.Liaw YF, Leung N, Kao JH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263–283. doi: 10.1007/s12072-008-9080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keeffe EB, Dieterich DT, Han SH, et al. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008;6:1315–1341. doi: 10.1016/j.cgh.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 9.Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678–686. doi: 10.1053/j.gastro.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 10.Chu CJ, Hussain M, Lok AS. Quantitative serum HBV DNA levels during different stages of chronic hepatitis B infection. Hepatology. 2002;36:1408–1415. doi: 10.1053/jhep.2002.36949. [DOI] [PubMed] [Google Scholar]
- 11.Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 12.Yuen MF, Yuan HJ, Wong DK, et al. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut. 2005;54:1610–1614. doi: 10.1136/gut.2005.065136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HC, Nam CM, Jee SH, Han KH, Oh DK, Suh I. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ. 2004;328:983. doi: 10.1136/bmj.38050.593634.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prati D, Taioli E, Zanella A, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 15.Lai CL, Yuen MF. The natural history and treatment of chronic hepatitis B: a critical evaluation of standard treatment criteria and end points. Ann Intern Med. 2007;147:58–61. doi: 10.7326/0003-4819-147-1-200707030-00010. [DOI] [PubMed] [Google Scholar]
- 16.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 17.Lai M, Hyatt BJ, Nasser I, Curry M, Afdhal NH. The clinical significance of persistently normal ALT in chronic hepatitis B infection. J Hepatol. 2007;47:760–767. doi: 10.1016/j.jhep.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 18.Yoo BC, Kim JH, Chung YH, et al. Twenty-four-week clevudine therapy showed potent and sustained antiviral activity in HBeAg-positive chronic hepatitis B. Hepatology. 2007;45:1172–1178. doi: 10.1002/hep.21629. [DOI] [PubMed] [Google Scholar]
- 19.Yoo BC, Kim JH, Kim TH, et al. Clevudine is highly efficacious in hepatitis B e antigen-negative chronic hepatitis B with durable off-therapy viral suppression. Hepatology. 2007;46:1041–1048. doi: 10.1002/hep.21800. [DOI] [PubMed] [Google Scholar]
- 20.Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442–2455. doi: 10.1056/NEJMoa0802878. [DOI] [PubMed] [Google Scholar]
- 21.Chang TT, Gish RG, de Man R, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 22.Gish RG, Lok AS, Chang TT, et al. Entecavir therapy for up to 96 weeks in patients with HBeAg-positive chronic hepatitis B. Gastroenterology. 2007;133:1437–1444. doi: 10.1053/j.gastro.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 23.Tenney DJ, Rose RE, Baldick CJ, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology. 2009;49:1503–1514. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 24.Yuen MF, Sablon E, Hui CK, Yuan HJ, Decraemer H, Lai CL. Factors associated with hepatitis B virus DNA breakthrough in patients receiving prolonged lamivudine therapy. Hepatology. 2001;34:785–791. doi: 10.1053/jhep.2001.27563. [DOI] [PubMed] [Google Scholar]
- 25.Keeffe EB, Zeuzem S, Koff RS, et al. Report of an international workshop: roadmap for management of patients receiving oral therapy for chronic hepatitis B. Clin Gastroenterol Hepatol. 2007;5:890–897. doi: 10.1016/j.cgh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Peters MG, Hann Hw H, Martin P, et al. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2004;126:91–101. doi: 10.1053/j.gastro.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 27.Lampertico P, Vigano M, Manenti E, Iavarone M, Sablon E, Colombo M. Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology. 2007;133:1445–1451. doi: 10.1053/j.gastro.2007.08.079. [DOI] [PubMed] [Google Scholar]
- 28.Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology. 2007;45:307–313. doi: 10.1002/hep.21534. [DOI] [PubMed] [Google Scholar]
- 29.Sherman M, Yurdaydin C, Simsek H, et al. Entecavir therapy for lamivudine-refractory chronic hepatitis B: improved virologic, biochemical, and serology outcomes through 96 weeks. Hepatology. 2008;48:99–108. doi: 10.1002/hep.22323. [DOI] [PubMed] [Google Scholar]
- 30.Ono SK, Kato N, Shiratori Y, et al. The polymerase L528M mutation cooperates with nucleotide binding-site mutations, increasing hepatitis B virus replication and drug resistance. J Clin Invest. 2001;107:449–455. doi: 10.1172/JCI11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Bommel F, Wunsche T, Mauss S, et al. Comparison of adefovir and tenofovir in the treatment of lamivudine-resistant hepatitis B virus infection. Hepatology. 2004;40:1421–1425. doi: 10.1002/hep.20464. [DOI] [PubMed] [Google Scholar]
- 32.Fung SK, Andreone P, Han SH, et al. Adefovir-resistant hepatitis B can be associated with viral rebound and hepatic decompensation. J Hepatol. 2005;43:937–943. doi: 10.1016/j.jhep.2005.05.037. [DOI] [PubMed] [Google Scholar]