Abstract
Background/Aims
Although various solutions are being tested for submucosal injection during endoscopic resection, ideal solution has not been established yet. We performed an animal study to evaluate the possibility of sodium alginate as an ideal submucosal injection solution for endoscopic mucosal resection (EMR).
Methods
To compare the lesion-lifting properties of different solutions, injection was done to the submucosal layer of porcine stomach. Then the height of mucosal elevation was measured. In addition, EMR was performed after submucosal injection of sodium alginate solution in six dogs. Two were euthanized after 30 minutes of endoscopic observation while the others were euthanized after 1-4 weeks. Retrieved stomachs were examined microscopically.
Results
Sodium alginate and sodium hyaluronate solutions maintained longer elevation of the submucosal layer than other solutions. There was no significant difference in the height between two solutions. A clear separation of the mucosal layer from the proper muscle layer was achieved by injecting sodium alginate solution. Histological examination of EMR-induced artificial ulcers revealed no apparent tissue damage and showed normal healing process.
Conclusions
Sodium alginate solution creates a sufficient submucosal fluid cushion without apparent tissue damage. It can be considered as an effective submucosal injection material.
Keywords: Endoscopic resection, Injection, Sodium alginate
INTRODUCTION
Endoscopic mucosal resection (EMR) has been widely accepted as a treatment modality for early stage gastrointestinal neoplasms.1-4 Creating a submucosal cushion by fluid injection is an essential process in EMR. Submucosal injection makes it easy to remove the lesion by lifting the mucosa. In addition, it reduces thermal injury as well as the risk of perforation and hemorrhage. Recently developed advanced procedure called endoscopic submucosal dissection (ESD) enables en-bloc resection in large lesions which were removed in piecemeal manners in the past. To perform ESD, a long-lasting submucosal cushion is needed, particularly when it requires long time. Submucosal cushion created by normal saline is maintained only for a short time, and thus requires repeated injections to maintain the mucosal elevation during the procedure.5 Other substances such as sodium hyaluronate,6 hydroxypropyl methylcellulose (HPMC),7 fibrinogen mixture,8 and glycerol,9 have been tested to create long-lasting submucosal cushion. Of those, sodium hyaluronate is reported to be a most effective solution for submucosal injection.10-12 However, it is expensive and requires a special container for storage.
Sodium alginate is a natural polysaccharide polymer isolated from brown seaweed (Phacophycase) which are used in food, cosmetics and pharmaceutical products for 100 years.13 In the acidic environment of the stomach, both alginate salts and alginic acids precipitate and form a low density viscous gel. Transformed form has an ability to accelerate the platelet aggregation and reduce the bleeding time. We presumed that this viscous solution and gel could act as a safe submucosal injection solution. In this study, we aimed to evaluate the effectiveness and the safety of sodium alginate as a novel submucosal injection solution.
MATERIALS AND METHODS
1. Submucosal injection solutions in a porcine stomach
Porcine stomachs were divided into approximately 5×5 cm, and stretched flat on a corkboard with pins within 24 hours. Using a 5 ml syringe with a 23-guage needle, 2 ml of solutions were injected into the submucosa through the resected margin. Injected solutions were normal saline, 10% dextrose solution, sodium hyaluronate solution (Hyruan plus injection, LG Life sciences, Korea), and sodium alginate solution (Lamina G, Taejoon Pharm, Korea). Sodium alginate was diluted with normal saline to a 1% concentration of sodium alginate while sodium hyaluronate was diluted to a 0.5% concentration.
Mucosal elevation was observed and recorded with a digital camera (E-20, Olympus, Tokyo, Japan) immediately and 5, 10, 15, 20, 25 and 30 minutes after the injection (Fig. 1). The height of mucosal elevation was measured through the recorded pictures using the image analysis software (TOMORO Scope Eye™, Version 3.5.197, Image Partnership Co., Ltd.). This experiment was repeated for four times. We compared the mean height of mucosal elevation made by each solution. Statistical analysis was performed with Kruskal-Wallis test using SPSS 12.0 (SPSS, Inc., Chicago, Illinois, USA). A p-value less than 0.05 was considered statistically significant.
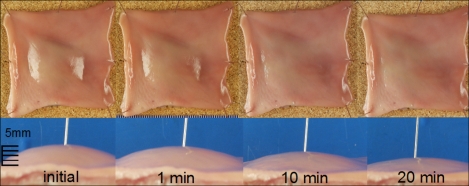
Fig. 1.
Chronological changes of mucosal elevation which is made by 1% sodium alginate submucosal injection in a resected porcine stomach.
2. EMR after submucosal injection in a canine stomach
A total of 6 dogs (5 kg) were prepared after 48 hours of fasting. They were placed in left lateral decubitus position after intravenous anesthesia and endoscopy was performed with a standard endoscope (GIF-Q230; Olympus Optical Co., Ltd., Tokyo, Japan). A disposable 22 gauge catheter injection needle (Injectra GSN-01-19-180; Medi-Globe GmbH, Achenmühle, Germany) was used to inject 5 ml of sodium alginate solution into the submucosal layer at the antrum or lower body. After the injection, endoclipping was performed behind the injected area to mark the location. The endoscope was kept in the stomach to observe the status of mucosal elevation for 30 minutes.
EMR was performed via cap-fitted method (EMR-C) after creating mucosal elevation by submucosal injection using sodium alginate solution at the antrum or lower body. A total of 6 EMR-C procedures were completed without any complications such as hemorrhage and perforation. All of the above experiments were recorded in digital videotapes.
Two dogs were euthanized immediate after EMR while the others were euthanized after 1-4 weeks after EMR. Retrieved stomachs were fixed with formalin and incised vertically to the sites of submucosal injection and EMR-induced ulcers. All resected specimens were embedded in paraffin. Histologic sections were made from the paraffin block and were stained with hematoxylin eosin (H&E). Histological examination was carried out to evaluate the histologic findings of the submucosal cushion, the effect of injection on the tissue, and effect on healing process of EMR-induced ulcers.
RESULTS
1. Comparison of submucosal injection solutions for maintaining mucosal elevation in a porcine stomach
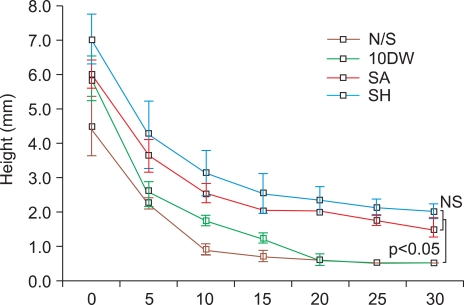
Chronological changes of mucosal elevation created by each solution are shown in Fig. 2. Although it was not statistically significant, sodium alginate and sodium hyaluronic acid solutions produced a higher initial submucosal elevation than normal saline and 10% dextrose solution. Sodium alginate and sodium hyaluronate solutions maintained longer submucosal elevations than normal saline and 10% glucose solution at 5, 10, 15, 20, 25 and 30 minutes after injection (p<0.05). There was no significant difference in the height of mucosal elevation between sodium alginate and sodium hyaluronate during the observation period.
Fig. 2.
Chronological changes of mucosal elevation after submucosal injection of various solutions (N/S; normal saline, 10DW; 10% glucose solution, SA; 1% sodium alginate solution, SH; 0.5% sodium hyaluronate solution). Both sodium hyaluronate and sodium alginate maintain a higher mucosal elevation between 5-30 minutes when compared to other solutions (p<0.05). There is no significant difference in the height of mucosal elevation between sodium hyaluronate and sodium alginate injection.
2. Result of EMR after submucosal injection of sodium alginate in canine stomachs
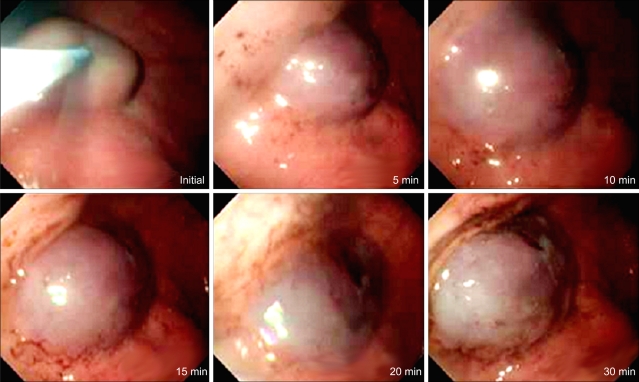
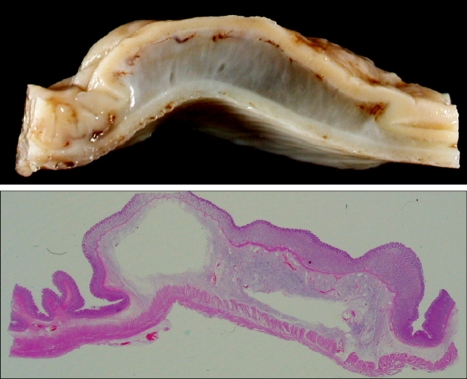
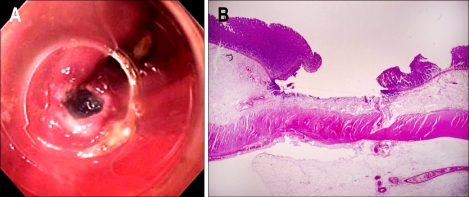
Endoscopic observation is shown in Fig. 3. The elevation was achieved by injecting sodium alginate solution to the submucosal layer of the stomach in living dogs. Mucosal elevations did not collapse completely at the end of the 30-minute endoscopic observation. Histologic features of mucosal elevation made by sodium alginate were similar with those by normal saline. Accumulated sodium alginate was noticed in the submucosa which separated the mucosa and the proper muscle (Fig. 4). Sodium alginate solution caused no apparent tissue damage as seen by microscopy.
Fig. 3.
Changes of gastric mucosal elevation after sodium alginate injection in a living dog.
Fig. 4.
Submucosal accumulation of sodium alginate. The mucosal layer and proper muscle layer are widely separated by submucosal accumulation of sodium alginate (upper: macroscopic finding of the injection site; lower: the entire mount view, H&E).
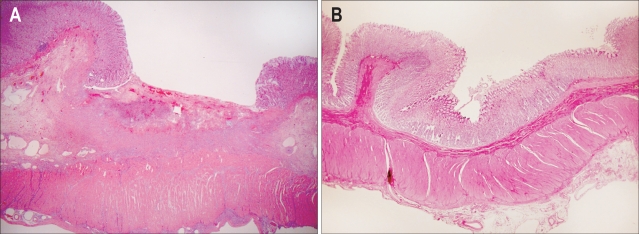
Histological findings of artificial ulcers made immediately after EMR-C showed complete elimination of the mucosal layer without tissue damage around the ulcer (Fig. 5). After 1-4 weeks, normal process of healing ulcer was seen by microscopy (Fig. 6). At 1 week after the procedure, findings of EMR-induced ulcer disclosed generation of granulation tissue consisted of inflammatory cells, newly developed vessels and fibroblasts on the ulcer beds as well as re-epithelization on the ulcer edges. Microscopic examination after 4 weeks revealed that EMR-induced ulcers were completely healed and were covered with normal gastric epithelium and glands.
Fig. 5.
Endoscopic mucosal resection (EMR)-induced ulcer. (A) An EMR-induced ulcer is noted after submucosal injection of sodium alginate in a canine stomach. (B) Histological findings of the mucosal resected site in a canine stomach demonstrates a mucosal defect without tissue damage (H&E, ×40).
Fig. 6.
Healing process of an endoscopic mucosal resection (EMR)-induced ulcer. (A) One week after the procedure, a large ulcer base is covered with necrotic inflammatory exudate and granulation tissue. (B) Four weeks after the procedure, previously noticed EMR-induced ulcer is completely replaced by regenerating epithelium (H&E, ×40).
DISCUSSION
EMR is designed to completely remove the diseased mucosa by resecting through the middle or deeper part of the submucosa. EMR has been accepted as the standard treatment modality of early cancers of gastrointestinal tract without lymph node metastasis. According to gastric cancer treatment guidelines issued by the Japanese Gastric Cancer Association (JGCA), EMR should be indicated for patients with small mucosal cancers (less than 2 cm in size) without concomitant lymph node metastasis.14 For gastric mucosal cancers larger than 2 cm in size without lymph node metastasis, the recently developed EMR technique, so called ESD enables en-bloc resection.15,16 En bloc resection is considered important since it provides more accurate histologic assessment and reduces the risk of local recurrence.17
A long-lasting submucosal cushion would be extremely useful when performing ESD, particularly in large lesions or in time-consuming difficult cases. However, the submucosal cushion created by normal saline is short-lived and requires repeated injections for extensive resection. Ideal solution for submucosal injection should meet several criteria; (i) adequate viscosity to provide a thick submucosal cushion, (ii) long durability to maintain the submucosal cushion during the endoscopic resection, and (iii) no toxic effect to the tissue specimen for precise pathologic staging. Generally, hypertonic solutions tend to make sufficient submucosal cushions.11 Various hypertonic solutions, such as sodium hyaluronate,6 HPMC,7 glycerol9 have been tried. Of those, sodium hyaluronate has been shown to create an effective and long-lasting submucosal cushion.10 However, its high cost, and requirement of an air-sealed container for storage has limited its use. Recently, it has been reported that sodium hyaluronate enhanced both tumor growth and CD44 expression of cancer cells at wound sites, suggesting that the use of sodium hyaluronate for EMR might stimulate the growth of residual tumor cells.18
Alginates block co-polymers of L-guluronic and D-mannuronic acid residues connected by 1:4 glycosidic linkages. Therefore, we hypothesized that sodium alginate may be used as a submucosal injection for EMR considering its ability to form viscous solutions and gels. Sodium alginate is known to be safe because it is not metabolized and absorbed in human body. In animal studies, its LD50 is 64-1,000 mg/kg in intravenous administration, 250-1,600 mg/kg in intraperitoneal administration, and greater than 5,000 mg/kg in oral administration.19-21
This study showed that sodium alginate was an effective and safe material for submucosal injection solution in animals. In the resected porcine stomach, sodium alginate created a longer lasting submucosal cushion than that of the normal saline, and was at least as effective as sodium hyaluronate. Although our study showed promising results, ex-vivo procine model has a limitation since it takes time for the submucosal cushion to disappear which is unnaturally prolonged.10 In addition, we assessed the effectiveness of sodium alginate as a submucosal injection solution in living dogs. This additional in-vivo experiment showed comparison on the efficacy of various submucosal injection solutions.
Although greater density of solution enables longer submucosal cushion durability, highly viscous solutions are difficult to inject. It is also possible that viscous injection material may damage the tissue and interrupt precise pathologic staging of the lesion. Furishiro et al11 reported that highly viscous solutions such as 3.75% NaCl solution and dextrose solution with a concentration greater than 20% create a toxic effect on tissue and delay the healing process of EMR-induced ulcers. Based on our experience, sodium alginate solution can be injected with a commonly used catheter injection needle without apparent tissue damage. Moreover, it has no effect on ulcer healing of EMR-induced ulcer on microscopic examination.
Lee et al8 have reported that submucosal injection made of fibrin mixture affects microvascular hemostasis and keeps visual field clear during EMR. Similarly, sodium alginate has a hemostatic effect through the aggregating platelets and red blood cells which enhance fibrin formation. Therefore, submucosal injection of sodium alginate might be useful for enhanced visual field during EMR theoretically. However, the evalution of visual field was not included in the current study. Further investigations are required to confirm the hemostatic effect of sodium alginate during EMR.
We anticipate that the need for endoscopic resection will increase as a modality of treatment for early gastrointestinal tumor. Since ESD is prevailing as a curative modality for early stage gastrointestinal cancer, an ideal submucosal injection solution that enables the endoscopist to carry out endoscopic resection slowly and meticulously is an important issue. Therefore, an ideal submucosal injection solution should be identified. Our study suggests that sodium alginate is effective, safe and convenient to use as a submucosal injection solution.
ACKNOWLEDGEMENTS
This paper was presented and entitled as "New method of endoscopic mucosal resection using submucosal injection of sodium alginate" at the 14th United European Gastroenterology Week meeting, held on 21-25 October 2006 in Berlin, Germany [Gut 2006;38(S2):A287].
References
- 1.Karita M, Tada M, Ota K, Kodama T. Endoscopic therapy for early colon cancer: the strip biopsy resection technique. Gastrointest Endosc. 1991;37:128–132. doi: 10.1016/s0016-5107(91)70669-x. [DOI] [PubMed] [Google Scholar]
- 2.Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy. 1993;25:455–461. doi: 10.1055/s-2007-1010367. [DOI] [PubMed] [Google Scholar]
- 3.Shim CS. Endoscopic mucosal resection: an overview of the value of different techniques. Endoscopy. 2001;33:271–275. doi: 10.1055/s-2001-12816. [DOI] [PubMed] [Google Scholar]
- 4.Tada M, Murakami A, Karita M, Yonai H, Okita K. Endoscopic resection of early gastric cancer. Endoscopy. 1993;25:445–450. doi: 10.1055/s-2007-1010365. [DOI] [PubMed] [Google Scholar]
- 5.Sakai P, Maluf Filho F, Iryia K, et al. An endoscopic technique for resection of small gastrointestinal carcinomas. Gastrointest Endosc. 1996;44:65–68. doi: 10.1016/s0016-5107(96)70232-8. [DOI] [PubMed] [Google Scholar]
- 6.Yamamoto H, Yube T, Isoda N, et al. A novel method of endoscopic mucosal resection using sodium hyaluronate. Gastrointest Endosc. 1999;50:251–256. doi: 10.1016/s0016-5107(99)70234-8. [DOI] [PubMed] [Google Scholar]
- 7.Feitoza AB, Gostout CJ, Burgart LJ, Burkert A, Herman LJ, Rajan E. Hydroxypropyl methylcellulose: A better submucosal fluid cushion for endoscopic mucosal resection. Gastrointest Endosc. 2003;57:41–47. doi: 10.1067/mge.2003.25. [DOI] [PubMed] [Google Scholar]
- 8.Lee SH, Cho WY, Kim HJ, et al. A new method of EMR: submucosal injection of a fibrinogen mixture. Gastrointest Endosc. 2004;59:220–224. doi: 10.1016/s0016-5107(03)02689-0. [DOI] [PubMed] [Google Scholar]
- 9.Uraoka T, Fujii T, Saito Y, et al. Effectiveness of glycerol as a submucosal injection for EMR. Gastrointest Endosc. 2005;61:736–740. doi: 10.1016/s0016-5107(05)00321-4. [DOI] [PubMed] [Google Scholar]
- 10.Conio M, Rajan E, Sorbi D, et al. Comparative performance in the porcine esophagus of different solutions used for submucosal injection. Gastrointest Endosc. 2002;56:513–516. doi: 10.1067/mge.2002.128107. [DOI] [PubMed] [Google Scholar]
- 11.Fujishiro M, Yahagi N, Kashimura K, et al. Tissue damage of different submucosal injection solutions for EMR. Gastrointest Endosc. 2005;62:933–942. doi: 10.1016/j.gie.2005.07.052. [DOI] [PubMed] [Google Scholar]
- 12.Fujishiro M, Yahagi N, Kashimura K, et al. Comparison of various submucosal injection solutions for maintaining mucosal elevation during endoscopic mucosal resection. Endoscopy. 2004;36:579–583. doi: 10.1055/s-2004-814517. [DOI] [PubMed] [Google Scholar]
- 13.McDowell RH. Properties of Alginates. London, UK: Alginate Industries Ltd.; 1968. p. 42. [Google Scholar]
- 14.Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002;5:1–5. doi: 10.1007/s101200200000. [DOI] [PubMed] [Google Scholar]
- 15.Tanabe S, Koizumi W, Mitomi H, et al. Clinical outcome of endoscopic aspiration mucosectomy for early stage gastric cancer. Gastrointest Endosc. 2002;56:708–713. doi: 10.1067/mge.2002.129085. [DOI] [PubMed] [Google Scholar]
- 16.Ono H, Gotoda T, Yamaguchi H, et al. A new method of EMR using insulation-tipped diathermic knife. Endoscopia digestiva. 1999;11:675–681. [Google Scholar]
- 17.Oyama T, Kikuchi Y, Hirasawa D, et al. Endoscopic submucosal dissection with hook knife. Stomach Intestine. 2004;39:35–38. [Google Scholar]
- 18.Matsui Y, Inomata M, Izumi K, Sonoda K, Shiraishi N, Kitano S. Hyaluronic acid stimulates tumor-cell proliferation at wound sites. Gastrointest Endosc. 2004;60:539–543. doi: 10.1016/s0016-5107(04)01890-5. [DOI] [PubMed] [Google Scholar]
- 19.Thienes CH, Skillen RG, Meredith OM, Fairchild MD, McCandless RS, Thienes RP. The hemostatic, laxative and toxic effects of alginic acid preparations. Arch Int Pharmacodyn Ther. 1957;1:167–181. [PubMed] [Google Scholar]
- 20.Solandt OM. Some observations upon sodium alginate. Quart J Exp Physiol. 1941;31:25. [Google Scholar]
- 21.Chenoweth MB. Toxicity of sodium alginate in rats. Ann Surg. 1948;127:1173. doi: 10.1097/00000658-194806000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]