Abstract
Background/Aims
The aim of this study was to determine whether the margin of early to be detected gastric cancer (EGC) and gastric adenoma is easier to be detected with autofluorescence imaging (AFI) than with white-light endoscopy (WLE).
Methods
A total of 102 lesions (48 EGCs and 54 gastric adenomas) found in 98 patients were removed endoscopically or surgically. The measured length of each pathology specimen was compared with the lengths estimated using WLE, AFI, and chromoendoscopy.
Results
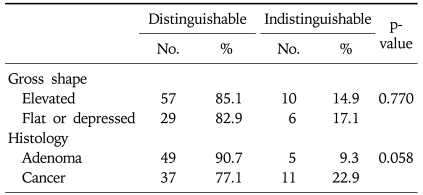
The lesions could be discriminated from surrounding mucosa by AFI in 86 cases (84.3%). The detection rates were similar for elevated lesions (85.1%) and flat/depressed lesions (82.9%, p=0.770). In terms of histology, the detection rate was slightly higher for adenomas (90.7%) than for cancer (77.1%, p=0.058). The estimated length was shorter than the pathologic length in 31.4% of cases when using WLE and 22.1% of cases when using AFI (p=0.168). The resection range was larger for EMR than for AFI in 24 of 80 cases (30.0%).
Conclusions
WLE tends to underestimate the size of EGCs, whereas AFI tends to overestimate their size.
Keywords: Fluorescence, Imaging, Gastric cancer, Adenoma, Resection
INTRODUCTION
Gastric cancer is one of the leading causes of cancer death in the world, and it is especially common in Eastern countries.1 One of the major factors for improving the survival of gastric cancer patients is the early detection.2 With the development of endoscopic instruments, early gastric cancers (EGCs) and gastric adenomas are frequently diagnosed.3,4 The endoscopic mucosal resection (EMR) has become one of the established treatment modalities for small early gastric cancers and gastric precancerous lesions.5-7 For successful EMR, clear delineation of the lateral margin of the lesion is very important. Using conventional white light endoscopy (WLE), some lesions are difficult to determine the exact border between the lesion and the normal surrounding mucosa. Many methods have been tried to clearly determine the exact margin of the EGCs and gastric adenomas.8-13
Autofluorescence imaging (AFI) is one of the newly developed technology which produces real-time pseudocolor images by detecting natural tissue fluorescence from endogenous fluorophores that is emitted by excitation light.11,14-19 Several studies have reported that AFI could reveal minute lesions of the stomach that were not detected in the WLE.14,20-23 Therefore, AFI might be helpful for the determination of the extent of small gastric lesions before treatments such as EMR. In the present study, we evaluated the role of AFI before the EMR of the EGC and/or gastric adenomas with emphasis on the determination of the margins.
MATERIALS AND METHODS
1. Patients
This is a multi-center prospective study in Korea performed between December 2005 and July 2006. Nine university hospitals participated in the study. In patients who are possible candidates for endoscopic treatment of gastric cancer and/or gastric adenoma, upper endoscopic examinations with AFI were done. There was no size limitation of the lesions in this study, but most lesions were less than 3 cm in diameter. Patients with inflammatory or hyperplastic polyps or advanced gastric cancers were excluded. A total of 98 patients were enrolled.
2. Endoscopic evaluation
The prototype videoendoscopes for AFI (XGIF-Q240FY; Olympus Co., Tokyo, Japan)24 were used for the present study. First, whole stomach was carefully evaluated with WLE, and the size and location of the lesion was recorded. Thereafter, the lesion was re-evaluated with AFI. In this system, normal mucosa appears bright green, whereas a tumor appears magenta. Areas containing more hemoglobin are displayed as dark green in the image.24 Biopsies for suspected lesions were performed after the whole evaluation with WLE, AFI, and chromoendoscopy. All examinations were performed by experienced endoscopists at the respective participating centers. Data were collected regarding location, size, diagnosis, and macroscopic shape of the lesions on the WLE and AFI mode, respectively. The lesions were divided into two types by the macroscopic shape; elevated type and flat or depressed type. The size of each lesion during endoscopic examination was determined using an open-biopsy forceps, and defined as the longest diameter.
3. Resection of the tumors
A total of 102 lesions in 98 patients (73 men, 25 women; mean age 61.7±8.2 years) were found. Four patients had two synchronous lesions. Ninety-four lesions were removed endoscopically and 8 lesions surgically. The final histological diagnosis of the lesions was early gastric cancer in 48 lesions and gastric adenoma in 54 lesions. On the pathologic examination, histology, depth of invasion, resection margin, lymphatic invasion and venous invasion were also evaluated.
To evaluate the clinical efficacy of AFI, we evaluated whether the size estimation of AFI was correct and whether the extent of endoscopic resection was changed based on the AFI findings. We compared the estimated size in endoscopy (WLE and AFI) and the length of the tumor in pathology specimen. If the difference between the estimated length and pathologic finding was no more than 3 mm, the estimated length was considered correct. We also evaluated whether the extent of endoscopic resection was increased, decreased, or not changed.
4. Statistical analysis
Continuous data are expressed as mean±standard deviation. Comparisons between groups were performed using the chi-square test or the Fisher's exact test for categorical variables and the Student's t-test or paired t-test for continuous variables. SPSS version 11.0 software was used for all analyses, and a p-value <0.05 was considered statistically significant.
RESULTS
1. Characteristics of gastric neoplasms
The location of the lesion was antrum in 54 lesions (52.9%), angle in 17 lesions (16.7%), body in 29 lesions (28.4%), and cardia in 2 lesions (2.0%). Macroscopically, 67 lesions (65.7%) were classified into the elevated type and 35 lesions (34.3%) were the flat or depressed type. Histologically, 48 lesions (47.1%) were cancer and 54 lesions (52.9%) were adenoma. The mean length of the 102 lesions was 1.74±1.19 cm and the mean length of the 48 cancers and 52 adenomas were 1.93±1.55 cm and 1.57±0.71 cm, respectively, in pathologic specimen. The mean length of the 67 elevated and 35 flat or depressed type lesions were 1.68±1.07 cm and 1.84±1.40 cm, respectively, in pathologic specimen. Among the cancerous lesions, well differentiated type accounted for 68.8% (n=33), moderately differentiated type 16.7% (n=8), poorly differentiated type 10.4% (n=5), and signet ring cell type 4.2% (n=2). For depth of cancer invasion, carcinoma in situ (CIS) accounted for 8.3%, lamina propria 52.1%, muscularis mucosa 33.3%, the mid-part of the submucosa (SM2) 4.2%, and the lower part of the submucosa (SM3) 2.1%. No cancer lesion showed tumor involvement of the resection margin or evidence of vascular invasion.
2. Overall rate of detection of the lesion by AFI
In 102 lesions, 86 lesions (84.3%) could be identified from the surrounding mucosa by AFI (Fig. 1). When the lesions were divided into elevated and flat/depressed lesions, the detection rates were similar (85.1% and 82.9%, respectively, p=0.770) (Table 1). When the lesions were divided by their histology, the detection rate of adenoma was slightly higher than that of cancer (90.7% and 77.1%, respectively, p=0.058) (Table 1). Among the 48 cancerous lesions, 78.0% (32/41) of well or moderately differentiated type lesions, and 71.4% (5/7) of poorly differentiated or signet ring cell type lesions could be detected using AFI (p=0.653). In selected case, AFI could highlight subtle lesions that were hardly detected even with magnification or after dye-spraying without significant increase in the procedure time.
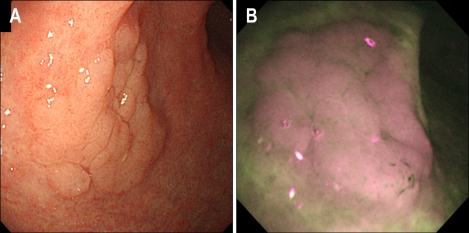
Fig. 1.
Autofluorescence imaging (AFI) of gastric adenoma. (A) Conventional white-light endoscopy (WLE) revealed a 2.2-cm flat, elevated lesion on the anterior aspect of the gastric antrum. (B) In AFI, the same lesion appeared purple, with the surrounding atrophic gastric mucosa appearing as a mixed area of green and white.
Table 1.
Discrimination Rate by Autofluorescence Imaging
In the 16 lesions that were indistinguishable from surrounding mucosa by AFI (Fig. 2), the mean length was 1.36±1.07 cm in pathologic specimen. The location of these lesions was the antrum in 6 lesions (37.5%), the angle in 1 lesion (6.3%), the body in 7 lesions (43.8%), and the cardia in 2 lesions (12.5%). Macroscopically, 10 lesions (62.5%) were classified into the elevated type and 6 lesions (37.5%) were the flat or depressed type. Histologically, 11 lesions (68.8%) were cancer and 5 lesions (31.3%) were adenoma. Among the cancerous lesions, there were 7 lesions (63.6%) of well differentiated type, 2 lesions (18.2%) of moderately differentiated type, and 2 lesions (18.2%) of poorly differentiated type.
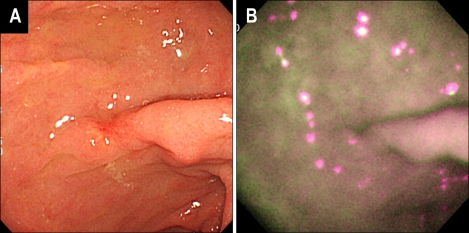
Fig. 2.
A case of early gastric cancer that could not be discriminated by the AFI. (A) Conventional WLE revealed an ill-defined, slightly depressed lesion on the anterior aspect of the gastric antrum, which histology revealed to be a well-differentiated adenocarcinoma. (B) In AFI, the color of the depressed area could not be discriminated from the surrounding mucosa, with the entire gastric mucosa appearing as a mixed surface of purple and green.
3. Evaluation of the margins of the lesions by WLE and AFI
Treatment options for early gastric cancer and adenoma differs greatly depending on the estimated size in the endoscopy. In this study, we considered the size estimation to be correct if the difference between the estimated length in endoscopy and the maximal length in histological specimen is no more than 3 mm. By these criteria, WLE correctly estimated the size of the lesions in 53.9% (55/102), and the percentage in AFI was 54.7% (47/86). In the 86 cases in which lesions could be discriminated by both WLE and AFI, the mean length of the lesions was 1.81±1.21 cm in pathologic specimen. The mean estimated length was 1.76±0.88 cm by WLE and 2.00±0.99 cm by AFI (p<0.01, paired t-test). Estimated length was shorter than pathologic length in 31.4% for WLE and 22.1% for AFI. No significant difference was found between this underestimation rate (p=0.168).
In the 29 cases of flat or depressed lesions in which lesions could be identified by both WLE and AFI, the mean length of the lesions was 1.95±1.45 cm in pathologic specimen. The mean estimated length was 1.69±0.69 cm by WLE and 1.98±0.83 cm by AFI (p<0.01, paired t-test).
4. Usefulness of AFI for decision making of treatment limits in EMR
In the 86 cases in which lesions could be discriminated by both WLE and AFI, EMR was performed in 80 cases. The extent of endoscopic resection was increased in 24 cases (30.0%) after AFI study. Among the 24 cases, the size was underestimated by WLE in 13 cases (6 cancers and 7 adenomas).
DISCUSSION
Endoscopic systems using the different fluorescence characteristics between normal and neoplastic tissue can be divided into two systems.3,11,17,22-24 In early endoscopic systems using fluorescence, photosensitizers should be administered before the endoscopic examination. However, the risk of photosensitization and the potential hepatotoxicity has limited its widespread use. In the autofluorescence endoscopy system, however, exogenous photosensitizers are not required because it uses endogenous biomolecules such as collagen, nicotinamide adenine dinucleotide (NADH), flavin adenine denucleotide (FAD), and porphyrins. Earlier systems of real-time autofluorescence imaging, such as the D-Light/AF system (Karl Storz, Tuttlingen, Germany),25 fiberoptic endoscopy with attached image-intensifying camera was used. In contrast, the AFI system of this study detects endogenous tissue autofluorescence by incorporated charge-coupled device (CCD) in the videoendoscope. Therefore, it can show brighter and clearer pseudocolor images than previous fiberoptic systems.
AFI uses the differences in tissue fluorescence properties, which depend on the concentration or depth distribution of endogenous fluorophores, and changes in the tissue microarchitecture.24 Therefore, it is expected that AFI might be useful for detecting minute lesions, especially flat lesion, and the determination of the lateral margin of the lesions. In addition, AFI can highlight subtle lesions without magnification or dye-spraying and could thus significantly reduce the procedure time.
In the present study, we used the AFI to examine patients with gastric adenoma and EGC to investigate its clinical efficacy before the EMR. Using AFI, the cancers could be distinguished from surrounding mucosa in 77.1% of the cases (Table 1). In a previous study, the detection rate of early gastric cancer by AFI was 94%.23 Direct comparison, however, seems to be impossible, because the characteristics of the lesions seems to be different. In this study, AFI was performed as a work-up examination before the endoscopic mucosal resection, so most of the lesions were small. This may have contributed to our less than expected detection rate of EGC. The reason for slightly higher detection rate for gastric adenoma is uncertain.
In the EMR procedures, determination of the extent of the lesion is very important.5 If the margins of the lesion are not determined correctly, it may cause high rate of incomplete resection. In the present study, we tried to evaluate the role of AFI in the determination of resection margins before the EMR for gastric neoplastic lesions. However, direct correlation of AFI findings with histopathologic findings could not be done because the study was done in the multi-center setting. Therefore, we compared the maximal length of the lesion estimated by various methods. If the difference between the estimated length and pathologic finding was no more than 3 mm, the estimated length was considered correct. The 3 mm criterion is arbitrary, but we considered that minimal target of cancer free resection margin is usually 2 mm.26,27 By the 3 mm criterion, the margin of the lesion was correctly estimated by AFI in 54.7% of the cases. We could not find any significant difference between the correct estimation rate of WLE and AFI for either elevated or flat or depressed lesions in this study. The efficacy of AFI in the determination of the EGC is difficult to compare with that of other studies. In the previous studies, it has been reported that the cancer extension was correctly estimated in 68-85% of the EGC cases.14,23 In our opinion, (1) differences in the definition of correct evaluation, (2) the characteristics of enrolled cases and (3) the model of endoscopes partially contributed these discrepancies.
In the present study, the estimated mean diameter of the lesions by AFI was larger than that by WLE (1.98±0.83 cm and 1.69±0.69 cm, respectively). Estimated length was shorter than pathologic length in 31.4% for WLE and 22.1% for AFI. In addition, the endoscopist of this study reported that the extent of endoscopic resection was increased in 24 cases (30.0%) after AFI. These findings suggest that AFI before EMR may increase the extent of the endoscopic treatment. It is not certain whether this increased treatment extent can lead to an increased complete resection rate determined by histology. Although data are lacking, we think that AFI may decrease the rate of incomplete resection caused by underestimation of the extent by WLE.
Recently, with the development of the video AFI system, the diagnostic yield of AFI has risen,15 and AFI technology has been markedly improving in the resolution and the formation of the pseudocolor image. Therefore, it is expected that AFI may become one of our major armament for diagnosing subtle gastric lesions soon. Recently, combination of AFI with NBI have been suggested for the early detection of Barrett esophagus.18 Further studies are necessary to evaluate whether combination of AFI and NBI improves the accuracy of endoscopic evaluation of gastric lesions.
In conclusion, AFI could identify color difference in most of small EGC and gastric adenomas. Compared to the maximal length of the lesion by pathology, WLE had a tendency to underestimate the size, whereas AFI had a tendency to overestimate it. In selected cases, AFI was useful for the determination of the treatment extent before EMR.
References
- 1.Plummer M, Franceschi S, Muñoz N. Epidemiology of gastric cancer. IARC Sci Publ. 2004;157:311–326. [PubMed] [Google Scholar]
- 2.Sung J. Early gastric cancer: diagnosis, treatment and prevention. Eur J Gastroenterol Hepatol. 2006;18:817–819. doi: 10.1097/00042737-200608000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson BC, Van Dam J. Gastrointestinal epithelial dysplasia: detection with new endoscopic techniques. Curr Opin Gastroenterol. 2002;18:581–586. doi: 10.1097/00001574-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Song LMWK, Wilson BC. Endoscopic detection of early upper GI cancers. Best Pract Res Clin Gastroenterol. 2005;19:833–856. doi: 10.1016/j.bpg.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490–4498. doi: 10.1200/JCO.2005.19.935. [DOI] [PubMed] [Google Scholar]
- 6.Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225–229. doi: 10.1136/gut.48.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Youn JC, Youn YH, Kim TI, et al. Factors affecting long-term clinical outcomes of endoscopic mucosal resection of early gastric cancer. Hepatogastroenterology. 2006;53:643–647. [PubMed] [Google Scholar]
- 8.Iishi H, Tatsuta M, Okuda S. Diagnosis of simultaneous multiple gastric cancers by the endoscopic Congo red-methylene blue test. Endoscopy. 1988;20:78–82. doi: 10.1055/s-2007-1018137. [DOI] [PubMed] [Google Scholar]
- 9.Park JH, Lee JH, Lim YJ, et al. Is chromoendoscopy with indigocarmine useful for detecting additional lesions in patients referred for endoscopic resection of gastric adenoma or cancer? Korean J Gastrointest Endosc. 2006;30:1–6. [Google Scholar]
- 10.Dinis-Ribeiro M, da Costa-Pereira A, Lopes C, et al. Magnification chromoendoscopy for the diagnosis of gastric intestinal metaplasia and dysplasia. Gastrointest Endosc. 2003;57:498–504. doi: 10.1067/mge.2003.145. [DOI] [PubMed] [Google Scholar]
- 11.Ohkawa A, Miwa H, Namihisa A, et al. Diagnostic performance of light-induced fluorescence endoscopy for gastric neoplasms. Endoscopy. 2004;36:515–521. doi: 10.1055/s-2004-814409. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka K, Toyoda H, Kadowaki S, et al. Features of early gastric cancer and gastric adenoma by enhanced-magnification endoscopy. J Gastroenterol. 2006;41:332–338. doi: 10.1007/s00535-005-1760-3. [DOI] [PubMed] [Google Scholar]
- 13.Dinis-Ribeiro M. Chromoendoscopy for early diagnosis of gastric cancer. Eur J Gastroenterol Hepatol. 2006;18:831–838. doi: 10.1097/00042737-200608000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Uedo N, Iishi H, Tatsuta M, et al. A novel videoendoscopy system by using autofluorescence and reflectance imaging for diagnosis of esophagogastric cancers. Gastrointest Endosc. 2005;62:521–528. doi: 10.1016/j.gie.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 15.Kara MA, Peters FP, Ten Kate FJ, Van Deventer SJ, Fockens P, Bergman JJ. Endoscopic video autofluorescence imaging may improve the detection of early neoplasia in patients with Barrett's esophagus. Gastrointest Endosc. 2005;61:679–685. doi: 10.1016/s0016-5107(04)02577-5. [DOI] [PubMed] [Google Scholar]
- 16.Abe S, Izuishi K, Tajiri H, Kinoshita T, Matsuoka T. Correlation of in vitro autofluorescence endoscopy images with histopathologic findings in stomach cancer. Endoscopy. 2000;32:281–286. doi: 10.1055/s-2000-7376. [DOI] [PubMed] [Google Scholar]
- 17.Stepp H, Sroka R, Baumgartner R. Fluorescence endoscopy of gastrointestinal diseases: basic principles, techniques, and clinical experience. Endoscopy. 1998;30:379–386. doi: 10.1055/s-2007-1001287. [DOI] [PubMed] [Google Scholar]
- 18.Kara MA, Peters FP, Fockens P, ten Kate FJ, Bergman JJ. Endoscopic video-autofluorescence imaging followed by narrow band imaging for detecting early neoplasia in Barrett's esophagus. Gastrointest Endosc. 2006;64:176–185. doi: 10.1016/j.gie.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 19.Stepinac T, Felley C, Jornod P, et al. Endoscopic fluorescence detection of intraepithelial neoplasia in Barrett's esophagus after oral administration of aminolevulinic acid. Endoscopy. 2003;35:663–668. doi: 10.1055/s-2003-41514. [DOI] [PubMed] [Google Scholar]
- 20.Bhunchet E, Shibata T. Proposal for two strategies to prevent remnants of gastric cancers after endoscopic mucosal resections: fluorescein electronic endoscopy and rapid stump diagnosis based on pit patterns. Gastric Cancer. 2004;7:221–232. doi: 10.1007/s10120-004-0296-1. [DOI] [PubMed] [Google Scholar]
- 21.Haringsma J, Tytgat GN, Yano H, et al. Autofluorescence endoscopy: feasibility of detection of GI neoplasms unapparent to white light endoscopy with an evolving technology. Gastrointest Endosc. 2001;53:642–650. doi: 10.1067/mge.2001.114419. [DOI] [PubMed] [Google Scholar]
- 22.Bhunchet E, Hatakawa H, Sakai Y, Shibata T. Fluorescein electronic endoscopy: a novel method for detection of early stage gastric cancer not evident to routine endoscopy. Gastrointest Endosc. 2002;55:562–571. doi: 10.1067/mge.2002.122031. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi M, Tajiri H, Seike E, et al. Detection of early gastric cancer by a real-time autofluorescence imaging system. Cancer Lett. 2001;165:155–159. doi: 10.1016/s0304-3835(01)00405-0. [DOI] [PubMed] [Google Scholar]
- 24.Uedo N, Iishi H, Ishihara R, Higashino K, Takeuchi Y. Novel autofluorescence videoendoscopy imaging system for diagnosis of cancers in the digestive tract. Digest Endosc. 2006;18:S131–S136. [Google Scholar]
- 25.Herth FJ, Ernst A, Becker HD. Autofluorescence bronchoscopy--a comparison of two systems (LIFE and D-Light) Respiration. 2003;70:395–398. doi: 10.1159/000072903. [DOI] [PubMed] [Google Scholar]
- 26.Tada M, Murakami A, Karita M, Yanai H, Okita K. Endoscopic resection of early gastric cancer. Endoscopy. 1993;25:445–450. doi: 10.1055/s-2007-1010365. [DOI] [PubMed] [Google Scholar]
- 27.Takekoshi T, Baba Y, Ota H, et al. Endoscopic resection of early gastric carcinoma: results of a retrospective analysis of 308 cases. Endoscopy. 1994;26:352–358. doi: 10.1055/s-2007-1008990. [DOI] [PubMed] [Google Scholar]