Abstract
Background/Aims
Cerulein pancreatitis is similar to human edematous pancreatitis with dysregulation of the production and secretion of digestive enzymes, edema formation, cytoplasmic vacuolization and the death of acinar cells. We hypothesized that membrane proteins may be altered as the early event during the induction of acute pancreatitis. Present study aims to determine the differentially expressed proteins in the membranes of cerulein-treated pancreatic acinar cells.
Methods
Pancreatic acinar AR42J cells were treated with 10-8 M cerulein for 1 hour. Membrane proteins were isolated from the cells and separated by two-dimensional electrophoresis using pH gradients of 5-8. Membrane proteins were identified by matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) analysis of the peptide digests. The differentially expressed proteins, whose expression levels were more or less than three-fold in cerulein-treated cells, were analyzed.
Results
Two differentially expressed proteins (mannan-binding lectin-associated serine protease-2, heat shock protein 60) were up-regulated while four proteins (protein disulfide isomerase, γ-actin, isocitrate dehydrogenase 3, seven in absentia homolog 1A) were down-regulated by cerulein treatment in pancreatic acinar cells. These proteins are related to cell signaling, oxidative stress, and cytoskeleton arrangement.
Conclusions
Oxidative stress may induce cerulein-induced cell injury and disturbances in defense mechanism in pancreatic acinar cells.
Keywords: Cerulein, Pancreatitis, Pancreatic acinar cells, Membrane proteome
INTRODUCTION
Pathogenesis of acute pancreatitis in humans has not been clarified yet. In human gallstone pancreatitis, plasma levels of gastrointestinal hormone cholecystokinin (CCK) are increased.1 In acute alcoholic pancreatitis, severe pancreatic insufficiency and the defect in CCK regulation were shown.2 Pancreatic exocrine dysfunction after acute pancreatitis is observed not only in patients with gallstone pancreatitis but also in those with alcoholic and idiopathic pancreatitis.3 These studies demonstrate that possible involvement of CCK, which affects exocrine function of pancreas, in the pathogenesis of acute pancreatitis.
Pancreatitis induced by cerulein, a CCK analogue, is one of the widely used experimental models of acute pancreatitis that exhibits the biochemical, morphological, and pathophysiological similarities to various aspects of human pancreatitis.4,5 Doses of cerulein beyond those that cause the maximum pancreatic secretion of amylase and lipase result in pancreatitis,6,7 which is characterized by dysregulation of the production and secretion of digestive enzymes, inhibition of pancreatic secretion, edema formation, infiltration of inflammatory cells into the pancreas, cytoplasmic vacuolization and the death of acinar cells.4,5,8 The cerulein-evoked increase in intracellular Ca2+ levels is believed to be the main intracellular signal for enzyme and fluid secretion.9 One interesting report showed the role of reactive oxygen species (ROS) on altering the cytoskeleton function in the pancreas.10 This cytoskeletal disruption may induce disturbances in the intracellular transport of digestive enzymes, leading to their premature intracellular activation.11 Our previous studies demonstrated that cerulein stimulates the production of ROS, NF-κB activation and the productions of the inflammatory cytokines (IL-1β, IL-6) in pancreatic acinar cells.12 The production of ROS was mediated with the activation of NADPH oxidase Ca2+-dependently in cerulein-stimulated pancreatic acinar cells.13-16 In our previous proteomic analysis, five differentially expressed proteins were identified in pancreatic acinar AR42J cells treated with cerulein (10-8 M) such as heat shock protein 90, mitochondrial ATP synthase beta chain precursor, tubulin beta chain, 3-mercaptopyruvate transferase, mitochondrial ATP synthase subunit d.17
Due to their interfacial position in cells, membrane proteins play important roles in various cellular processes.18 Transmembrane proteins represent around 30% of total proteins.19 Membrane proteins are important in the aspect of pharmacological action of drugs, and many successful drugs target on the activity of membrane proteins. Therefore, the identification of altered membrane proteins by specific stimuli is required to understand the pathophysiologic mechanism of the disease.
Present study aims to determine the differentially expressed proteins in the membranes of cerulein-treated pancreatic acinar cells by two-dimensional electrophoresis (2-DE) using pH gradients of 5-8 and matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) analysis. In addition, Western blot analysis was performed for the proteins which were differentially expressed by cerulein treatment, determined by proteomics.
AR 42J cells, used in the present study, is the only currently available cell line that maintains many characteristics of normal pancreatic acinar cells, such as the synthesis and secretion of digestive enzymes.20 Therefore, this cell line has been widely used as an in vitro model to study cellular secretion, growth, proliferation, and apoptosis of the exocrine pancreas.20-23
MATERIALS AND METHODS
1. Reagents and materials
Electrophoresis reagents including acrylamide solution (25%), N,N'-methylenebisacrylamide, N,N,N',N'-tetramethylethylene diamine, tris (hydroxylmethyl) aminomethane (Tris) base, glycine, sodium dodecyl sulfate (SDS), ammonium persulfate, dithiothreitol (DTT), 3-[(3-cholamidopropyl) dimethylamino]-1-propanesulfonate, urea, thiourea, Bio-lyte, sulfobetaine 3-10, tributyl phosphine, Immobiline Dry Strips, immobilized pH gradient (IPG) buffer, IPG cover mineral oil, iodoacetamide and trifluoroacetic acid (TFA) were purchased from Bio-Rad Laboratories (Hercules, CA, USA). Coomassie Brilliant Blue (CBB) G-250 was purchased from Amersham Biosciences (Piscataway, NJ, USA). Trypsin (modified) was obtained from Promega (Madison, WI, USA). ZipTipC18 microcolumn was purchased from Millipore (Bedford, MA, USA). α-Cyano-4-hydroxy-trans-cinnamic acid was purchased from Sigma (St. Louis, MO, USA). Antibodies against Nox, aldolase A, γ-actin, isocitrate dehydrogenase 3 (ICD 3), seven in absentia homolog 1A (SIAH 1A), and actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) while antibodies against heat shock protein 60 (Hsp 60) and protein disulfide isomerase (PDI) were obtained from Cell Signaling (Danvers, MA, USA). Other analytically pure reagents were domestically produced.
2. Cell culture
The rat pancreatic acinar AR42J cells (ATCC CRL 1492) were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's medium (Sigma) supplemented with 10% fetal bovine serum (GIBCO-BRL, Granad Island, NY, USA) and antibiotics (100 U/mL penicillin and 100 µg/mL streptomycin) in a 5% CO2 and 95% O2 atmosphere.
3. Experimental protocol
The acinar cells were plated at a density of 2×106/mL in a 100 mm culture plate (Falcon 3047; Becton Dickinson Labware, Lincoln Park, NJ, USA) and allowed to attach for 12 hours. The cells were treated with cerulein at a concentration of 10-8 M and cultured for 1 hour (for proteomic analysis) and 1.5 hour (for Western blotting for the differentially expressed proteins). After determination of membrane fraction by Western blot analysis for Nox as membrane marker, the membrane proteome was separated by two-dimensional electrophoresis (2-DE) using pH gradients of 5-8 and identified by matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF MS) analysis of the peptide digests. In addition, the differentially expressed proteins, determined by proteomic analysis, were verified by Western blotting. The dose of cerulein and duration of exposed time to the cells were adapted from our previous studies showing that cerulein activated NF-κB and inflammatory cytokine expression.13-16
4. Protein extraction and fractionation
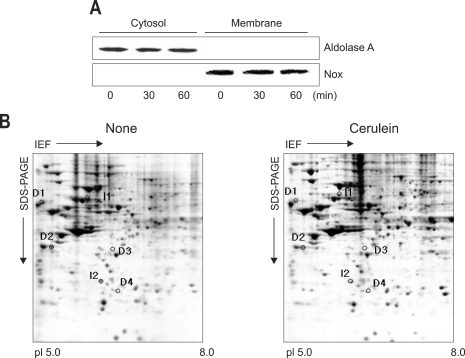
The AR42J cells were trypsinized and washed with PBS buffer and then centrifuged at 500 rpm for 5 minutes. The cells were resuspended with lysis buffer containing 10 mM Tris, pH 7.4, 50 mM NaCl, 1 mM EDTA, 50 µM leupeptin, 25 µM pepstatin, 25 µM aprotin, and 1 M PMSF and extracted by drawing the cells through a 1 mL syringe with several rapid strokes. Nuclei and intact cells were sedimented down at 2,000 × g for 10 minutes, and cytosolic and membrane fractions were separated further by centrifugation at 100,000 × g for 1 hour. Western blot analysis was performed to identify the cytosolic fractions and membrane factions using aldolase A and Nox1 as the cytosolic and membrane markers for the cells cultured in the presence of cerulein for 1 hour (Fig. 1A). The membrane fractions were resuspended and then protein contents were determined by Bradford protein assay.
Fig. 1.
Western blot analysis for aldolase A and Mox1 (A) and 2-DE gel map derived from non-treated and cerulein-treated cells (B). (A) The cells (2×106/mL in a 100 mm culture) were treated with cerulein for the indicated time periods. Cytosolic and membrane fractions were isolated from the cells. Protein levels of aldolase 1 and Nox were determined by Western blotting using anti-aldolase A and anti-Nox antibodies. Aldolase A and Nox were used for the markers of cytosolic and membrane fractions, respectively. (B) The protein (300 µg in 300 µL) was applied to pH 5-8 linear IPG strips (17 cm), with 11% linear vertical SDS-PAGE as the second dimension. The gel was visualized by Coomassie staining. The numbered proteins are those that were differentially expressed between non-treated cells (none) and cerulein-treated cells (cerulein). The numbers I 1-4 indicate up-regulated proteins while the numbers D 1-2 denote down-regulated proteins by cerulean treatment.
IEF, isoelectric focusing.
5. Western blot analysis
The membrane fractions were separated by 11% SDS-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (Amersham Inc, Arlington Heights, IL, USA) by electroblotting. After blocking of nonspecific binding with 5% nonfat dry milk in TBS-T (Trisbuffered saline containing 0.15% Tween 20) for 2 hours at room temperature, the proteins were detected with antibodies for aldolase A (1:500; sc-12059; Santa Cruz Biotechnology), Nox (1:1,000; sc-34699; Santa Cruz Biotechnology), Hsp 60 (1:1,000; Catalog No. 4870; Cell Signaling, Danvers, MA), PDI (1:1,000; Catalog No. 2446; Cell Signaling), γ-actin (1:1,000; sc-65634; Santa Cruz Biotechnology), ICD 3 (1:1,000; sc-55670; Santa Cruz Biotechnology), SIAH 1A (1:1,000; sc-5505; Santa Cruz Biotechnology), and actin (1:1,000; Santa Cruz Biotechnology) diluted in TBS-T containing 5% dry milk, and incubated at 4℃ overnight. Primary antibodies were detected using horseradish peroxidase-conjugated secondary antibodies, and visualized by the ECL detection system (Santa Cruz Biotechnology) according to the manufacturer's instruction. Since there is no commercially available antibody for mannan-binding lectin-associated serine protease-2 (MASP-2), the protein could not be verified by Western blotting.
6. Isoelectric focusing (IEF) and 2-DE separation
Three hundred µg of membrane protein (in 300 µL) was adsorbed onto a 17 cm IPG strip (pH 3-10, pH 5-8), and then electrophoresed on a IEF cells (Bio-Rad) for 70,000 Vh at 20℃. Following IEF, the IPG strips were subjected to equilibration for 15 minutes in equilibration buffer (375 mM Tris-HCl, pH 8.8, containing 6 M urea, 2% w/v SDS, 20% v/v glycerol, and 2% w/v DTT). Strips were then re-equilibrated for 15 minutes in the same buffer containing 2.5% w/v iodoacetamide in place of DTT. In all cases, molecular weight separation was achieved using Protean II xi cell gel SDS-PAGE system (Bio-Rad).
7. Coomassie Brilliant Blue (CBB) G-250 staining
Duplicate samples were separated on 11% linear SDS polyacrylamide gel. Proteins in one gel were CBB stained. After overnight fixation (50% ethanol, 2% w/v phosphoric acid), the gels were washed three times for 20 min in double-distilled water and incubated for at least 48 hours in a solution containing 34% methanol, 17% ammonium sulfate, 3% w/v phosphoric acid and CBB G-250 powder (0.1%). The stained gels were digitalized using a GS 690 Imaging densitometer (Bio-Rad) at a resolution of 400×400 dpi.
8. Image analyses
The digitalized images from the CBB stained gels from non-treated cells and cerulein-treated cells were analyzed using the 2-DE gel analysis program PDQuest (Bio-Rad). A comparison report of qualitative and quantitative differences of the samples for each set of data was then generated.
9. In-gel digestion
In-gel digestion of proteins from the CBB stained gels was performed as follows. Spots were excised to 1-2 mm2 slices using a blade, destained with freshly prepared 15 M potassium ferricyanide/50 mM sodium thiosulfate, washed with 25 mM ammonium bicarbonate/50% acetonitrile, and dried in a SpeedVac Plus SC100A (Savant, Holbook, NY, USA) vacuum concentrator. The dried gel pieces were rehydrated with 3-10 µL of 20 ng/µL trypsin solution (Promega), the solution volume being enough for the dried gel to be reswelled. Digestion was continued at 37℃ for 14-18 hours. Tryptic peptides were first extracted using 5% TFA for 40℃ for 1 hour, then 2.5% TFA/50% acetonitrile at 30℃ for 1 hour. The extracted solutions were mixed in an Eppendorf tube, and dried in a vacuum concentrator.
10. MALDI-TOF MS identification of peptide mixtures
The peptide mixture was solubilized with 0.5% TFA for MS analysis. MS was performed on a Micromass M@LDI-TOF (Manchester, UK) with saturated α-cyano-4-hydroxy-trans-cinnamic acid solution in 0.1% TFA/50% acetonitriles as matrix. Mass spectra were externally calibrated with autodigest peaks of trypsin (MH+: 906.505 Da, 1020.504 Da, 1153.574 Da, 2163.057 Da, 2273.160 Da). The peptide mass maps produced by MALDI-TOF MS were searched against the published databases by means of the MS-Fit module in Protein Prospector (http://prospectorucsf.edu/ucsfhtml3.4/msfit.htm) and Mascot (Matrix Science, http://www.matrixscience.com).
11. Statistical analysis
The statistical differences were determined using one-way ANOVA by Newman Keuls test. All values were expressed as means±S.E. of three different experiments. A value of p<0.01 was considered statistically significant.
RESULTS
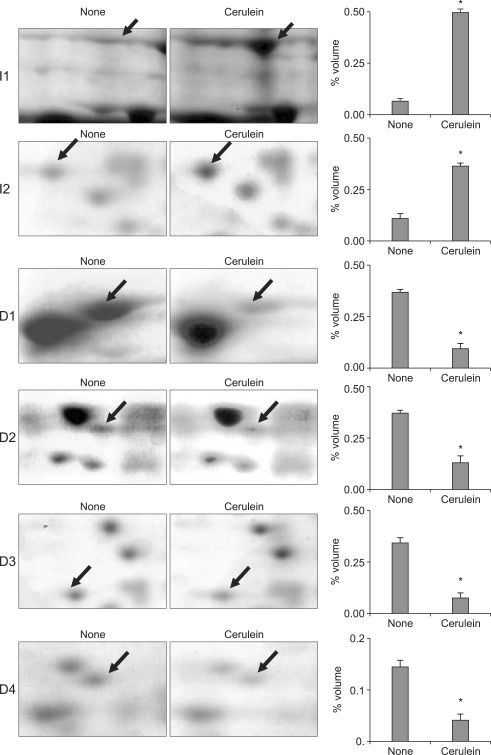
Western blot analysis showed the constitutively expressed aldoase A in cytosolic fractions and Nox in membrane fractions of pancreatic acinar cells at the beginning of the experiment, which was not changed with cerulein treatment for 1 hour (Fig. 1A). The results demonstrate that there was no contamination of cytosol in the membrane fraction used in the present study. 2-DE separation of membrane proteins extracted from non-treated cells and cerulein-treated cells were carried out to determine the proteins differentially expressed by cerulein treatment. For the protein expression profile, 2-DE separation was first performed in the pH range of 3-10 (data not shown). Since major protein changes were shown in the range of pH 5-8, further 2-DE separation was repeatedly performed from the protein extracts of non-treated cells and cerulein-treated cells between pH 5 and pH 8. Fig. 1B shows the representative of three separate experiments. The CBB stained gels were scanned with the GS 690 Imaging densitometer at 400 ppi grayscale level. After spot detection, background subtraction and volume normalization, six differentially expressed proteins were detected in membrane fraction of cerulein-treated cells as compared with non-treated cells (Fig. 1B). The differentially expressed proteins whose expression level was more than three-fold increase or decrease in cerulein-treated cells as compared to non-treated cells were selected for the further analysis. The expression level was determined by the relative spot volume of the proteins as compared to total amount of protein in the gel (Fig. 2).
Fig. 2.
Segments of 2-DE gel map derived from non-treated (none) and cerulein-treated cells (cerulein). The arrows indicate the differentially expressed proteins whose expression level was more than three times higher or lower in cerulein-treated cells than non-treated cells. The expression level was determined by the relative spot volume of the proteins compared with the total amount of the protein in the gel, and is expressed as the percentage volume (right panel). The proteins were identified by the MALDI-TOF MS analysis. A representative gel image and expression level (percentage volume) for each spot is shown. For each spot, the percentage volume was averaged and expressed as a mean±S.E. from three different experiments.
*p<0.01 versus the none-treated cells. The proteins identified with MALDI-TOF MS were (I1) Hsp60, (I2) MASP-2, (D1) PDI, (D2) γ-actin, (D3) ICD3, and (D4) SIAH 1A. Hsp60, heat shock protein 60; PDI, protein disulfide isomerase; ICD3, isocitrate dehydrogenase 3; SIAH 1A, seven in absentia homolog 1A; MASP-2, mannan-binding lectin-associated serine protease-2.
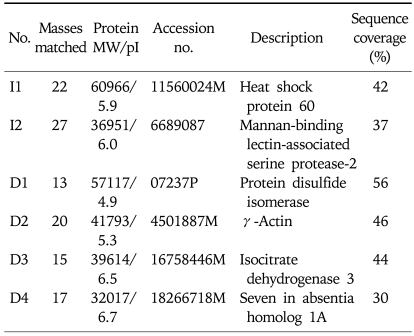
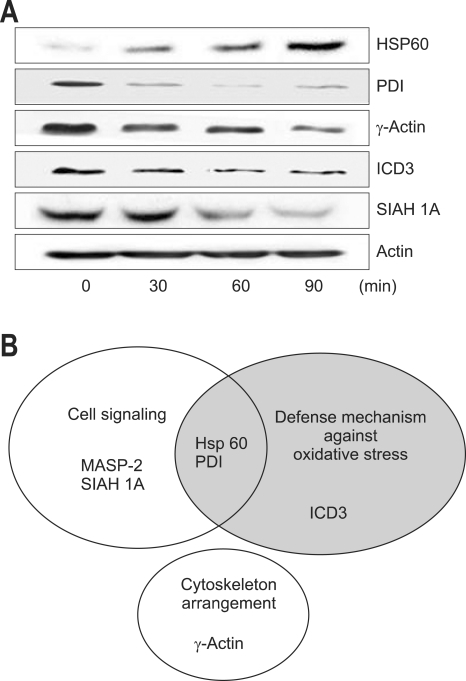
Differentially expressed proteins between non-treated cells and cerulein-treated cells were excised from 2-DE gels and identified using peptide mass fingerprinting. Mascot search using the peptide mass fingerprinting data indicated that the differentially expressed proteins are up-regulated two proteins (MASP-2, Hsp 60) and down-regulated four proteins (PDI, γ-actin, ICH 3, SIAH 1A) by cerulein treatment in pancreatic acinar cells (Table 1). After proteomic analysis, the differentially expressed proteins were verified by Western blot analysis (Fig. 3A). The results confirmed that Hsp 60 was up-regulated while PDI, γ-actin, ICD 3, and SIAH 1A were down-regulated by cerulein in AR42J cells. Total actin as a loading control was not changed by cerulein treatment. MASP-2 was not verified by Western blotting due to lack of the commercially available antibody for the protein.
Table 1.
Proteins That Were Analyzed with MALDI-TOF MS
The differentially expressed proteins whose expression level was more or less than three times in cerulein-treated cells than non-treated cells were shown. Details about the MOWSE program can be found at http://matrixscience.com/help/history.html and http://srs.hgmp.mrc.ac.uk/cgi-bin/mowse. Mass matched expresses the number of peptides identically matched between identified peptides of the in-gel digested samples by MALDI-TOF and the peptides of the known protein; the numbers in parentheses indicate the percentage of peptides identically matched compared with the total numbers of the identified peptide of the sample by MALDI-TOF (http://prospector.ucsf.edu/ucsfhtml4.0/misfit.htm) and Mascot (Matrix Science, http://matrixscience.com).
Fig. 3.
Western blot analysis for the differentially expressed proteins (A) and diagram according to the functional role of the proteins (B). (A) The differentially expressed proteins, determined by proteomics, were assessed by Western blot analysis. The cells (2×106/mL in a 100 mm culture) were treated with cerulein for the indicated time periods. The membrane fractions were isolated from the cells. Protein levels of Hsp60, PDI, γ-actin, ICD3, SIAH 1A, and actin were determined by Western blotting. Since there is no commercially available antibody for MASP-2, the protein could not be verified by Western blotting. (B) Diagram of the proteins shows the overlap among the proteins whose expression levels were more than three-fold different between non-treated cells and cerulean-treated cells. The proteins having cell defense mechanism against cellular stress including oxidative stress include Hsp60, PDI and ICD3. The proteins related to cell signaling are MASP-2, SIAH 1A, Hsp60 and PDI. The protein showing a role in cytoskeleton arrangement is γ-actin. Some proteins share their functional roles. Hsp60 and PDI have functions in cellular defense mechanism and signal transduction.
Hsp60, heat shock protein 60; PDI, protein disulfide isomerase; ICD3, isocitrate dehydrogenase 3; SIAH 1A, seven in absentia homolog 1A; MASP-2, mannan-binding lectin-associated serine protease-2.
The proteins having cell defense mechanism against oxidative stress include Hsp 60, PDI and ICD 3. The proteins related to cell signaling are MASP-2, SIAH 1A, Hsp 60 and PDI. The protein mediating cytoskeleton arrangement is γ-actin. Some proteins share their functional roles. Hsp 60 and PDI have functions in cellular defense mechanism and cell signaling (Fig. 3B). Among the proteins involved in cell defense mechanism, Hsp 60 was induced while PDI and ICD 3 were down-regulated by cerulein treatment in pancreatic acinar cells. Therefore, cerulein may induce oxidative stress which may mediate inflammatory signaling and induce cytoskeletal changes in pancreatic acinar cells.
DISCUSSION
For the pathogenesis of pancreatitis, the activation of oxidant-sensitive transcription factor such as NF-κB and chemokine expression in pancreatic acinar cells have been studied.24-26 Since plasma levels of CCK are increased in the patients with gallstone pancreatitis27 and the defect in CCK regulation was shown in the patients with alcoholic pancreatitis,2 CCK may have a critical role in the development of acute pancreatitis. CCK is a peptide hormone that has important physiologic functions mediated through specific membrane-spanning receptors.28 Binding of CCK to CCK receptor stimulates intracellular Ca2+ levels and mediates the physiologic functions such as pancreatic enzyme secretion including amylase and lipase.29,30 The severity of pancreatitis is related to CCK level and the oxidative indices such as lipid peroxides in serum.31 Since CCK receptor is present in pancreatic acinar cells,32 not in pancreatic ductal cells or islet cells,33 cerulein-induced alterations in protein expression may affect the exocrine function of pancreas by changing physiological roles of pancreatic acinar cells.
In the present study, increase in Hsp 60 was observed in the membrane of cerulein-treated pancreatic acinar cells. Heat shock proteins (Hsp or stress proteins) are produced in response to various stimuli including oxidative stress.34,35 Hsp deals with denatured proteins and to act as scavengers that trap disorderedly polypeptides, thereby protecting the stressed cells from disastrous cascading denaturation. Another important physiological role of Hsp is unfolding and refolding of proteins, which is required for the transport of the proteins across cell membranes. Recent study demonstrated that prior thermal stress can protect the animals from the subsequent development of secretagogue-induced pancreatitis36 and arginine-induced pancreatitis.37 Thermal stress induced intrapancreatic upregulation of Hsp 70 expression.37,38 Water immersion stress increased the expression of Hsp 60 and Hsp 70 and prevented the development of acute pancreatitis in animals.39 The possible role of Hsp 70 in the protection against cholecystokinin-induced pancreatitis has been suggested for its inhibitory effect on pro-inflammatory cytokine expression.40 In contrast, heat-pretreatment had no preventive role on development of trypsin-induced acute pancreatitis in rats.41 In early stage of pancreatitis, digestive enzymes including trypsinogen are prematurely activated within the pancreas. Hsp 70 expression was related to suppression on spontaneous activation of trypsinogen by inhibiting the phosphorylation of extracellular signal-regulated kinase in the pancreas of the rats.42 Previously, Hsp 90 increased in whole cell extracts of pancreatic AR42J cells treated with cerulein (10-8 M) for 24 hours.17 Since Hsp 60 is located at mammalian cell surfaces, we could detect up-regulated Hsp 60 in membrane of cerulein-treated AR42J cells in the present study. Hsp 60 is known to be highly expressed in ovarian cancer43 and ischemia/reperfusion injury of rat heart.44 Since Hsp are induced by oxidative stress as molecular chaperones, Hsp 60 might be induced for its defensive role against cerulein-induced cell damage in the membranes and possibly initiate the intracellular signaling pathways to disease-related gene expression.
The other increased protein MASP-2 is capable of activating complement system and destruction of infectious agents as well as modulating pro-inflammatory cytokines.45 The lectin pathway is part of the innate immune system providing the first line of defense against infections. Mannose-binding lectin forms a complex with MASP-2, which activates complement pathway.46 Cerulein-induced MASP-2 may modulate inflammatory cytokines and it may be involved in initiation of signaling since mannose-binding lectin indirectly interacts with cell surface receptors.
Endoplasmic reticulum (ER) plays a central role in the synthesis and distribution of many cellular proteins. Before proteins can be transported towards their final destination, disulfide bonds essential for a proper folding have to be formed.47 In human fibroblasts, hydrogen peroxide oxidizes the proteins including PDI, which are important in the protein folding machinery in ER.48 Oxidation of the protein-folding machinery may lead to improper folding and/or accumulation of proteins to be secreted since only correctly folded proteins exit the ER. In correctly folded proteins are retained and will be degraded. Therefore, improper functioning of the ER can have serious consequences for the cells. Since cerulein induced oxidative stress to the pancreatic acinar cells, this may lead to damage ER resident chaperone PDI. PDI is reported to regulate signaling by sequestering inactive and activated Stat3.49 PDI expression is highly increased in pancreatic ductal carcinoma.49 These reports demonstrate the possible involvement of PDI in carcinogenesis. Present cerulein-induced decrease in ER chaperone PDI may indicate loss of defensive mechanism of the cells against oxidative stress. Further study should be focused on the relation between acute pancreatitis and ER protein to determine pathophysiologic mechanism of acute pancreatitis.
The cerulein-evoked increase in intracellular Ca++ and ROS are believed to be the main intracellular signal for enzyme and fluid secretion,10 and cytoskeleton function in the pancreas.10 Present cerulein-induced decrease in γ-actin may contribute to cytoskeletal disturbances in the intracellular transport of digestive enzymes, leading to their premature intracellular activation. Cytoskeletal proteins generate a variety of intracellular movements, including intracellular particle transport during mitosismeiosis.50 Possible role of cytoskeletal proteins on cell death or cell differentiation has been suggested.51 Even though total actin mainly consists of β and γ-actin, the gene regulation of β and γ-actin is different in response to thyroid hormone52 and during differentiation.53 γ-actin decreases more quickly than of β-actin during differentiation.53 Present decrement of γ-actin in the membrane may be related to cytoskeletal changes in pancreatic acinar cells during cerulein treatment.
Oxidative stress induces the peroxidation of membrane lipids. ICD plays an important role against lipid peroxidation-mediated oxidative damage through the removal of ROS.54 NAD(P)+-dependent ICDs are enzymes that reduce NAD(P)+ to NAD(P)H using isocitrate as electron donor. ICD provides NAD(P)H, an essential reducing equivalent for antioxidant systems in alcoholic induced liver injury54 and fatty acid synthesis in hepatic cells.55 Since this enzyme appears to be involved in cellular defense system,54-56 the present decrease in ICD 3 in membrane may demonstrate the loss of defense mechanism in cerulein-treated pancreatic acinar cells.
Another decreased protein, seven in absentia homolog (SIAH), is an integral membrane protein functioning as ubiquitin-protein ligase.57 It competitively interacts with Ca++/calmodulin for glutamate receptors.58 Further study should be performed to identify the role of seven in absentia homolog in the membrane of pancreatic acinar cells.
Cerulein increases intracellular Ca2+ levels, which may activate various intracellular signaling mediators for enzyme and fluid secretion.9 Ca2+ may activate ROS production, which may induce the alteration of the cytoskeleton function.10 Changes in cytoskeletons disturb the intracellular transport of digestive enzymes, leading to premature activation of the digeative enzymes.11 Antioxidants including glutathione, SOD and catalase or Ca2+ chelators BAPTA-AM suppressed cytokine expression induced by cerulein in pancreatic acinar cells.12 Taken together, the production of ROS may be mediated by intracellular Ca2+. ROS scavengers or Ca2+ chelators may modify cerulein-induced changes in protein expression of pancreatic acinar cells.
We found that the differentially expressed proteins in the membrane of cerulein-treated cells are related to cell signaling, oxidative stress, and cytoskeleton arrangement. Oxidative stress may induce cerulein-induced cell injury and disturbances in defense mechanism (increase in Hsp 60 and decrease in ICD 3 and PDI) in pancreatic acinar cells. Cerulein may initiate the intracellular signaling pathways to the expression of disease-related genes including MASP-2. Since ROS produced in pancreatic acinar cells may recruit inflammatory cells including neutrophils into the damaged tissues, the early event induced by cerulein in pancreatic acinar cells may affect the behavior of other cells such as immune cells and influence later propagation of inflammatory process in pancreas. The present results are supported by the previous proteomic analysis for whole cell extracts showing that five differentially expressed proteins are related to oxidative stress, cytoskeletal function, and cell signaling.17 With the proteins differentially expressed in the present study using cerulein treatment, the further signaling or pathologic mechanism studies would be performed to determine pathophysiologic mechanism of acute pancreatitis.
ACKNOWLEDGEMENTS
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (R11-2007-040-01002-0) (to H. Kim). H. Kim is grateful to Brain Korea 21 Project, College of Human Ecology, Yonsei University.
References
- 1.Shirohara H, Otsuki M. Plasma cholecystokinin levels in acute pancreatitis. Pancreas. 1997;14:249–254. doi: 10.1097/00006676-199704000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Koide M, Okabayashi Y, Hasegawa H, et al. Plasma cholecystokinin concentration in patients with chronic pancreatitis measured by bioassay. Nippon Shokakibyo Gakkai Zasshi. 1989;86:2419–2424. [PubMed] [Google Scholar]
- 3.Mitchell CJ, Playforth MJ, Kelleher J, McMahon MJ. Functional recovery of the exocrine pancreas after acute pancreatitis. Scand J Gastroenterol. 1983;18:5–8. doi: 10.3109/00365528309181549. [DOI] [PubMed] [Google Scholar]
- 4.Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402–G1414. doi: 10.1152/ajpgi.1998.275.6.G1402. [DOI] [PubMed] [Google Scholar]
- 5.Willemer S, Elsasser HP, Adler G. Hormone-induced pancreatitis. Eur Surg Res. 1992;24(Suppl 1):29–39. doi: 10.1159/000129237. [DOI] [PubMed] [Google Scholar]
- 6.Jensen RT, Wank SA, Rowley WH, Sato S, Gardner JD. Interaction of CCK with pancreatic acinar cells. Trends Pharmacol Sci. 1989;10:418–423. doi: 10.1016/0165-6147(89)90192-2. [DOI] [PubMed] [Google Scholar]
- 7.Sato S, Stark HA, Martinez J, Beaven MA, Jensen RT, Gardner JD. Receptor occupation, calcium mobilization, and amylase release in pancreatic acini: effect of CCK-JMV-180. Am J Physiol. 1989;257:G202–G209. doi: 10.1152/ajpgi.1989.257.2.G202. [DOI] [PubMed] [Google Scholar]
- 8.Lerch MM, Adler G. Experimental animal models of acute pancreatitis. Int J Pancreatol. 1994;15:159–170. [PubMed] [Google Scholar]
- 9.Kanno T. Ca2+ signaling in the pancreatic acinus. Pancreas. 1998;16:273–276. [PubMed] [Google Scholar]
- 10.Jungermann J, Lerch MM, Weidenbach H, Lutz MP, Krüger B, Adler G. Disassembly of rat pancreatic acinar cell cytoskeleton during supramaximal secretagogue stimulation. Am J Physiol. 1995;268:G328–G338. doi: 10.1152/ajpgi.1995.268.2.G328. [DOI] [PubMed] [Google Scholar]
- 11.Dabrowski A, Konturek SJ, Konturek JW, Gabryelewicz A. Role of oxidative stress in the pathogenesis of caerulein-induced acute pancreatitis. Eur J Pharmacol. 1999;377:1–11. doi: 10.1016/s0014-2999(99)00421-5. [DOI] [PubMed] [Google Scholar]
- 12.Yu JH, Kim KH, Kim DG, Kim H. Diphenyleneiodonium suppresses apoptosis in cerulein-stimulated pancreatic acinar cells. Int J Biochem Cell Biol. 2007;39:2063–2075. doi: 10.1016/j.biocel.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 13.Yu JH, Kim KH, Kim H. Role of NADPH oxidase and calcium in cerulein-induced apoptosis: involvement of apoptosis-inducing factor. Ann N Y Acad Sci. 2006;1090:292–297. doi: 10.1196/annals.1378.031. [DOI] [PubMed] [Google Scholar]
- 14.Yu JH, Lim JW, Kim KH, Morio T, Kim H. NADPH oxidase and apoptosis in cerulein-stimulated pancreatic acinar AR42J cells. Free Radic Biol Med. 2005;39:590–602. doi: 10.1016/j.freeradbiomed.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 15.Seo JY, Kim H, Seo JT, Kim KH. Oxidative stress induced cytokine production in isolated rat pancreatic acinar cells: effects of small-molecule antioxidants. Pharmacology. 2002;64:63–70. doi: 10.1159/000056152. [DOI] [PubMed] [Google Scholar]
- 16.Kim H, Seo JY, Roh KH, Lim JW, Kim KH. Suppression of NF-kappaB activation and cytokine production by N-acetylcysteine in pancreatic acinar cells. Free Radic Biol Med. 2000;29:674–683. doi: 10.1016/s0891-5849(00)00368-3. [DOI] [PubMed] [Google Scholar]
- 17.Yu JH, Yun SY, Lim JW, Kim H, Kim KH. Proteome analysis of rat pancreatic acinar cells: implication for cerulein-induced acute pancreatitis. Proteomics. 2003;3:2446–2453. doi: 10.1002/pmic.200300545. [DOI] [PubMed] [Google Scholar]
- 18.Santoni V, Molloy M, Rabilloud T. Membrane proteins and proteomics: un amour impossible? Electrophoresis. 2000;21:1054–1070. doi: 10.1002/(SICI)1522-2683(20000401)21:6<1054::AID-ELPS1054>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 19.Paulsen IT, Sliwinski MK, Nelissen B, Goffeau A, Saier MH., Jr Unified inventory of established and putative transporters encoded within the complete genome of Saccharomyces cerevisiae. FEBS Lett. 1998;430:116–125. doi: 10.1016/s0014-5793(98)00629-2. [DOI] [PubMed] [Google Scholar]
- 20.Christophe J. Pancreatic tumor cell line AR42J cell: an amphicrine model. Am J Physiol. 1994;266:G963–G971. doi: 10.1152/ajpgi.1994.266.6.G963. [DOI] [PubMed] [Google Scholar]
- 21.Sata N, Klonowski-Stumpe H, Han B, Häussinger D, Niederau C. Cytotoxicity of peroxynitrite in rat pancreatic acinar AR4-2J cells. Pancreas. 1997;15:278–284. doi: 10.1097/00006676-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Piiper A, Leser J, Lutz MP, Beil M, Zeuzem S. Subcellular distribution and function of Rab3A-D in pancreatic acinar AR42J cells. Biochem Biophys Res Commun. 2001;287:746–751. doi: 10.1006/bbrc.2001.5651. [DOI] [PubMed] [Google Scholar]
- 23.Masamune A, Sakai Y, Satoh A, Fujita M, Yoshida M, Shimosegawa T. Lysophosphatidylcholine induces apoptosis in AR42J cells. Pancreas. 2001;22:75–83. doi: 10.1097/00006676-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Okumura N, Sakakibara A, Hayakawa T. Pancreatic endocrine function in experimental pancreatolithiasis in dogs. Am J Gastroenterol. 1982;77:392–396. [PubMed] [Google Scholar]
- 25.Aho HJ, Nevalainen TJ, Havia VT, Heinonen RJ, Aho AJ. Human acute pancreatitis: a light and electron microscopic study. Acta Pathol Microbiol Immunol Scand A. 1982;90:367–373. [PubMed] [Google Scholar]
- 26.Neurath MF, Becker C, Barbulescu K. Role of NF-kappaB in immune and inflammatory responses in the gut. Gut. 1998;43:856–860. doi: 10.1136/gut.43.6.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takiguchi S, Suzuki S, Sato Y, et al. Role of CCK-A receptor for pancreatic function in mice: a study in CCK-A receptor knockout mice. Pancreas. 2002;24:276–283. doi: 10.1097/00006676-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Evander A, Ihse I, Lundquist I. Influence of hormonal stimulation by caerulein on acute experimental pancreatitis in the rat. Eur Surg Res. 1981;13:257–268. doi: 10.1159/000128192. [DOI] [PubMed] [Google Scholar]
- 29.Niederau C, Liddle RA, Ferrell LD, Grendell JH. Beneficial effects of cholecystokinin-receptor blockade and inhibition of proteolytic enzyme activity in experimental acute hemorrhagic pancreatitis in mice. Evidence for cholecystokinin as a major factor in the development of acute pancreatitis. J Clin Invest. 1986;78:1056–1063. doi: 10.1172/JCI112661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez G, Townsend CM, Jr, Green DW, et al. Protective action of luminal bile salts in necrotizing acute pancreatitis in mice. J Clin Invest. 1990;86:323–331. doi: 10.1172/JCI114703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park BK, Chung JB, Lee JH, et al. Role of oxygen free radicals in patients with acute pancreatitis. World J Gastroenterol. 2003;9:2266–2269. doi: 10.3748/wjg.v9.i10.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niederau C, Luthen R, Heintges T. Effects of CCK on pancreatic function and morphology. Ann N Y Acad Sci. 1994;713:180–198. doi: 10.1111/j.1749-6632.1994.tb44065.x. [DOI] [PubMed] [Google Scholar]
- 33.Smith JP, Yelamarty RV, Kramer ST, Cheung JY. Effects of cholecystokinin on cytosolic calcium in pancreatic duct segments and ductal cells. Am J Physiol. 1993;264:G1177–G1183. doi: 10.1152/ajpgi.1993.264.6.G1177. [DOI] [PubMed] [Google Scholar]
- 34.Li N, Karin M. Is NF-kappaB the sensor of oxidative stress? FASEB J. 1999;13:1137–1143. [PubMed] [Google Scholar]
- 35.Kaufmann SH, Schoel B, van Embden JD, et al. Heat-shock protein 60: implications for pathogenesis of and protection against bacterial infections. Immunol Rev. 1991;121:67–90. doi: 10.1111/j.1600-065x.1991.tb00823.x. [DOI] [PubMed] [Google Scholar]
- 36.Frossard JL, Bhagat L, Lee HS, et al. Both thermal and non-thermal stress protect against caerulein induced pancreatitis and prevent trypsinogen activation in the pancreas. Gut. 2002;50:78–83. doi: 10.1136/gut.50.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tashiro M, Ernst SA, Edwards J, Williams JA. Hyperthermia induces multiple pancreatic heat shock proteins and protects against subsequent arginine-induced acute pancreatitis in rats. Digestion. 2002;65:118–126. doi: 10.1159/000057713. [DOI] [PubMed] [Google Scholar]
- 38.Bhagat L, Singh VP, Song AM, et al. Thermal stress-induced HSP70 mediates protection against intrapancreatic trypsinogen activation and acute pancreatitis in rats. Gastroenterology. 2002;122:156–165. doi: 10.1053/gast.2002.30314. [DOI] [PubMed] [Google Scholar]
- 39.Takacs T, Rakonczay Z, Jr, Varga IS, et al. Comparative effects of water immersion pretreatment on three different acute pancreatitis models in rats. Biochem Cell Biol. 2002;80:241–251. doi: 10.1139/o02-006. [DOI] [PubMed] [Google Scholar]
- 40.Rakonczay Z, Jr, Takacs T, Mandi Y, et al. Water immersion pretreatment decreases pro-inflammatory cytokine production in cholecystokinin-octapeptide-induced acute pancreatitis in rats: possible role of HSP72. Int J Hyperthermia. 2001;17:520–535. doi: 10.1080/02656730110081785. [DOI] [PubMed] [Google Scholar]
- 41.Rakonczay Z, Jr, Takacs T, Ivanyi B, et al. Induction of heat shock proteins fails to produce protection against trypsin-induced acute pancreatitis in rats. Clin Exp Med. 2002;2:89–97. doi: 10.1007/s102380200012. [DOI] [PubMed] [Google Scholar]
- 42.Schafer C, Williams JA. Stress kinases and heat shock proteins in the pancreas: possible roles in normal function and disease. J Gastroenterol. 2000;35:1–9. doi: 10.1080/003655200750024443. [DOI] [PubMed] [Google Scholar]
- 43.Kimura E, Enns RE, Thiebaut F, Howell SB. Regulation of HSP60 mRNA expression in a human ovarian carcinoma cell line. Cancer Chemother Pharmacol. 1993;32:279–285. doi: 10.1007/BF00686173. [DOI] [PubMed] [Google Scholar]
- 44.Sakai J, Ishikawa H, Kojima S, Satoh H, Yamamoto S, Kanaoka M. Proteomic analysis of rat heart in ischemia and ischemia-reperfusion using fluorescence two-dimensional difference gel electrophoresis. Proteomics. 2003;3:1318–1324. doi: 10.1002/pmic.200300432. [DOI] [PubMed] [Google Scholar]
- 45.Turner MW. The role of mannose-binding lectin in health and disease. Mol Immunol. 2003;40:423–429. doi: 10.1016/s0161-5890(03)00155-x. [DOI] [PubMed] [Google Scholar]
- 46.Moller-Kristensen M, Jensenius JC, Jensen L, et al. Levels of mannan-binding lectin-associated serine protease-2 in healthy individuals. J Immunol Methods. 2003;282:159–167. doi: 10.1016/j.jim.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Braakman I, Helenius J, Helenius A. Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO J. 1992;11:1717–1722. doi: 10.1002/j.1460-2075.1992.tb05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Vlies D, Pap EH, Post JA, Celis JE, Wirtz KW. Endoplasmic reticulum resident proteins of normal human dermal fibroblasts are the major targets for oxidative stress induced by hydrogen peroxide. Biochem J. 2002;366:825–830. doi: 10.1042/BJ20020618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cecconi D, Astner H, Donadelli M, et al. Proteomic analysis of pancreatic ductal carcinoma cells treated with 5-aza-2'-deoxycytidine. Electrophoresis. 2003;24:4291–4303. doi: 10.1002/elps.200305724. [DOI] [PubMed] [Google Scholar]
- 50.Goldsmith M, Yarbrough L, van der Kooy D. Mechanics of motility: distinct dynein binding domains on alpha- and beta-tubulin. Biochem Cell Biol. 1995;73:665–671. doi: 10.1139/o95-074. [DOI] [PubMed] [Google Scholar]
- 51.Ogawa M. Anticancer drugs and pharmacologic actions. Nippon Rinsho. 1997;55:1017–1023. [PubMed] [Google Scholar]
- 52.Sarkar S, Chaudhury S, Sarkar PK. Regulation of beta- and gamma- actin mRNA by thyroid hormone in the developing rat brain. Neuroreport. 1997;8:1267–1271. doi: 10.1097/00001756-199703240-00042. [DOI] [PubMed] [Google Scholar]
- 53.Lloyd C, Gunning P. Beta- and gamma-Actin genes differ in their mechanisms of down-regulation during myogenesis. J Cell Biochem. 2002;84:335–342. doi: 10.1002/jcb.10014. [DOI] [PubMed] [Google Scholar]
- 54.Yang JH, Park JW. Oxalomalate, a competitive inhibitor of NADP+-dependent isocitrate dehydrogenase, enhances lipid peroxidation-mediated oxidative damage in U937 cells. Arch Biochem Biophys. 2003;416:31–37. doi: 10.1016/s0003-9861(03)00291-1. [DOI] [PubMed] [Google Scholar]
- 55.Lee JH, Yang ES, Park JW. Inactivation of NADP+-dependent isocitrate dehydrogenase by peroxynitrite: implications for cytotoxicity and alcohol-induced liver injury. J Biol Chem. 2003;278:51360–51371. doi: 10.1074/jbc.M302332200. [DOI] [PubMed] [Google Scholar]
- 56.Jung UJ, Kwon OS, Park YB, Huh TL, Lee MK, Choi MS. Effect of oxalomalate on lipid metabolism and antioxidant defense system in rats. J Biochem Mol Toxicol. 2003;17:295–302. doi: 10.1002/jbt.10092. [DOI] [PubMed] [Google Scholar]
- 57.Wheeler TC, Chin LS, Li Y, Roudabush FL, Li L. Regulation of synaptophysin degradation by mammalian homologues of seven in absentia. J Biol Chem. 2002;277:10273–10282. doi: 10.1074/jbc.M107857200. [DOI] [PubMed] [Google Scholar]
- 58.Ishikawa K, Nash SR, Nishimune A, Neki A, Kaneko S, Nakanishi S. Competitive interaction of seven in absentia homolog-1A and Ca2+/calmodulin with the cytoplasmic tail of group 1 metabotropic glutamate receptors. Genes Cells. 1999;4:381–390. doi: 10.1046/j.1365-2443.1999.00269.x. [DOI] [PubMed] [Google Scholar]