Abstract
Background/Aims
Endoscopic treatment as an alternative to surgery has become increasingly popular for improving the quality of life. Although photodynamic therapy (PDT) has been used for the endoscopic treatment of digestive cancer, its curative efficacy remains unclear. We evaluated the curative efficacy of PDT in superficial esophageal cancer in inoperable patients.
Methods
Ten male patients with histologically proven early esophageal cancer (surgery was contraindicated for age > 80 years, surgery was contraindicated, Karnofsky performance status of at least 30%, or refusal of surgery) were intravenously injected with a hematoporphyrin derivative (2 mg/kg), and PDT was performed 48 h later. The response to treatment was assessed by gastroscopy with biopsies.
Results
The mean follow-up period was 27.6 months (range, 9.6-58.7 months). Endoscopic ultrasonography revealed that all ten cases were at tumor stage T1. Complete remission (CR) to initial and subsequent PDT was observed in all patients. For the CR cases, the recurrence rate was 10% (1/10) and the time from initial PDT to recurrence was 9.6 months.
Conclusions
For patients in whom surgery is risky or refused, PDT may represent an acceptable alternative treatment modality, especially for superficial esophageal cancer without lymph node metastasis. However, a study involving long-term follow-up in a large population is needed for confirmation.
Keywords: Photodynamic therapy, Endoscopic treatment, Early esophageal cancer, Hematoporphyrin
INTRODUCTION
The conventional therapy for esophageal cancer is esophagectomy, a complex surgical procedure with a mortality rate of 3 to 5%.1-3 Superficial esophageal cancer is not usually associated with locoregional lymph node involvement or distant metastasis.4,5 Therefore, local treatment of superficial esophageal cancer may reasonably considered as a solution for the cure. However, the success of local treatment depends on accurate staging, which might be difficult with currently existing technology. Before the introduction of endosonography, the diagnosis of an early stage cancer in the gastrointestinal tract was established by pathological examination of the resected surgical specimen. High-resolution endosonography allows the diagnosis to be made in situ with a sensitivity and specificity of nearly 90%.6,7 The endosonographic in situ diagnosis of an early cancer offers local endoscopic therapy to inoperable patients with intention for cure, after evaluating the histological classification. Endoscopic treatments such as endoscopic mucosal resection (EMR), photodynamic therapy (PDT), and non-selective laser destruction or electrodestruction have become increasingly popular as an alternative to surgery in the hope of offering the patient a superior quality of life by avoiding a surgical procedure and its postoperative problems.8,9
The PDT is a means of selective sensitization of precancerous or malignant lesions using a systemically applicable photosensitizer with subsequent, endoscopically controlled, photochemically induced tissue ablation.10 Several investigators have reported the use of PDT in early esophageal cancer with encouraging results.11,12 We report on the application of PDT with intention for cure in selected patients with superficial esophageal cancer.
MATERIALS AND METHODS
1. Patients
Between January 2001 and November 2007, ten patients with superficial esophageal cancer (T1) were included in a prospective study. All treated patients were either ineligible for or had refused conventional surgery. All patients were hospitalized for the diagnostic work-up and PDT until at least 2 days after PDT. Early esophageal cancer was categorized endoscopically as superficial erosive/plaque-like (the cancerous area appeared as a slightly depressed lesion against a reddish background or slightly elevated, with a granular or coarse knobby surface), congestive/flat (a flat patch of localized edema and congestion), or papillary type (protruded or circumscribed polypoid lesion).13 The tumor extension was assessed using computed tomography (CT) and endoscopic ultrasonography (EUS; Olympus GF-UM2000; Olympus, Tokyo, Japan) in all patients (no extension beyond the muscular layer or metastasis to regional lymph nodes). The tumor staging was based on the depth of invasion to the digestive wall which was delineated as having five-layer echoic patterns. The tumor was classified as uT1 when the middle hyperechoic layer was intact (mucosal or submucosal invasion only). All patients were informed of this clinical study and gave written informed consent.
2. Photodynamic therapy
The hematoporphyrin derivative was administered intravenously at a dose of 2 mg/kg body weight. For light distribution, we used flexible cylindrical diffuser probes (biolitec, Stirling, UK) mounted on a 400-µm quartz fiber with an active length of 1.0 to 2.0 cm at the distal tip. The light source was a diode laser system (Ceralas PDT 633, CeramOptec, Bonn, Germany) with a maximum power output of 2 W and a wavelength of 633±3 nm. The power emitted by the diffuser tip was calibrated to 400 mW/cm before PDT was conducted and was checked subsequently using an integrating sphere power meter. The laser irradiation was performed 48 h after injecting the drug. The mean irradiation time was 450 s (400-540 s), and the energy dose was 120-200 (mean 160) J/cm2 of the diffuser length.
3. Evaluating the tumor response
A complete tumor response was defined as having normal or cicatrical mucosa on endoscopic examination, with negative for malignancy, which was proven by biopsy at the previous tumor site. Chromoendoscopy was applied during follow up to assist the identification of small residual or recurrent lesions. The procedure was done using Lugo's iodine solution diluted 1:1 and sprayed over the esophageal mucosa beginning at the site proximal to the original area of early esophageal cancer and beyond it.
The tumor response was defined as a failure (incomplete) if there was evidence of a residual tumor in endoscopic biopsies (4-6) 1 month after PDT. In cases with incomplete responses, a second PDT was performed. The response was evaluated 6 months after the first PDT session in all living patients as an overall assessment of the efficacy of the treatment.
4. Follow-up
A follow-up endoscopy was performed 48 hours after PDT to determine the initial therapeutic effect. Subsequent endoscopies with biopsies (4-6) were performed 1 and 2 months after PDT to assess the evolution of tumor necrosis and the existence of residual tumor and at 3-month intervals up to 1 year, and at 6-month intervals thereafter. EUS and CT were performed at 3, 6, and 12 months.
RESULTS
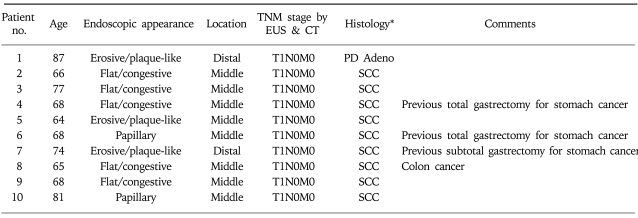
Ten male patients with superficial esophageal tumors were included in the study (mean age 71.3 years; range 64-82 years; Table 1). In five patients, surgical treatment was abandoned due to severe associated medical illnesses, including cardiovascular diseases in three and chronic respiratory failure in two patients. In other patients, the choice of PDT was made due to the cancer at another sites (previous surgical treatment of three stomach cancers and one colon cancer), age older than 80, or the patient's refusal of surgery. Nine tumors were squamous cell carcinoma and one was poorly differentiated adenocarcinoma. Endoscopic appearance was determined as erosive/plaque-like in cases, flat/congestive in five cases, and papillary-type in two cases. Eight tumors were located at the mid esophagus, and two were located at the distal esophagus.
Table 1.
Characteristics of Ten Male Patients Evaluated in this Study
*SCC, squamous cell carcinoma; PD Adeno, poorly differentiated adenocarcinoma.
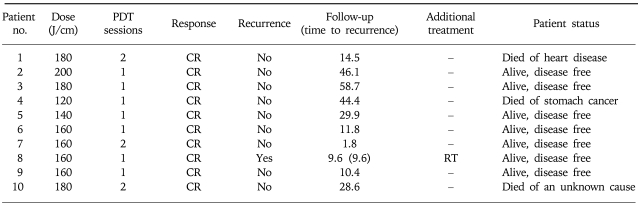
According to the TNM classification, all ten patients were diagnosed as having endosonographic uT1 (Table 2). Severe necrotic change was observed 2 days after PDT. Mucosal healing and complete re-epithelialization took an average of 4 weeks (Fig. 1). A complete response was obtained in all ten patients (100%). Seven patients received a single session of the treatment and three patients received two sessions due to residual tumors determined by n endoscopic biopsies 4 weeks after the initial PDT.
Table 2.
Results of Photodynamic Therapy (PDT)
CR, complete response; RT, radiotherapy.
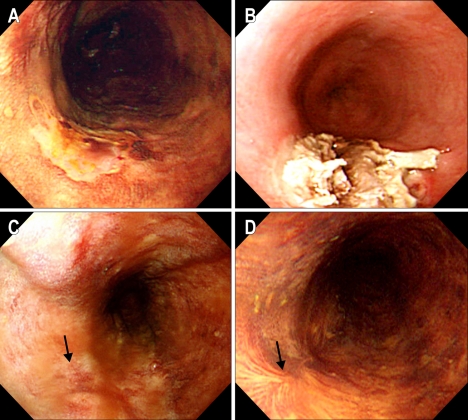
Fig. 1.
(Case #9) (A) Before PDT. A slightly depressed lesion unstained with Lugol's solution is seen on the mid-esophagus. (B) Two days after PDT. Endoscopy shows coagulation necrosis with ulcer at the PDT treated lesion. (C) One month after PDT. The previously PDT-induced ulcerative lesion have healed. (D) Five months after PDT. The scar well stained with Lugol's solution is seen at the previously cancerous lesion, and there is no remaining tumor in the biopsied specimens.
The mean follow-up period was 27.6 months (9.6-58.7 months). Local recurrence occurred in one patient (10%) after 9.6 months (case #8). During the follow-up, three patients died of cause not related to the esophageal cancer. Case #1 was uT1 with poorly differentiated adenocarcinoma and died at 14.5 months from a heart attack (he had underlying two-vessel coronary disease). Case #4 died from advanced stomach cancer. Case #10 died from an unknown cause.
Cutaneous photosensitization and pigmentation occurred in four patients, but was not severe. Esophageal stenosis occurred in one patient who received circumferential illumination of the esophagus, and required repeated dilation followed by stenting (Fig. 2).
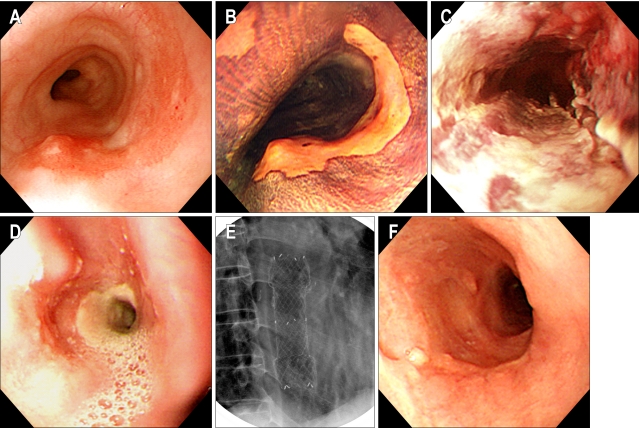
Fig. 2.
(Case #1) (A, B) A flat reddish lesion unstained with Lugol's solution is seen on the mid-esophagus (the biopsied shows specimen squamous cell carcinoma). (C) Two days after photodynamic therapy. Endoscopy shows circumferential coagulation necrosis with an ulcer at the PDT treated lesion. (D) Two months after PDT. Endoscopy shows luminal narrowing with fibrous scarring at the site of the PDT-treated lesion. (E) Fluoroscopic image shows a metal stent at the site of the esophageal stricture. (F) Endoscopy shows the improvement at the stricture site 2 months after stent removal.
DISCUSSION
Based on numerous surgical reports, a favorable postoperative outcome in terms of survival is currently expected in early gastrointestinal cancer.1,2,14 In an effort to obtain a better quality of life, less invasive treatment is more commonly chosen for such cancer with a low possibility of accompanying metastatic lymph nodes.4,5 In larger studies, the reported morbidity and mortality rates were 20-40% and 4-10%, respectively.3,15 Endoscopic treatment is another treatment option in gastrointestinal oncology, and its therapeutic efficacy is now being evaluated from various perspectives. In addition to reports on endoscopic mucosal resection (EMR), that have been provided almost exclusively by sources from Asia,16-18 there have been contributions concerning non-selective Nd:YAG-laser vaporization and case studies on electrosurgical thermocoagulation of early cancer.19 However, the implementation of these techniques for palliative purposes does not appear to be justified. A major advantage of EMR is in that the pathologist can examine the resected specimen in order to establish the absence of tumor tissue. However, the technique bears the risk of esophageal perforation and hemorrhage, and requires endoscopic expertise.
By contrast, PDT allows selective tumor destruction in all accessible sections of the gastrointestinal tract. PDT makes use of the ability of light to activate photosensitizing compounds stored in tissue. The treatment of early-stage esophageal cancers with PDT has had promising results. PDT of small esophageal tumors achieved a complete response in 87% (99/114) of patients.11 These patients either had refused surgery or were judged to be unsuitable for surgery on medical grounds. PDT of 31 early squamous cell carcinomas (SCCs) in 24 patients resulted in a complete response in 26 tumors (84%), without recurrence during the mean follow-up of 2 years. In our series, PDT achieved a high rate (100%) of complete tumor response in selected patients with superficial esophageal tumors.
Accurate staging of esophageal tumors by endoscopy is difficult, and incorrect staging may reduce the apparent efficacy of PDT. For PDT to be successful in early-stage esophageal cancer the lesion should be visible on endoscopy, and the peripheral margins should be well delineated. Tumor invasion should be limited to within 1 cm of the lumen surface since depth of 1 cm is the limit of tissue penetration by 630 nm wavelength light.20 For example, PDT of 36 early-stage SCCs of the esophagus led to a complete response in 31 tumors after one or two sessions. Two tumors that did not respond or that recurred were subsequently found to be infiltrating the submucosa and had therefore been staged incorrectly.21 In this study, all uT1 cases achieved CR after PDT. The rate of complete response in terms of the T stage with EUS staging for uT1 was 100% (10/10) Therefore, PDT seems best suited for the complete destruction of small tumors of limited depth (mucosa or submucosa), i.e., superficial or T1 cancer.
A major drawback of PDT using a hematoporphyrin derivative is the inconvenience caused by patients having to avoid direct exposure to the sun for at least 1 month. In our series, the frequency of cutaneous complications such as hyperpigmentation due to the hematoporphyrin derivative was 33% (4/12), although none was severe. The most problematic complication is the development of esophageal strictures. Strictures typically present with the development of progressive solid food dysphagia within 3 weeks after PDT and occur in 27 to 34% of treated individuals.10,22 The major PDT-related complication was esophageal stenosis, which occurred in one patient who received circumferential illumination of the esophagus, and required repeated sessions of dilation and stenting.
In conclusion, PDT effectively destroyed small superficial esophageal tumors, as shown by the 100% complete response rate at 6 months. However, one case of local recurrence (10%) occurred after 9.6 months. This recurrence rate seems to be higher compared to surgery. Our study had several limitations. There were not enough study cases. In addition, the patients were elderly, and evaluating the survival benefit of PDT was not feasible. However, in patients for whom the surgery may have surmounting risks or who refuse the surgery, PDT may be a reasonable alternative treatment modality, especially in superficial tumors without lymph node metastasis.
References
- 1.Nomura S, Kaminishi M. Surgical treatment of early gastric cancer. Dig Surg. 2007;24:96–100. doi: 10.1159/000101895. [DOI] [PubMed] [Google Scholar]
- 2.Park CH, Song KY, Kim SN. Treatment results for gastric cancer surgery: 12 year's experience at a single institute in Korea. Eur J Surg Oncol. 2007 doi: 10.1016/j.ejso.2007.03.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Thomas P, Doddoli C, Lienne P, et al. Changing patterns and surgical results in adenocarcinoma of the esophagus. Br J Surg. 1997;84:119–125. [PubMed] [Google Scholar]
- 4.Aurello P, D'Angelo F, Rossi S, et al. Classification of lymph node metastases from gastric cancer: comparison between N-site and N-number systems. Our experience and review of the literature. Am Surg. 2007;73:359–366. [PubMed] [Google Scholar]
- 5.Rice TW, Zuccaro G, Jr, Adelstein DJ, et al. Esophageal carcinoma: depth of tumor invasion is predictive of regional lymph node status. Ann Thorac Surg. 1998;65:787–792. doi: 10.1016/s0003-4975(97)01387-8. [DOI] [PubMed] [Google Scholar]
- 6.Fockens P, van den Brande JHM, van Dullemen HM, et al. Endosonographic T-staging of esophageal carcinoma: a learning curve. Gastrointest Endosc. 1996;44:58–62. doi: 10.1016/s0016-5107(96)70230-4. [DOI] [PubMed] [Google Scholar]
- 7.Natsugoe S, Yoshinaka H, Moringa T, et al. Assessment of tumor invasion of the distal esophagus in carcinoma of the cardia using endoscopic ultrasonography. Endoscopy. 1996;28:750–755. doi: 10.1055/s-2007-1005599. [DOI] [PubMed] [Google Scholar]
- 8.Pech O, May A, Gossner L, et al. Curative endoscopic therapy in patients with early esophageal squamous-cell carcinoma or high-grade intraepithelial neoplasia. Endoscopy. 2007;39:30–35. doi: 10.1055/s-2006-945040. [DOI] [PubMed] [Google Scholar]
- 9.Takeshita K, Tani M, Inoue H, et al. Endoscopic treatment of early oesophageal or gastric cancer. Gut. 1997;40:123–127. doi: 10.1136/gut.40.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang KK, Kim JY. Photodynamic therapy in Barrett's esophagus. Gastrointest Endosc Clin N Am. 2003;13:483–489. doi: 10.1016/s1052-5157(03)00049-7. [DOI] [PubMed] [Google Scholar]
- 11.Sibille A, Lambert R, Souquet JC, et al. Long-term survival after photodynamic therapy for esophageal cancer. Gastroenterology. 1995;108:337–344. doi: 10.1016/0016-5085(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 12.Regula J, MacRobert AJ, Gorchein A, et al. Photosensitization and photodynamic therapy of esophageal, duodenal, and colorectal tumors using 5-aminolevulinic acid induced protoporphyrin IX: a pilot study. Gut. 1995;36:67–75. doi: 10.1136/gut.36.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sivak MV. Gastroenterologic endoscopy. 2nd ed. Pennsylvania: Saunders; 2000. pp. 510–511. [Google Scholar]
- 14.Lo SS, Wu CW, Chen JH, et al. Surgical results of early gastric cancer and proposing a treatment strategy. Ann Surg Oncol. 2007;14:340–347. doi: 10.1245/s10434-006-9077-x. [DOI] [PubMed] [Google Scholar]
- 15.Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908–914. doi: 10.1056/NEJM199903253401202. [DOI] [PubMed] [Google Scholar]
- 16.Takekoshi T, Baba Y, Ota H, et al. Endoscopic resection of early gastric cancer: results of a retrospective analysis of 308 cases. Endoscopy. 1994;26:352–358. doi: 10.1055/s-2007-1008990. [DOI] [PubMed] [Google Scholar]
- 17.Nakagoe T, Tanaka K, Yasutake T, et al. Long-term outcomes of intragastric endoscopic mucosal resection using a modified Buess technique for early gastric cancer. Dig Surg. 2003;20:141–147. doi: 10.1159/000069391. [DOI] [PubMed] [Google Scholar]
- 18.Youn JC, Youn YH, Kim TI, et al. Factors affecting long-term clinical outcomes of endoscopic mucosal resection of early gastric cancer. Hepatogastroenterology. 2006;53:643–647. [PubMed] [Google Scholar]
- 19.Sibille A, Descamps C, Jonard P, et al. Endoscopic Nd:YAG treatment of superficial gastric carcinoma: experience in 18 Western inoperable patients. Gastrointest Endosc. 1995;42:340–345. doi: 10.1016/s0016-5107(95)70134-6. [DOI] [PubMed] [Google Scholar]
- 20.Okunaka T, Kato H, Conaka C, et al. Photodynamic therapy of esophageal carcinoma. Surg Endosc. 1990;4:150–153. doi: 10.1007/BF02336594. [DOI] [PubMed] [Google Scholar]
- 21.Grosjean P, Savary JF, Mizeret J, et al. Photodynamic therapy for cancer of the upper aerodigestive tract using tetra(m-hydroxyphenyl)chlorine. Clin Laser Med Surg. 1996;14:281–287. doi: 10.1089/clm.1996.14.281. [DOI] [PubMed] [Google Scholar]
- 22.Wolfsen HC, Hemminger LL, Wallace MB, et al. Clinical experience of patients undergoing photodynamic therapy for Barrett's dysplasia or cancer. Aliment Pharmacol Ther. 2004;20:1125–1131. doi: 10.1111/j.1365-2036.2004.02209.x. [DOI] [PubMed] [Google Scholar]