Abstract
Background/Aims
The aim of this study was to examine the reproducibility of methane and hydrogen in exhaled air breath after a per-oral load of lactulose.
Methods
Methane was present in the exhaled breath of 21 of 50 healthy subjects recruited by advertisement. Three methane breath tests were performed in 12 women (aged 23.6±0.5 years, mean±SEM) after they consumed 10 g of lactulose dissolved in 300 ml of water. Short- and medium-term reproducibilities were assessed by paired examinations taken 3 and 17 days (median) apart, respectively.
Results
High values of coefficients of variation for paired examinations (CVp) indicated a poor short-term reproducibility of parameters characterizing either the methane or hydrogen excretion in breath air: CVp values of the maximum net increments over baseline in methane (max CH4_net), and in hydrogen (max H2_net), were 34% and 41%, respectively. Moreover, the reproducibility consistently deteriorated with increasing time gap between repeat measurements (CVp: 60% for max CH4_net and 64% for max H2_net).
Conclusions
The low reproducibility of parameters characterizing quantitative methane breath excretion suggests that caution is necessary when judging the clinical usefulness of the methane breath test after a per-oral lactulose load for the purpose of diagnosing and classifying functional bowel disorders.
Keywords: Breath tests, Hydrogen, Lactulose, Methane, Reproducibility
INTRODUCTION
Measurement of hydrogen concentrations in breath air is a basis for a battery of breath tests currently applied in gastroenterology for the diagnosis of small intestinal bacterial overgrowth or malabsorption of different carbohydrates on the one hand, and the measurement of the orocaecal transit time on the other.1-3 Addition of the determination of methane content in the expiratory air may contribute to increase the diagnostic yield in the case of the former group of applications of those breath test.1,2,4
A very promising appears to be a recent finding that a subpopulation of irritable bowel syndrome (IBS) patients with predominant constipation may be detected on the basis of an increment in methane within the breath air after a per oral lactulose load.5-7 Thus that breath test gained a rank of a decision-influencing diagnostic measure because a positive result thereof in an IBS patient would encourage a clinician to undertake a course of an antimicrobial therapy.6,7 More recently a quantitative result of a methane breath test, expressed in terms of an area under a curve of methane concentration in expiratory air after per-oral lactulose load, was advocated a measure of the degree of constipation in IBS patients.8
What may, however, cast some doubt as to the above reasoning is a potential instability of methane elimination with breath air, as previously was signalized by Minocha and Rashid.9 Also, in our Laboratory of Breath Tests, we observed a noticeable inconsistency of a methane-producing status in a number of subjects. Therefore, we decided to undertake a prospective study aiming at the examination of the reproducibility of quantitative parameters characterizing methane breath air elimination in the course of a breath test involving a per oral lactulose load.
MATERIALS AND METHODS
In response to an advertisement made public at the University, fifty subjects volunteered to participate in the study. At an initial screening with a dedicated gas chromatograph, 21 of them were found to have a fasted breath concentration of methane ≥1 ppm. After having been made familiar with the aim, protocol and methodology of the study twelve methane-producing females (age 23.6±0.5 [SE] years, body mass index 21.05±0.62 kg·m-2) gave a written consent to participate. They declared themselves as being in full health according to the World Health Organisation criteria.10 Exclusion criteria comprised use of antibiotics and any other antimicrobial agents currently, as well as within a period of three months preceding the study, a history of an abdominal surgery affecting the anatomical integrity of the digestive tract, except for appendectomy and pregnancy.5-8 The research project was conducted according to the Helsinki Declaration and was approved by the Bioethics Committee of the Medical University of Silesia.
1. Study protocol
The research was performed on patients reporting to the laboratory in the morning, after a 12-h overnight fast and abstaining from cigarette smoking (this requirement pertained to three female smokers). Moreover, they were instructed to resign from dietary components which might evoke a rise in basal breath H2 and CH4 concentrations as from the afternoon of the preceding day, which included dishes made of white or red beans, green peas, cabbage, or large amounts of complex carbohydrates.9,11 Every volunteer took part at three examination sessions held on separate days. The time gap between two sessions amounted to 3 days (median, range 2-3 days) for a short-term reproducibility (STR), whereas a third session was accomplished at a median of 17 days (range 16-21) apart (medium-term reproducibility, MTR); the order of sessions for the STR/MTR or MTR/STR was randomly assigned to the subjects. The volunteers did not take any medication throughout the whole period of the study involving participation in the three examination sessions.
At the beginning the subjects were requested to perform a thorough 4-min cleaning of their teeth with the use of an antibacterial tooth paste (Colgate Total, Colgate-Palmolive, Poland). Then, after a 15-min rest in a sitting position, necessary for stabilization of the metabolism, a basal probe of expiratory air was collected into an aluminium covered plastic bag of about 1 l capacity (Fisher Analysen Instrumente GmbH, Leipzig, Germany); the procedure of collecting the breath air was standardized: the subjects took in breath and held it for 20 seconds, then steadily blew the air through a mouthpiece equipped with a valve into the bag until full, which was tightly closed with a plastic stopper immediately at the end of the exhalation. At the time point designated "0" the subjects drank 285 still mineral water into which 15 ml of Normase syrup (Molteni Farmaceutici, Italy), containing 10 g lactulose, was added. Next the volunteers were asked to rinse theirs mouths with tap water. Probes of breath air ware collected at 15-min intervals from 30 min and until 4 hour after time zero. The subjects remained fasted during that period. They rested in a comfortably furnished room and were allowed to watch video films.
2. Measurement of breath hydrogen and methane
The H2 and CH4 concentrations in the expiratory breath samples were measured on the day of their collection with a dedicated chromatograph (Microlyzer Model DP, QuinTron Instrument Co., Milwaukee, WI, USA) calibrated with a reference gas containing 49 ppm CH4 and 99 ppm H2 in air (Quingas-2, QuinTron Instrument Co., Milwaukee, WI, USA) according to the manufacturer's recommendations.
From the curves reflecting the H2 and CH4 concentrations in the breath air the following parameters were derived:
basal fasted hydrogen (H2_bas) and methane (CH4_bas) concentration,
maximum net increment over baseline in hydrogen (max H2_net) and methane (max CH4_net) after per oral lactulose intake (Fig. 1),
areas under the curves of net increments of hydrogen (AUC H2_net) and methane (AUC CH4_net) concentrations (Fig. 1).
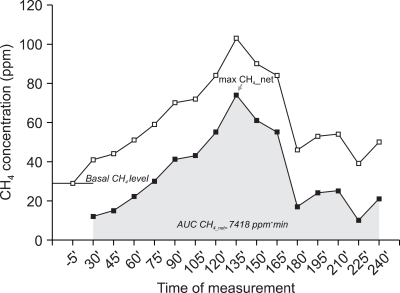
Fig. 1.
Graphical explanation of the computation of parameters used in the paper based on an example of a methane breath concentration curve. The basal concentration is subtracted from the original curve (open squares) at every time point to produce a curve of the net increment over baseline (solid squares), from which a maximum net increment over baseline is determined (arrow); subsequently the area under the curve of the net increments over baseline is computed according to the trapezoidal rule (shaded area), with the occasional negative values set to 0.
3. Statistical analysis
The data obtained were subjected to the Bland and Altman statistic for calculation of the repeatability coefficients.12 Moreover, coefficients of variation for paired examinations (CVp) were computed.13
RESULTS
From 36 examinations in 12 volunteers, the medians of the basal concentration, the net increment over baseline, the area under the curve of the net increment of the breath hydrogen were 2 ppm (interquartile range, IQR: 2-7), 36 ppm (IQR: 26-48), and 3,424 ppm.min (IQR: 2,466-5,404), respectively. Whereas, the medians of the basal concentration, the net increment over baseline, the area under the curve of the net increment of the breath methane were 15 ppm (interquartile range, IQR: 7-21), 19 ppm (IQR: 8-31), and 1,988 ppm.min (IQR: 752-3,619), respectively.
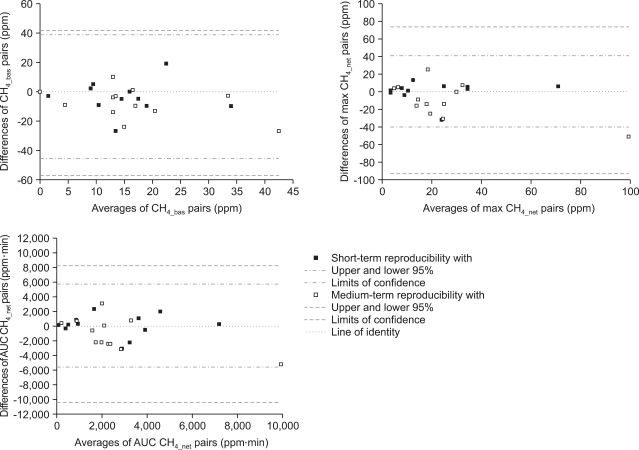
High values of coefficients of variation for paired examinations, as well as the Bland and Altman repeatability coefficients disclosed a poor short- and medium-term of the parameters characterizing either the hydrogen or methane excretion in breath air after per oral lactulose load (Table 1, 2). Bland and Altman plots of differences between pairs of the parameters considered against their means clearly indicated that the unsatisfactory short-term reproducibility of the hydrogen or methane elimination with expiratory air consistently deteriorated with increased time gap between the repeat measurements (Fig. 2, 3).
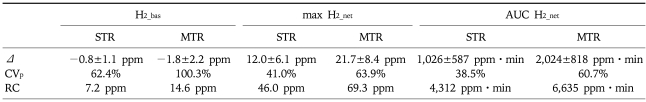
Table 1.
Reproducibility of Breath Hydrogen Concentrations under the Fasted Condition and after a 10-g Per-oral (p.o.) Load of Lactulose
H2_bas, basal fasted hydrogen; max H2_net, maximum net increment over baseline in hydrogen after peroral lactulose intake; AUC H2_net, area under the curve of the net increment of hydrogen; STR, short term reproducibility with a median time gap of 3 days (range 1-3) between repeat sessions; MTR: medium term reproducibility with examinations separated by a median of 17 days (range 16-21); Δ, difference between repeat examinations (mean±SE); CVp, coefficient of variation for paired examinations; RC, Bland and Altman repeatability coefficient.
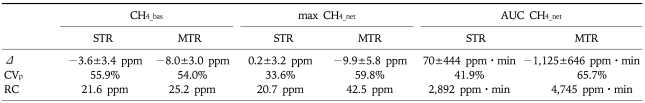
Table 2.
Reproducibility of Breath Methane Concentrations Under the Fasted Condition and after a 10-g Per-oral (p.o). Load of Lactulose
CH4_bas, basal fasted methane; max CH4_net, maximum net increment over baseline in methane after peroral lactulose intake; AUC CH4_net, area under the curve of the net increment of methane; STR, short term reproducibility with a median time gap of 3 days (range 1-3) between repeat sessions; MTR, medium term reproducibility with examinations separated by a median of 17 days (range 16-21); Δ, difference between repeat examinations (mean±SE); CVp, coefficient of variation for paired examinations; RC, Bland and Altman repeatability coefficient.
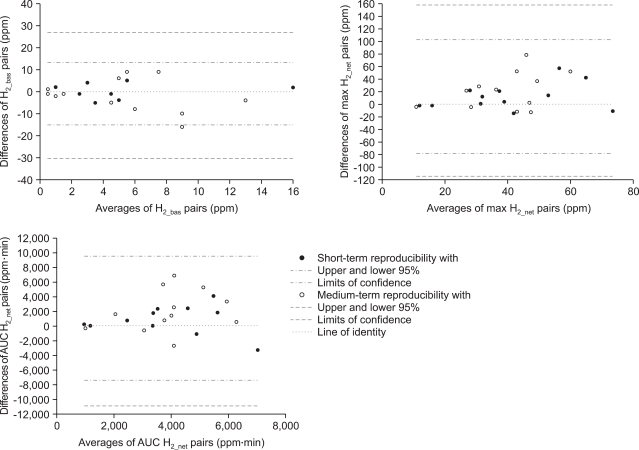
Fig. 2.
Bland and Altman statistics (plot of differences between pairs vs. their means) of the short-term (filled circles) and medium-term (open circles) reproducibilities of parameters characterizing hydrogen breath excretion after a p.o. lactulose load.
H2_bas: basal fasted hydrogen; max H2_net: maximum net increment over baseline; AUC H2_net: area under the curve of the net increment of hydrogen; the respective borders of the 95% confidence intervals are plotted in each panel (cf. the legend in the bottom panel).
Fig. 3.
Bland and Altman statistics (plot of differences between pairs vs. their means) of the short-term (filled squares) and medium-term (open squares) reproducibilities of parameters characterizing methane breath excretion after a p.o. lactulose load.
CH4_bas: basal fasted hydrogen; max CH4_net: maximum net increment over baseline; AUC CH4_net: area under the curve of the net increment of hydrogen; the respective borders of the 95% confidence intervals are plotted in each panel (cf. the legend in the bottom panel).
DISCUSSION
When pursuing the recruitment of volunteers for our study, there were 21 methane-producers out of 50 (42%) screened healthy volunteers. This fraction is similar to 50% established by Peled et al.14 and to 45% found by Minocha and Rashid.9 In the female healthy subjects examined in the study of Peled et al., the average basal fasted methane concentration amounted to 11±9 ppm which is slightly less than in our twelve volunteers who had 15±12 ppm. Both the quoted research groups pointed out to a great variability in fasted state breath methane concentrations on repeat measurements. Peled et al. had opportunity to re-test 23 methane-producers after elapse of 10-25 months, of whom two converted to a non-producer status then.14 As a quantitative representation of reproducibility Minocha and Rashid calculated an average inter-day coefficient of variation which amounted to 49.7±9.9% and to 69.4±16.7% in the case of the hydrogen and methane fasted state breath concentrations, respectively.9 Our data entirely corroborate those results.
The novelty brought about by the current study is the evaluation of the reproducibility of the methane (and hydrogen) response in terms of breath air concentrations observed after per oral load of lactulose. Both short- and medium-term reproducibilities of the methane breath test were poor. Factors that may affect the methane-producing status of an individual can be antibiotic therapy and bowel rinsing for colonoscopy.11 Obviously, those situations did not pertain to our volunteers. Our group of volunteers was consisted of women only - a fact which was accounted for by the predominance of female students at the school of pharmacy where the recruitment took place. This should not, however, be considered any drawback of the study. On the contrary, if a methane breath test after a lactulose load were to be accepted as a valid diagnostic tool among IBS patients, its reliability would have to be scrutinized among the female population, which knowledgeably constitutes the majority of IBS-afflicted subjects. Nevertheless the gender of our volunteers may probably account for the deteriorated medium-term compared to the short-term reproducibility of the methane breath test. All the subjects examined were young, regularly menstruating women. We had assured that with a 2-3 days break between the repeat examinations the short-term reproducibility examinations took place within the same phase of their menstrual cycle. Whereas the 16- to 21-day time gap between a pair of the medium-term examinations had caused that the breath test was performed then during a different phase of the menstrual cycle. Thus, somehow in a natural way, the effect of gender upon the reproducibility of the methane breath test after a lactulose load was apparently disclosed.
The appearance of methane in the baseline probes of exhaled breath taken before the lactulose load would indicate that an on-going fermentation was already taking place involving some pre-existing amount of fermentable substrate. Accordingly, it would suggest that methanogenesis was already generating this gas methane before the administration of the set dose of lactulose. Levitt et al. pointed out previously that a considerable amount of ingested complex carbohydrates may escape assimilation by the small intestine so that they are then available for colonic fermentation.15 Following the methodology outlined by Pimentel et al.,7 we adopted a 12-h fasting period before performance of the lactulose breath test, as well as instructed the subjects to avoid dietary components which might evoke a rise in basal breath H2 and CH4 concentrations as from the afternoon of the preceding day. Nevertheless neither in the Pimentel's work,7 nor in the current study did the subjects avoid complex carbohydrates for a more prolonged, let's say lasting several days, period. Therefore the determined unsatisfactory reproducibility of the methane breath test may be explained by the fact that under the experimental conditions adopted, an unknown amount of fermentable substrates may had been already in place in the colon before the ingestion of the fixed dose of 10 g lactulose on the three examination days. Therefore in reality the methane excretion on any of the repeated examination may have reflected the fermentation of lactulose plus an unknown and variable amount of fermentable complex carbohydrates residing in the colon.15
From the result of this study, we suggest that cautious interpretation is needed in judging a clinical usefulness of a methane breath test after a per oral lactulose load for the purpose of diagnosis and classification of functional gastrointestinal disorders.
ACKNOWLEDGEMENTS
A financial support of the project was provided by the Medical University of Silesia (contracts NN-5-180/04, NN-2/278/05, and NN-2/220/06). The authors greatly acknowledge the gift of the lactulose syrup (Normase) from the Molteni Farmaceutici Polska Sp. z o.o.
References
- 1.Yang CY, Chang CS, Chen GH. Small-intestinal bacterial overgrowth in patients with liver cirrhosis, diagnosed with glucose H2 or CH4 breath tests. Scand J Gastroenterol. 1998;33:867–871. doi: 10.1080/00365529850171549. [DOI] [PubMed] [Google Scholar]
- 2.Farup PG, Monsbakken KW, Vandvik PO. Lactose malabsorption in a population with irritable bowel syndrome: prevalence and symptoms: a case-control study. Scand J Gastroenterol. 2004;39:645–649. doi: 10.1080/00365520410005405. [DOI] [PubMed] [Google Scholar]
- 3.Sojka E, Jonderko K, Magiera B, Błońska-Fajfrowska B. Reproducibility of the orocaecal transit time measured with the use of a miniaturized portable breath hydrogen analyzer. In: Singer MV, Krammer HJ, editors. Neurogastroenterology: falk symposium 112. Dordrecht: Kluwer Academic Publishers; 2000. pp. 549–553. [Google Scholar]
- 4.Mishkin DS, Mishkin S, Blank D, Yalovsky M. Does the addition of methane determinations increase the yield of hydrogen breath tests for sugar malabsorption? Am J Gastroenterol. 2004;99:761. doi: 10.1111/j.1572-0241.2004.04120.x. [DOI] [PubMed] [Google Scholar]
- 5.Pimentel M, Mayer AG, Park S, Chow EJ, Hasan A, Kong Y. Methane production during lactulose breath test is associated with gastrointestinal disease presentation. Dig Dis Sci. 2003;48:86–92. doi: 10.1023/a:1021738515885. [DOI] [PubMed] [Google Scholar]
- 6.Pimentel M, Chow AJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–419. doi: 10.1111/j.1572-0241.2003.07234.x. [DOI] [PubMed] [Google Scholar]
- 7.Pimentel M, Chatterjee S, Chow EJ, Park S, Kong Y. Neomycin improves constipation-predominant irritable bowel syndrome in a fashion that is dependent on the presence of methane gas: subanalysis of a double-blind randomized controlled study. Dig Dis Sci. 2006;51:1297–1301. doi: 10.1007/s10620-006-9104-6. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee S, Park S, Low K, Kong Y, Pimentel M. The degree of breath methane production in IBS correlates with the severity of constipation. Am J Gastroenterol. 2007;102:837–841. doi: 10.1111/j.1572-0241.2007.01072.x. [DOI] [PubMed] [Google Scholar]
- 9.Minocha A, Rashid S. Reliability and reproducibility of breath hydrogen and methane in male diabetic subjects. Dig Dis Sci. 1997;42:672–676. doi: 10.1023/a:1018832117482. [DOI] [PubMed] [Google Scholar]
- 10.Preamble to the Constitution of the World Health Organization as adopted by the International Health Conference; 19-22 June, 1946; New York. signed on 22 July 1946 by the representatives of 61 States (Official Records of the World Health Organization, no. 2, p. 100) and entered into force on 7 April 1948. [Google Scholar]
- 11.Hamilton LH. Breath tests and gastroenterology. 2nd ed. Milwaukee (WI): QuinTron Instrument Co; 1998. [Google Scholar]
- 12.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 13.Loo FD, Palmer DW, Soergel KH, Kalbfleisch JH, Wood CM. Gastric emptying in patients with diabetes mellitus. Gastroenterology. 1984;86:485–494. [PubMed] [Google Scholar]
- 14.Peled Y, Weinberg D, Hallak A, Gilat T. Factors affecting methane production in humans: gastrointestinal diseases and alterations of colonic flora. Dig Dis Sci. 1987;32:267–271. doi: 10.1007/BF01297052. [DOI] [PubMed] [Google Scholar]
- 15.Levitt MD, Hirsh P, Fetzer CA, Sheahan M, Levine AS. H2 excretion after ingestion of complex carbohydrates. Gastroenterology. 1987;92:383–389. doi: 10.1016/0016-5085(87)90132-6. [DOI] [PubMed] [Google Scholar]