Abstract
Background/Aims
The relationship between Helicobacter pylori infection and ghrelin is controversial. We compared ghrelin levels in gastric mucosa and plasma between H. pylori-positive and -negative subjects, and between before and after H. pylori eradication.
Methods
We compared the ghrelin levels in the antrum, body, and fundus between H. pylori-positive and -negative subjects; in stomach tissues between before and after H. pylori eradication; and in plasma and tissue in 10-person cohorts between before and after H. pylori eradication therapy. Body mass index, age, and sex were controlled for when comparing ghrelin levels.
Results
Stomach ghrelin levels (in the antrum, body, and fundus) did not differ significantly between H. pylori-positive and -negative samples (p=0.095, 0.316, and 0.897, respectively), or between before and after H. pylori eradication (p=0.19, 0.178, and 0.513, respectively). In the ten-person cohort study, plasma ghrelin levels in the eight H. pylori-positive subjects were 2,260 pg/mL (range, 1,280-3,770 pg/mL) and 1,900 pg/mL (range, 1,350-5,200 pg/mL) before and after eradication therapy (p=0.871). Stomach ghrelin levels did not differ significantly in the eight H. pylori-positive subjects between before and after H. pylori eradication (p=0.732, 0.618, and 0.435 in the antrum, body, and fundus, respectively), or between six eradicated and two noneradicated subjects (p=0.071, 0.857, 0.429, and 0.857 in the antrum, body, fundus, and plasma, respectively).
Conclusions
These results show that H. pylori infection has no effect on stomach ghrelin levels and that eradication therapy does not influence plasma or tissue ghrelin levels.
Keywords: Helicobacter pylori, Ghrelin, Eradication
INTRODUCTION
Ghrelin is an orexigenic hormone secreted by gastric endocrine cells/oxyntic glands,1 and has been implicated in both the sensation of mealtime hunger and the long-term regulation of body weight.2-7 Its levels are low in obesity,7 high in malnutrition,8 high after an overnight fast,7,8 and suppressed by food intake.7,8 Levels show sexual dimorphism, women in the late follicular stage having higher levels than men.9 It is also known that ghrelin secretion is suppressed by somatostatin, and that growth hormone does not influence ghrelin secretion.9
Helicobacter pylori is known to play a crucial role in gastritis, gastroduodenal ulcer disease, and in gastric carcinoma. Moreover, chronic corpus gastritis and mucosal atrophy reduce gastric acid secretions, and gastrin and somatostatin are regulatory hormones that affect gastric acid secretion in the gastric mucosa.10 H. pylori infection has been shown to reduce plasma somatostatin11 and increase plasma gastrin concentrations.10 Recently, a study revealed that central ghrelin administration inhibits gastric acid secretion, but that peripheral ghrelin administration does not modify gastric acid secretion in rats.12
Thus the possibility exists that ghrelin and H. pylori infection may be associated. In the literature, there is a debate regarding the relation of ghrelin levels to H. pylori infection in humans. Gokcel et al found that H. pylori has no effect on plasma ghrelin concentrations.13 The authors studied 39 H. pylori-positive and -negative women, whose plasma ghrelin levels were measured by enzyme immunoassay; however, the plasma ghrelin concentrations of H. pylori-positive and -negative groups did not differ significantly. On the other hand, Nwokolo et al showed that plasma ghrelin increases significantly after H. pylori eradication, and that this leads to an increased appetite and weight gain, and contributes to increasing obesity and gastroesophageal reflux disease.14 They measured plasma ghrelin before and after H. pylori eradication in 10 patients, and found that plasma ghrelin increased profoundly in asymptomatic subjects after cure.
In the present study, we determined ghrelin levels in the gastric mucosa and in plasma, and compared these findings for H. pylori-positive and -negative subjects, and before and after H. pylori eradication.
MATERIALS AND METHODS
1. Study design
The study was composed of three stages. First, we compared the ghrelin levels of the antrum, body, and fundus in H. pylori-positive and -negative subjects. Second, the ghrelin levels of stomach tissues were compared before and after H. pylori eradication. Finally, we measured the plasma and tissue ghrelin levels in 10 cohorts before and after H. pylori eradication therapy.
2. Subjects
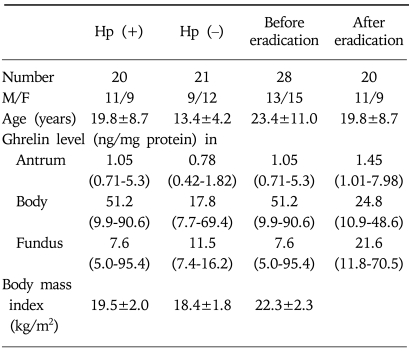
To compare tissue ghrelin levels in H. pylori-positive and -negative subjects, biopsied tissues (antrum, body, and fundus) stored at -70℃ were used. The number of specimens was 16 in antrum, 21 in body, and 8 in fundus of H. pylori-negative subjects (age: 13.4±4.2 years, male to female ratio: 9/12), and 19 in antrum, 20 in body, and 10 in fundus of H. pylori-positive subjects (age: 19.8±8.7 years, M/F: 11/9, Table 1).
Table 1.
Characteristics of Subjects in Comparison Study
Hp, Helicobacter pylori.
Ghrelin levels are expressed as the median (interquartile range).
Biopsied tissues (antrum, body, and fundus) stored at -70℃ were also used to compare tissue ghrelin levels before and after H. pylori eradication. The number of specimens was 15 in antrum, 28 in body, and 5 in fundus of pre-eradication subjects (age: 23.4±11.0 years, M/F: 13/15), and 19 in antrum, 20 in body, and 10 in fundus of post-eradication subjects (age: 19.8±8.7 years, M/F: 11/9, Table 1).
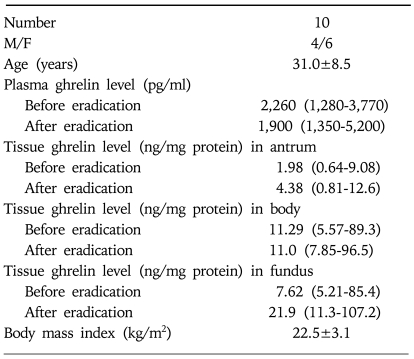
Ten volunteers (age: 31.0±8.5 years, male to female ratio: 4/6) were then recruited and enrolled for the cohort study (Table 2). Criteria for enrollment included the presence of a history of significant gastric discomfort, such as dyspepsia, and no history of undergoing endoscopy or taking peptic ulcer medication. Informed consent was obtained from each of subjects, and the study design was reviewed and approved by the Samsung Medical Center Institutional Review Board.
Table 2.
Characteristics of Subjects in Cohort Study
Ghrelin levels are expressed as the median (interquartile range).
3. Controlling for body mass index (BMI), age, and sex
BMI was calculated as body weight (kg) divided by height2 (m2), and obesity was defined as a BMI >25. Subjects were divided into younger (≤11 years) and older (≥12 years) groups. Obesity, age, and sex were considered confounding variables for ghrelin level comparisons, and they were adjusted for in the statistical analysis.
4. Gastroduodenal endoscopy and biopsy samples
Ten volunteers underwent gastroduodenal endoscopy after overnight fasting. Blood sampling was also performed for a fasting plasma ghrelin study. Six biopsy specimens were taken at three sites along the greater curvature of the stomach, 2 from the antrum, 2 from the body, and 2 from the fundus. Three specimens from the 3 different sites were stored at -70℃ for ghrelin measurement by ELISA and the DNA identification of H. pylori. The other 3 specimens were forwarded to a pathologist for Giemsa staining. Eight of the 10 subjects were H. pylori-positive and two were negative.
5. DNA identification of H. pylori
H. pylori positivity was defined as a positive vacA s1 by PCR amplification and by the identification of the microorganism by Giemsa staining. The primers used in this study were Korean specific VASK-F (5'-MTKRTTTCTCTCGCTTT-3') and VASK-R (5'-GGGATYTGTATAAGTCGTATT-3').15 All samples stored were reexamined for vacA s1.
6. H. pylori eradication
Ten volunteers including the two who were H. pylori-negative received a triple regimen, consisting of amoxicillin 1 g twice daily, metronidazole 500 mg twice daily, plus bismuth subcitrate 600 mg twice daily, for 14 days. Follow-up endoscopy and blood sampling was conducted 4 weeks after the triple regimen. Biopsy specimens were reexamined for H. pylori vacA and ghrelin levels. The two H. pylori-negative subjects were administered the triple regimen to investigate its potential effect on ghrelin levels in the stomach in the absence of H. pylori infection.
7. Peptide extraction from stomach tissue
Peptides were extracted from stomach biopsy specimens using an enzyme immunoassay kit and instructions obtained from (http://www.PhoenixPeptide.com). Briefly, 200 µL of 0.1 M acetic acid was added to each tissue sample, which was then transferred to a boiling water bath for 10 min. After cooling on ice, samples were homogenized in polypropylene tubes, and centrifuged at 13,000 g for 15 min. The pellets obtained were resuspended in 3 N NaOH (200 µL) and analyzed for total protein using a bicinchoninic acid (BCA) assay kit (Sigma, St. Louis, MO, USA).
8. Enzyme-linked immunosorbent assay (ELISA) for ghrelin
Ghrelin levels in stomach biopsy specimens and in plasma were measured using a commercially available human ghrelin ELISA kit (Phoenix Pharmaceuticals, Belmont, CA, USA). The immunoplate in this kit was pre-coated with a secondary antibody; nonspecific binding sites were blocked. 50 µL standard or samples, 25 µL of primary antiserum, and 25 µL of biotinylated ghrelin were added to each well and incubated at room temperature for 2 hours. After washing the immunoplate 5 times with assay buffer, 100 µL of streptavidin-horseradish peroxidase (SA-HRP) solution was added, and the mixture was then incubated at room temperature for 1 hour. After washing the immunoplate 6 times with assay buffer, 100 µL of 3,3'5,5'-tetramethylbenzidine (TMB) solution was added, and incubated at room temperature for 1 hour. The enzymesubstrate reaction was stopped using hydrogen chloride (HCl) and optical density (OD) at 450 nM was determined using a microplate reader (Thermomax, Columbia, MD, USA). All samples were measured in duplicate.
9. Statistical analysis
Statistical analysis was performed using SAS statistics software, version 8.2 (SAS Institute Inc., Cary, NC, USA). The Mann-Whitney test and Wilcoxon's signed rank test were used to compare ghrelin levels in the groups. The effects of confounding variables such as obesity, age, and sex on ghrelin levels were analyzed by multiple linear regression with variable transformation for normality (Box-Cox power transformation). Data are expressed as medians and interquartile ranges.
RESULTS
1. Comparison between H. pylori-positive and -negative subjects
The median (interquartile range) ghrelin levels in the antrum, body, and fundus of H. pylori-positive samples were 1.05 (0.71-5.30), 51.2 (9.9-90.6), and 7.6 ng/mg protein (5.0-95.4 ng/mg protein), respectively (Table 1). The corresponding values in H. pylori-negative samples were 0.78 (0.42-1.82), 17.8 (7.7-69.4), and 11.5 ng/mg protein (7.4-16.2 ng/mg protein). No significant difference in stomach ghrelin levels was found between H. pylori-positive and -negative samples (p=0.095, 0.316, and 0.897, respectively). Multiple linear regression analysis after adjusting for obesity, age, and sex also showed no significant difference between the ghrelin levels of H. pylori-positive and -negative samples (p=0.164, 0.451, and 0.157, respectively).
2. Ghrelin levels before and after H. pylori eradication
The median (interquartile range) ghrelin levels in antrum, body, and fundus before H. pylori eradication were 1.05 (0.71-5.30), 51.2 (9.9-90.6), and 7.6 ng/mg protein (5.0-95.4 ng/mg protein), respectively (Table 1). Corresponding values in H. pylori-eradicated samples were 1.45 (1.01-7.98), 24.8 (10.9-48.6), and 21.6 ng/mg protein (11.8-70.5 ng/mg protein), respectively. These values were not significantly different (p=0.19, 0.178, and 0.513, respectively).
3. Comparison of the 10 cohorts before and after H. pylori eradication
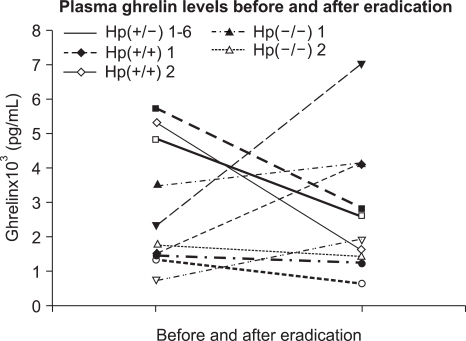
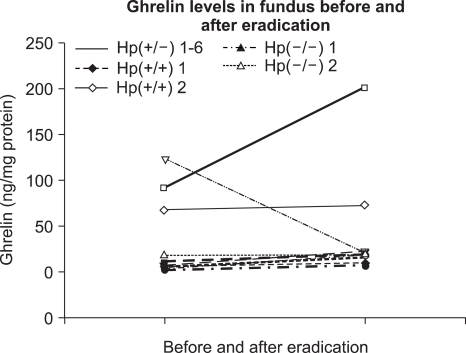
Two subjects were H. pylori-negative and 8 had H. pylori gastritis. Two of the 8 H. pylori-positive cohorts failed eradication. All subjects received the above triple regimen and underwent follow-up endoscopy. Plasma ghrelin levels of the 8 H. pylori-positive subjects were 2,260 (1,280-3,770) and 1,900 pg/mL (1,350-5,200 pg/ml), before and after eradication therapy, which was not significantly different (p=0.871, Fig. 1). Of the two that failed the eradication, one showed an increase in ghrelin levels and the other a decrease. The median (interquartile range) ghrelin levels in antrum, body, and fundus of the 8 H. pylori-positive subjects before eradication were 1.98 (0.64-9.08), 11.29 (5.57-89.3), and 7.62 ng/mg protein (5.21-85.4 ng/mg protein), respectively (Table 2). After eradication these were 4.38 (0.81-12.6), 11.0 (7.85-96.5), and 21.9 ng/mg protein (11.3-107.2 ng/mg protein), respectively, which were not significantly different (p=0.732, 0.618, and 0.435, respectively, Fig. 2). The difference in ghrelin levels between the 6 eradicated and 2 non-eradicated subjects was not statistically significant (antrum p=0.071, body p=0.857, fundus p= 0.429, and plasma p=0.857). In the cases of the two that were initially H. pylori-negative, one showed a ghrelin reduction in the antrum and body, and an increase in the fundus and plasma after eradication therapy. The other showed a reduction in ghrelin levels in the body and plasma, and an increase in the antrum and fundus.
Fig. 1.
Plasma ghrelin levels before and after eradication in 10 cohorts (p=0.871). Hp(+/-) means that the subject is initially H. pylori (+) and H. pylori (-) after eradication.
Fig. 2.
Fundus ghrelin levels before and after eradication in 10 cohorts (p=0.618). Hp(+/-) means that the subject is initially H. pylori (+) and H. pylori (-) after eradication.
DISCUSSION
Our results show that H. pylori infection has no effect on stomach ghrelin levels and that eradication therapy does not influence plasma or tissue ghrelin levels. Ghrelin levels were measured in both random and cohort samples. All data revealed the absence of a significant relationship between H. pylori infection and ghrelin levels.
The limitation of our study is that the number of cohorts was small. However, ghrelin levels showed no tendency to change in either, plasma or tissue samples. Moreover, data obtained from the cohort study is supported by the results of the random storage samples.
We did not measure plasma ghrelin in the random subjects because their plasma samples were unavailable. However, we believe that this does not influence our conclusion, because plasma ghrelin concentrations reflect gastric ghrelin production, which is supported by the finding that gastrectomy reduces plasma ghrelin concentrations by 65%.16
Because gastric ghrelin production is regulated by multiple nutritional and hormonal factors,17-19 it is not easy to control for the variables that may effect ghrelin levels, in terms of statistical analysis. Fasting plasma ghrelin concentrations in obesity are low and correlate negatively with body mass index.20 Ghrelin changes significantly throughout development and also correlates with anthropometric and metabolic parameters.21 Its secretion is sexually dimorphic in humans, with women in the late follicular stage have higher levels than men.9 Therefore, variables such as obesity, age, and sex were analyzed by a test, named multiple linear regression with variable transformation for normality.
One investigator disagreed with Nwokolo's findings,14 which showed that plasma ghrelin increased after H. pylori cure. He indicated that the Nwokolo's suggestion that H. pylori eradication leads to a hyperghrelinemic state, which drives an increased appetite is not physiologically feasible, as any transient appetite increase would be counterbalanced by any increase in adiposity, which in turn would suppress ghrelin levels.22
An interesting study concerning H. pylori infection and gastric and plasma ghrelin in Mongolian gerbils was reported recently.23 Although no significant difference was found in total gastric ghrelin levels between a control and H. pylori group at four weeks after inoculation, the ghrelin level was found to be significantly lower in the H. pylori group than in the control group at 17-23 weeks after inoculation. Also, they reported that fasting plasma total ghrelin levels were significantly higher in the H. pylori group than in controls 17 weeks after H. pylori inoculation, though this difference was not significant at 4 or 23 weeks. Their data in gerbils was not consistent with the results of two recent human studies,13,14 which were also discrepant. These discrepancies might be attributable to differences in the methods of measuring ghrelin peptide, patient selection, or interspecies differences in the distribution of extragastric ghrelin.
In conclusion, our results show that ghrelin levels in plasma and stomach tissue are not directly related to H. pylori infection. We speculate that ghrelin levels are not determined by H. pylori infection alone, and that multiple factors including H. pylori infection, nutritional change, and hormonal factors should be investigated in future studies to evaluate the role of H. pylori infection in determining ghrelin levels.
References
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 2.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 3.Wren AM, Small CJ, Ward HL, et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology. 2000;141:4325–4328. doi: 10.1210/endo.141.11.7873. [DOI] [PubMed] [Google Scholar]
- 4.Asakawa A, Inui A, Kaga T, et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- 5.Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86:5992. doi: 10.1210/jcem.86.12.8111. [DOI] [PubMed] [Google Scholar]
- 6.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 7.Cummings DE, Weigle DS, Frayo RS, et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623–1630. doi: 10.1056/NEJMoa012908. [DOI] [PubMed] [Google Scholar]
- 8.Nagaya N, Uematsu M, Kojima M, et al. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/catabolic factors. Circulation. 2001;104:2034–2038. doi: 10.1161/hc4201.097836. [DOI] [PubMed] [Google Scholar]
- 9.Barkan AL, Dimaraki EV, Jessup SK, Symons KV, Ermolenko M, Jaffe CA. Ghrelin secretion in humans is sexually dimorphic, suppressed by somatostatin, and not affected by the ambient growth hormone levels. J Clin Endocrinol Metab. 2003;88:2180–2184. doi: 10.1210/jc.2002-021169. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko H, Konagaya T, Kusugami K. Helicobacter pylori and gut hormones. J Gastroenterol. 2002;37:77–86. doi: 10.1007/s005350200000. [DOI] [PubMed] [Google Scholar]
- 11.Moss SF, Legon S, Bishop AE, Polak JM, Calam J. Effect of Helicobacter pylori on gastric somatostatin in duodenal ulcer disease. Lancet. 1992;340:930–932. doi: 10.1016/0140-6736(92)92816-x. [DOI] [PubMed] [Google Scholar]
- 12.Sibilia V, Pagani F, Guidobono F, et al. Evidence for a central inhibitory role of growth hormone secretagogues and ghrelin on gastric acid secretion in conscious rats. Neuroendocrinology. 2002;75:92–97. doi: 10.1159/000048225. [DOI] [PubMed] [Google Scholar]
- 13.Gokcel A, Gumurdulu Y, Kayaselcuk F, et al. Helicobacter pylori has no effect on plasma ghrelin levels. Eur J Endocrinol. 2003;148:423–426. doi: 10.1530/eje.0.1480423. [DOI] [PubMed] [Google Scholar]
- 14.Nwokolo CU, Freshwater DA, O'Hare P, Randeva HS. Plasma ghrelin following cure of Helicobacter pylori. Gut. 2003;52:637–640. doi: 10.1136/gut.52.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee JH, Choe YH, Jeon BH, et al. Genotypes of the Helicobacter pylori vacA signal sequence differ with age in Korea. Helicobacter. 2004;9:54–58. doi: 10.1111/j.1083-4389.2004.00198.x. [DOI] [PubMed] [Google Scholar]
- 16.Ariyasu H, Takaya K, Tagami T, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 17.Inui A. Ghrelin: an orexigenic and somatotrophic signal from the stomach. Nat Rev Neurosci. 2001;2:551–560. doi: 10.1038/35086018. [DOI] [PubMed] [Google Scholar]
- 18.Lee HM, Wang G, Englander EW, Kojima M, Greeley GH., Jr Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology. 2002;143:185–190. doi: 10.1210/endo.143.1.8602. [DOI] [PubMed] [Google Scholar]
- 19.Pinkney J, Williams G. Ghrelin gets hungry. Lancet. 2002;359:1360–1361. doi: 10.1016/S0140-6736(02)08387-3. [DOI] [PubMed] [Google Scholar]
- 20.Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 21.Soriano-Guillen L, Barrios V, Chowen JA, et al. Ghrelin levels from fetal life through early adulthood: relationship with endocrine and metabolic and anthropometric measures. J Pediatr. 2004;144:30–35. doi: 10.1016/j.jpeds.2003.08.050. [DOI] [PubMed] [Google Scholar]
- 22.Murray CD, Emmanuel AV. Ghrelin and Helicobacter pylori. Gut. 2004;53:315. [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki H, Masaoka T, Hosoda H, et al. Helicobacter pylori infection modifies gastric and plasma ghrelin dynamics in Mongolian gerbils. Gut. 2004;53:187–194. doi: 10.1136/gut.2003.021568. [DOI] [PMC free article] [PubMed] [Google Scholar]