Abstract
Background/Aims
We conducted this study to identify the risk factors for finding gallbladder polyps (GBP) in Korean subjects during health screening, and to determine the nature of the association between the presence of metabolic syndrome (MS) and the development of GBP
Methods
A total of 1,523 subjects were enrolled, comprising 264 with GBP (81 women and 183 men) and 1,259 controls (696 women and 563 men with normal GB). Body mass index (BMI), waist circumference (WC), blood pressure (BP), insulin, fasting blood sugar (FBS), lipids, liver enzymes, hepatitis B antigens (HBs Ag), and hepatitis C antibodies (HCV Ab) were measured. MS was considered to be present when three or more of the NCEP-ATPIII (National Cholesterol Education Program-Adult Treatment Panel III) criteria were satisfied. Insulin resistance was calculated by homeostasis model assessment of insulin resistance (HOMA-IR). Independent risk factors were analyzed by logistic regression analysis.
Results
Univariate analysis revealed that the risk factors for GBP were age, sex, WC, smoking history, BP, BMI, FBS, serum lipids, HOMA-IR score, and MS. Multivariate logistic regression analysis revealed that the risk factors for GBP were presence of MS (Odds Ratio (OR)=2.35, 95%Confidence Interval (CI)=1.53-3.60), being male (OR=2.34, 95%CI=1.72-3.18), HOMA-IR score>2.5 (OR=1.64, 95%CI=1.19-2.26), and higher WC (OR=1.4, 95%CI=1.05-1.88). MS was present in 20.8% and 5.9% of GBP patients and controls, respectively, and was the highest risk factor for GBP.
Conclusions
MS, male, insulin resistance, and abdominal obesity are probably risk factors for GBP, with MS appearing to be strongly associated with GBP in Koreans.
Keywords: Gallbladder polyp, Risk factor, Metabolic syndrome, Insulin resistance
INTRODUCTION
Polypoidal lesions of the gallbladder (GBP) may be defined as elevations of gall bladder (GB) mucosa1 and are usually found incidentally by ultrasonography (USG) or in resected GB after cholecystectomy. The detection of GBP has increased particularly since the widespread use of USG as a diagnostic modality. Such polypoid lesions have been found in 0.004 to 13.8% of resected GB2 and observed in 3-12.8% of GB assessed by USG.3,4
We occasionally found that GBP observed incidentally by the USG during health screening disappeared during follow-up, and the majority of these cases have undergone successful weight reduction and improved lipid profiles. In terms of prevalence of GBP, ethnic differences and even temporal differences in same area have been reported.4 Obesity,5 glucose intolerance,6 or increased BMI7,8 has been reported in the English literature to be related to the prevalence of GBP. These reports indicate that the risk factors of GBP are probably related to lifestyle factors such as eating habits or activities. Moreover, obesity and impaired glucose intolerance are also components of metabolic syndrome, which is related to lifestyle factors. No previous study has been conducted on the relation between GBP and metabolic syndrome. This study was carried out to explore the association between the two as well as to identify the risk factors of GBP found by USG on health screening in the Korean population.
MATERIALS AND METHODS
1. Materials
We conducted a retrospective, cross-sectional study on individuals that had undergone health screening at the Healthcare System Gangnam Center of Seoul National University Hospital. To assess the prevalence rate of GBP, we investigated subjects who had received USG of abdomen from October 2003 to March 2007.
To investigate the risk factors of GBP, the study included 264 subjects (the GBP group) found to have GBP by USG of abdomen and 1,259 subjects (the control group) with a normal GB by USG screened from February to April 2007. Lab results including insulin level were available for all subjects. Those with GBP and other benign diseases of the hepatobiliary or renal system such as hepatic cysts or renal cysts were included in the GBP group. However, those without a GB due to previous cholecystectomy were excluded from the control group.
2. Methods
1) Diagnosis of GBP
After 10 hours of fasting, abdominal USG was performed using a SEQUOIA 512 (Acuson, Charleston road, CA, USA) with 3.5 MHz convex probe. Nine radiologists were involved. GBP were diagnosed as immobile echoes protruding from inside the GB wall into the lumen.3 Diameters of the largest polyps, polyp numbers, and the presence of gallstones were recorded.
2) Analysis of risk factors
(1) Questionnaire: We reviewed age, sex, smoking history, drinking history, and past medical history including hypertension, diabetes and hyperlipidemia for all 1,523 study subjects.
(2) Physical examination: Body weights and heights were measured, and body mass indexes (BMI) were calculated (weight (Kg) divided by height (m) squared). Waist circumference (WC) was measured at the midpoint between the lower border of the rib cage and the iliac crest, and body fat percentages were measured using bipolar electric impedance (In Body 4.0, Seoul Korea). Blood pressure readings were obtained after a 10 min rest.
(3) Biochemical laboratory test: After at least 10 hours of fasting, blood sample was drawn to determine fasting glucose (FBS), GOT, GPT, alkaline phosphatase, total cholesterol, triglyceride, high density lipoprotein cholesterol (HDL-C), insulin, HBsAg, anti hepatitis C antibody (HCV Ab), thyroid function test (FT4, TSH) and tumor markers (CA 19-9, CEA, AFP).
(4) Insulin resistance: The homeostasis model assessment (HOMA-IR) was used to assess insulin resistance.9 HOMA-IR was calculated using the following formula: HOMA index=[fasting insulin (µU/mL)·fasting glucose (mmol/L)]/22.5, high index of insulin resistance a value >2.5.9 HOMA-IR is known to closely correlate with the insulin sensitivity index as determine using the standard euglycemic hyperinsulinemic clamp, as shown by Matthews et al.9
(5) Definition of metabolic syndrome: According to NCEP-ATP III criteria10 metabolic syndrome is defined if three or more of the following criteria are satisfied: ① Abdominal obesity; Waist circumference (WC) in men>102 cm and in women>88 cm. ② Hypertriglyceridemia; ≥1.7 mmol/L (150 mg/dL). ③ Low HDL cholesterol; <1.03 mmol/L (40 mg/dL) in men and <1.29 mmol/L (50 mg/dL) in women. ④ High blood pressure; ≥130/85 mmHg. ⑤ High fasting plasma glucose; ≥6.1 mmol/L (110 mg/dL). We also applied WHO-APR (World Health Organization-Asian Pacific Region) criteria11 for abdominal obesity (WC in men>90 cm and in women>80 cm) rather than the NCEP-ATP III criteria (also called the modified ATP III criteria). Cases that were under antihypertensive treatment were considered as having a high BP, and those receiving antidiabetic treatment were regarded to have a high FBS.
6) Categorization of each variable: BMI was grouped into 4 categories11; BMI <18.5 kg/m2 as underweight, ≥18.5 kg/m2 but <23 kg/m2 as normal weight, ≥23 kg/m2 but <25 kg/m2 as overweight, and ≥25 kg/m2 as obesity.
FBS was also grouped into 3 categories; <110 mg/dL as normal, ≥110 mg/dL but <126 mg/dL as impaired, and ≥126 mg/dL as diabetic.
Total cholesterol was categorized using the NCEP-ATPIII (National Cholesterol Education Program, ATPIII)10 criteria into 3 groups; <200 mg/dL, ≥200 mg/dL but <240 mg/dL, ≥240 mg/dL.
For HDL-C, 2 categories were made; <40 mg/dL and ≥40 mg/dL or more in men, and <50 mg/dL and ≥50 mg/dL or more in women.
Triglyceride was also categorized into 2 groups; <150 mg/dL, and ≥150 mg/dL.
3. Statistical analyses
Results are expressed as mean±SD. The Chi square-test was utilized to analyze the categorical differences. The t-test was used to test differences in characteristics between the groups. Univariate analysis results are expressed by the odds ratio (OR) and 95% confidence interval (CI). To evaluate the putative potential risk factors associated with GBP, a stepwise multivariate analysis with logistic regression was performed. A value of p<0.05 was considered statistically significant. Analyses were performed using the Statistical Package for the Social Sciences (SPSS v10.0, Chicago, IL, USA).
RESULTS
1. Prevalence rate
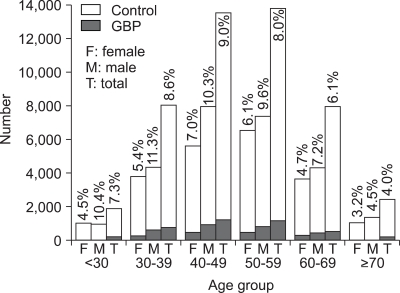
Among 43,606 subjects who received USG of abdomen at our center from October 2003 to March 2007, 3,701 subjects (8.5%) were found to have GBP. GBP were found in 2,468 subjects (10.5%) of 23,555 men and in 1,233 subjects (6.2%) of 20,051 women. An analysis of prevalence rates by age showed peaks in the fifth decade for men (10.3%) and in women (7.0%) (Fig. 1). The overall prevalence rate of GBP during the three month-study period (February to April 2007) was 7.9% (459/5,827). Prevalence of GBP during the study period was 9.2% (294/3,209) in men and 6.3% (165/2,618) in women.
Fig. 1.
The prevalence rate of gallbladder polyps according to gender and age from October 2003 to March 2007.
% means prevalence rate; F, female; M, male; T, total.
2. Risk factors
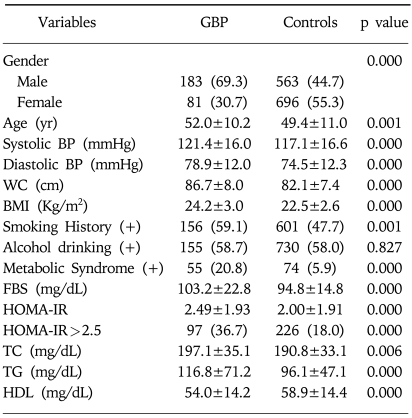
The proportion of men in the GBP group was significantly higher (69.3 vs. 44.7%) than in control group. Cases in the GBP group were older (52 vs. 49 years), had a greater waist circumference (86.7 vs. 82.1 cm), body mass index (24.2 vs. 22.5 kg/m2), and higher systolic (121 vs. 117 mmHg) and diastolic (79 vs. 75 mmHg) blood pressures, FBS (103 vs. 95 mg/dL), HOMA-IR (2.5 vs. 2.0) and lipid profiles (total cholesterol, triglyceride, and inverse HDL-C values)(Table 1). The frequencies of a smoking history and the presence of metabolic syndrome were also significantly higher in the GBP group than in the control group (59.1 vs. 47.7% and 20.8 vs. 5.9%, respectively). However, no differences were observed in the mean of body fat or of liver enzymes, or presence of HBs Ag or HCV-Ab (data not shown). There was no difference in the frequency of alcohol consumption, either (data not shown). Moreover, the mean of three tumor markers and the frequency of elevation of tumor markers were similar in the two groups (data not shown).
Table 1.
Mean Differences or Frequency Comparison of Variables between Gallbladder Polyp and Control Groups
Data are n (%) or mean±S.E.
GBP, gallbladder polyps; yr, year; BP, blood pressure; WC, waist circumference; BMI, body mass index; FBS, fasting blood glucose; HOMA-IR, homeostasis model assessment of insulin resistance; TC, total cholesterol; TG, triglyceride; HDL, high density lipoprotein cholesterol.
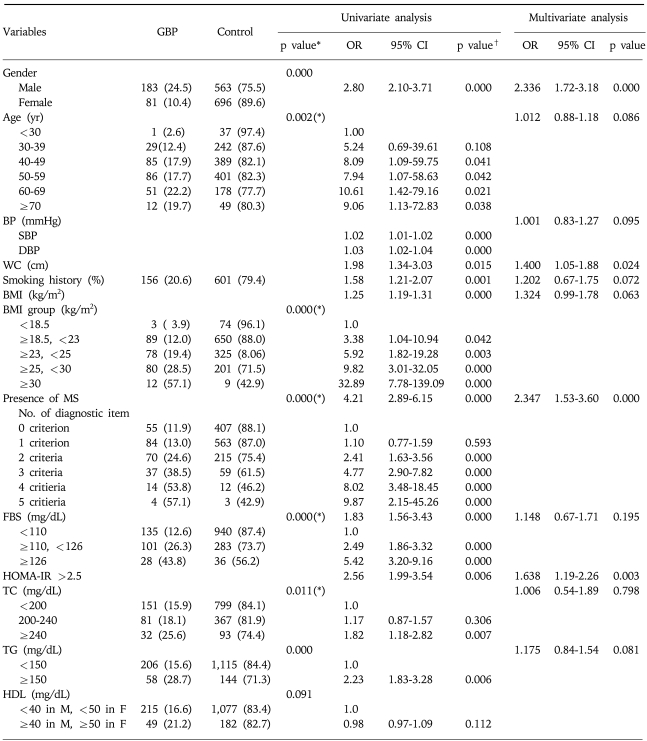
Table 2 shows the crude (non-controlled) risks determined by univariate analysis. When anthropometric variables were tested, a higher obesity grade, an older age, a higher BMI category, a greater number of diagnostic metabolic syndrome criteria, a higher FBS, and a higher total cholesterol were associated with a higher probability of GBP. Male gender, high blood pressure, waist circumference, smoking history, BMI, metabolic syndrome, and a HOMA-IR of >2.5 were found to be significantly associated with higher risk of GBP. However, liver enzymes, viral hepatitis markers, thyroid function profiles, and tumor markers were not associated with GBP (data not shown.).
Table 2.
Univariate and Multivariate Analysis of the Risk Factors for Gallbladder Polyp
Data are n (%).
*Data are analysed by chi-squre test, esp.(*): p for trend.
†Data are analysed by logistic regression test.
Items with Bold character show statistical significance according to multivariate logistic regression analysis.
GBP, gallbladder polyps; OR, odds ratio; CI, confidence interval; yr, year; BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; WC, waist circumference; (+), presence; BMI, body mass index; MS, metabolic syndrome; FBS, fasting blood glucose; HOMA-IR, homeostasis model assessment of insulin resistance; TC, total cholesterol; TG, triglyceride; HDL, high density lipoprotein cholesterol.
Stepwise multivariate logistic regression analysis of the risk factors identified by univariate analysis confirmed four factors as being associated with GBP (Table 2). Metabolic syndrome increased GBP risk, with an OR of 2.35 (95%CI, 1.53-3.60; p=0.000). The other three significant anthropometric variables were: male gender (OR 2.34, 95%CI 1.72-3.18, p=0.000), insulin resistance (HOMA-IR>2.5) (OR 1.64, 95%CI 1.19-2.26, p=0.003), and abdominal obesity (WC) (OR 1.40, 95%CI 1.05-1.88, p=0.024).
3. Characteristics of GBP
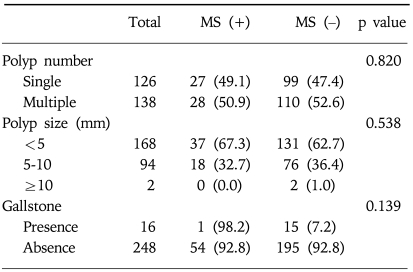
No statistical relations were found among polyp number or size, or gallstones and the presence of metabolic syndrome in the GBP group (Table 3).
Table 3.
Difference of Characteristics of Gallbladder Polyps according to Presence of Metabolic Syndrome within Gallbladder Polyp Group
Data are n (%).
GBP, gallbladder polyps; MS, metabolic syndrome.
DISCUSSION
When GBP are detected by USG, their clinical significance lies in the malignant potential. However, the majority of GBP cases are benign, and are most commonly cholesterol polyps with a relative frequency of about 46-70% of all GBP cases.1-3 Cholesterol polyp is a polypoid form of cholesterolosis, and is characterized by mucosal villous hyperplasia with excessive cholesterol esters accumulation within epithelial macrophages, and may be either be diffuse or polypoid in form.1 Cholesterol polyps are typically small (2-10 mm in diameter) and pedunculated, and have no neoplastic potential.1,12 The majority (99.2%) of the GBP in this study were less than 10 mm in size, and thus, were probably cholesterol polyps. The etiology of this condition is poorly understood but is believed to be associated with the absorption of cholesterol from the bile or from blood. Holzbach et al13 suggested that cholesterolosis might be derived from the direct deposition of cholesterol from the blood, in a manner analogous to the plaque formation in atherosclerosis. Other studies by Tilvis et al14 have suggested that alterations in hepatic cholesterol metabolism and altered mucosal esterification of free sterols from bile may contribute to the development of cholesterolosis of the GB.15
Studies on the prevalence of GBP can be classified roughly into 2 types: studies based on resected GB and USG findings in healthy subjects. We also conducted this study in healthy adults and obtained a prevalence rate was 8.5%, which is similar to those of the studies conducted in New Zealand,16 China6 and Japan.17 However, compared to two previous studies in Korea, this prevalence of 8.5% is high; the previously reported rates were 2.9%7 and 2.2%.8 This difference may have been due to different places of residence, i.e., a rural versus an urban location, and period of time during which study was conducted. For example, Hayashi et al4 reported regional and temporal differences in the prevalence rate of GBP in Japan to be 3.9% in a rural area in 1988 and 12.3% in an urban area in 1993. Rates in healthy adults according to gender generally indicated4-8,17,18 a higher prevalence in men, which concurs with our findings, although one study in the Danish population19 showed no gender difference. Hence, it appears reasonable to conclude that Asian men have a higher risk of developing GBP. However, the mechanism that mediates this gender-determined risk remains unknown.
A review of the literatures regarding the risk factors of GBP revealed that results are contradictory. Schinchi et al18 and Jorgensen et al19 reported that smoking tends to be inversely associated with GBP, and the prevalence of GBP is not associated with age, sex, social factors, weight factors, intake of alcohol, glucose tolerance or plasma lipids. However, studies in China,6 Korea,7,8 and Japan5 concluded that the life-style related factors such as BMI, glucose intolerance, and obesity are also risk factors of GBP. The present study shows metabolic syndrome, insulin resistance, and abdominal obesity are significantly associated with the risk of GBP. Based on the above-mentioned hypotheses concerning the pathogenesis of cholesterol polyps, blood lipid profile seems to be related to the risk factors of GBP found in healthy adults. However, we found no association between lipid profile, glucose, or BMI and the risk of GBP by multivariate analysis despite their significance by univariate analysis.
Metabolic syndrome, which has become a major public-health concern worldwide, was found to be most strongly associated with the risk of GBP in the present study. Moreover insulin resistance was also found to be associated with the risk of GBP. We found that metabolic syndrome was associated with a 2.5-fold risk of GBP, and that insulin resistance (HOMA-IR>2.5) had a 1.6-fold risk in developing GBP. In addition, the presence of three metabolic syndrome criteria was found to confer a 4.8-fold increased risk of GBP, and the presence of five criteria were found to confer a 10-fold risk.
Lim et al20 reported that the age-adjusted prevalence of metabolic syndrome according to NCEP-ATP III was significantly higher in the 2001 survey than in 1998 survey (28.0 vs. 23.6%, p<0.01) conducted in the Korean population.
These findings that metabolic syndrome is a risk factor of GBP and that the prevalence of metabolic syndrome is increasing, explain the results of Hayashi et al4 and the difference between the prevalence rates found by Shim et al7 and those found during the present study.
In the present study, 16 cases (6.1%) of GBP group had concomitant gallstones or sludge, but only one had metabolic syndrome, indicating no significant interaction between gallstones and metabolic syndrome in GBP group in this study.
This present study has several limitations. First of all, GBP histologic type was not determined. Second, the sample size was small. Third, the study was conducted on subjects at single healthcare center. Last, it provides few details concerning the mechanism of the associations of metabolic syndrome or insulin resistance and the development of GBP. Nevertheless, this is the first study to show an association between GBP and metabolic syndrome or insulin resistance in a population.
In conclusion, GBP found incidentally during health screening appear to be strongly associated with metabolic syndrome similar to that for cardiovascular disease and diabetes mellitus. Moreover, our findings also imply that insulin resistance plays an important role in the pathogenesis of these diseases. In the future, there is a need to elucidate the association of metabolic syndrome to GBP according to its histologic classification.
ACKNOWLEDGMENTS
We thank MRCC (medical research collaborating center) of Seoul National University Hospital for assistance in statistical analysis.
References
- 1.Persley KM. Feldman: Sleisenger & Fordtran's gastrointestinal and liver disease. 8th ed. W.B. Saunders; 2006. Acalculous cholecystitis, cholesterolosis, adenomyomatosis, and polyps of the gallbladder; pp. 1448–1456. [Google Scholar]
- 2.Yang HL, Sun YG, Wang Z. Polypoid lesions of the gallbladder: diagnosis and indications for surgery. Br J Surg. 1992;79:227–229. doi: 10.1002/bjs.1800790312. [DOI] [PubMed] [Google Scholar]
- 3.Lee KF, Wong J, Li JCM, Lai PBS. Polypoid lesions of the gallbladder. Am J Surg. 2004;188:186–190. doi: 10.1016/j.amjsurg.2003.11.043. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi Y, Liu JH, Moriguchi H, et al. Prevalence of polypoid lesions of the gallbladder in urban and rural areas of Japan: comparison between 1988 and 1993. J Clin Gastroenterol. 1996;23:158–159. doi: 10.1097/00004836-199609000-00021. [DOI] [PubMed] [Google Scholar]
- 5.Segawa K, Arisawa T, Niwa Y, et al. Prevalence of gallbladder polyps among apparently healthy Japanese: ultrasonographic study. Am J Gastroenterol. 1992;87:630–633. [PubMed] [Google Scholar]
- 6.Chen CY, Lu CL, Chang FY, Lee SD. Risk factors for gallbladder polyps in the Chinese population. Am J Gastroenterol. 1997;92:2066–2068. [PubMed] [Google Scholar]
- 7.Shim SG, Lee KT, Lee JK, et al. Prevalence and risk factors of gallbladder polyps in health screening subjects. Korean J Med. 1999;57:1014–1020. [Google Scholar]
- 8.Kim SY, Lee HS, Lee YS, et al. Prevalence and risk factors of gallbladder polyp in adults living in Daegu and Gyeongbuk provinces. Korean J Gastroenterol. 2006;48:344–350. [PubMed] [Google Scholar]
- 9.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 10.National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 11.Western Pacific Regional Office of the World Health Organization; The International Obesity Task Force. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia; 2000. [Google Scholar]
- 12.Majeski JA. Polyps of the gallbladder. J Surg Oncol. 1986;32:16–18. doi: 10.1002/jso.2930320105. [DOI] [PubMed] [Google Scholar]
- 13.Holzbach RT, Marsh M, Tang P. Cholesterolosis: physical-chemical characteristics of human and diet-induced canine lesions. Exp Mol Pathol. 1977;27:324–338. doi: 10.1016/0014-4800(77)90004-1. [DOI] [PubMed] [Google Scholar]
- 14.Tilvis RS, Aro J, Strandberg TE, Lempinen M, Miettinen TA. Lipid composition of bile and gallbladder mucosa in patients with acalculous cholesterolosis. Gastroenterology. 1982;82:607–615. [PubMed] [Google Scholar]
- 15.Watanabe F, Hanai H, Kaneko E. Increased AcylCoA-cholesterol ester acyltransferase activity in galllbladder mucosa in patients with gallbladder cholesterolosis. Am J Gastroenterol. 1998;93:1518–1523. doi: 10.1111/j.1572-0241.1998.00473.x. [DOI] [PubMed] [Google Scholar]
- 16.Collett JA, Allan RB, Chisholm RJ, Wilson IR, Burt MJ, Chapman BA. Gallbladder polyps: prospect ive study. J Ultrasound Med. 1998;17:207–211. doi: 10.7863/jum.1998.17.4.207. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto M, Yamagata Z, Takeda Y, YodaY, Kobayashi K, Fujino MA. The relationship between gallbladder disease and smoking and drinking habits in middle-aged Japanease. J Gastroenterol. 2002;37:455–462. doi: 10.1007/s005350200066. [DOI] [PubMed] [Google Scholar]
- 18.Schinchi K, Kono S, Honjo S, Imanishi K, Hirohata T. Epidemiology of gallbladder polyps: an ultrasonographic study of male self-defense officials in Japan. Scand J Gastroenterol. 1994;29:7–10. doi: 10.3109/00365529409090429. [DOI] [PubMed] [Google Scholar]
- 19.Jorgensen T, Jensen KH. Polyps in the gallbladder: a prevalence study. Scand J Gastroenterol. 1990;25:281–286. [PubMed] [Google Scholar]
- 20.Lim S, Park KS, Lee HK, Cho SI. Changes in the characteristics of metabolic syndrome in Korea over the period 1998-2001 as determined by Korean national health and nutrition examination surveys. Diabetes Care. 2005;28:1810–1812. doi: 10.2337/diacare.28.7.1810. [DOI] [PubMed] [Google Scholar]