Abstract
Background/Aims
It is difficult to detect early gastric cancer (EGC) during endoscopic surveillance because the remnant stomach is usually deformed after surgical resection and the mucosa at the gastric stump are changed due to bile reflux. In this study, we aimed to determine the characteristic endoscopic findings of cancer in the remnant stomach.
Methods
Fifty-five remnant gastric cancer (RGC) patients were classified into three groups according to location and elapsed time after surgery. Among 32 RGCs that developed less than 10 years after surgery, 21 lesions were located close to the anastomosis site (recurrent cancers), whereas 11 lesions were not (residual cancers). Twenty-three cancers developed at least 10 years after surgery (newly developed cancers). The endoscopic features were compared among these groups.
Results
Most patients (29/32, 91%) with residual or recurrent cancer developed their tumors within five years after surgery, and the proportion of EGC was 43.8% (14/32). However, 91.3% (21/23) of newly developed cancers were advanced gastric cancers. When classified according to the Japanese classification system for EGC, 71% (5/7) of the residual cancers were of the elevated type, whereas 86% (6/7) of the recurrent cancers were of the depressed type (p=0.00).
Conclusions
During the first 5 years after subtotal gastrectomy, endoscopists should mainly try to find depressed lesions on the anastomosis site as well as elevated lesions on the non-anastomosis site.
Keywords: Gastric cancer, Stump, Gastrectomy
INTRODUCTION
The number of patients who undergo endoscopic surveillance after subtotal gastrectomy has recently been increasing, which is due to the improved survival rate in gastric cancer patients. However, it is difficult to detect early gastric cancer (EGC) during endoscopic surveillance because the remnant stomach is usually deformed after surgical resection, and the mucosal changes at the gastric stump are severe due to bile reflux.1 Therefore, it would be very helpful if the endoscopic features of EGC that exist on a specific location of the gastric remnant such as at the anastomosis site or at a non-anastomosis site, could be characterized.
The definition of remnant gastric cancer (RGC) has changed since the first description of gastric cancer after partial gastrectomy for benign peptic ulcer disease in the early 1920s.2,3 Recent definition of RGC is any gastric cancer that develops on the remnant gastric mucosa after a partial gastrectomy because of gastric cancer or benign gastric disease. Kaminishi et al have classified RGCs into three subsets (Table 1)4; (i) a newly developed cancer (a cancer developing more than 10 years after subtotal gastrectomy for benign or malignant disease), (ii) a recurrent cancer (a cancer developing on the anastomosis site less than 10 years after surgery for gastric malignancy), and (iii) a residual cancer (a cancer developing less than 10 years after surgery for gastric malignancy except at the anastomosis site, or less than 10 years after benign gastric surgery). This classification is based on the time interval between subtotal gastrectomy and tumor recurrence, and also on the location of the tumor recurrence (at the anastomosis site or non-anastomosis site) in order to differentiate recurrent and primary cancers. Therefore, in this study, we aimed to know if there are characteristic RGC endoscopic findings according to the criteria of Kaminishi's classification. In addition, different clinicopathologic findings between Kaminishi's subgroup classifications were also analyzed.
Table 1.
Classification of Remnant Gastric Cancer by Kaminishi et al.4
MATERIALS AND METHODS
1. Subjects
For 8 years, 55 patients underwent surgery for RGC in our institution. The data on the following clinical features were collected according to the age, gender, time interval between operations, tumor size, Borrman's type, the depth of invasion, lymph node metastasis, and the histological differentiation classified by the Japanese Research Society for Gastric Cancer (JRSGC) as well as Lauren's classification. Macroscopic appearances of early gastric cancers (EGCs) were analyzed by the Japanese classification system,5 and advanced gastric cancers (AGCs) were categorized according to the Borrman's classification.6 RGC patients were divided into three groups according to Kaminishi's classification; (i) a newly developed cancer, (ii) a recurrent cancer, or (iii) a residual cancer (Table 1).4 Comparisons were then made on the clinical characteristics and the endoscopic findings among these three groups.
2. Statistical analysis
All analyses were performed using SPSS (version 11.5.0, SPSS Inc.). The chi-squared test was used to compare frequencies and one-way ANOVA testing was used to compare the mean patient ages, the time intervals and the sizes of the lesions. The level of significance was defined as p<0.05.
RESULTS
Of 55 RGC patients, 23 patients were diagnosed as newly developed cancer while 11 of 21 patients were classified as having residual and recurrent cancers, respectively (Table 2). The mean age of the patients with residual cancer and newly developed cancer (63.5 and 59.5 years, respectively) was significantly higher than that of the patients with recurrent cancer (51.3 years) (p<0.01). However, there was no difference in gender ratio among three groups. The median time between the first operation and a newly developed cancer was not different for those patients with residual and recurrent cancer. Most of the patients (29/32, 91%) with residual or recurrent cancer developed their tumors within five years after the surgery.
Table 2.
Clinical Features of Residual, Recurrent and Newly Developed Cancer of the Remnant Stomach
*Time interval between the first operation and the development of recurrent cancer.
†B-I, Billroth I; B-II, Billroth II.
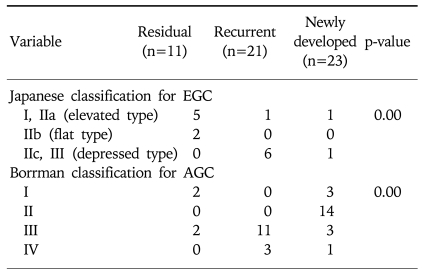
Gastric cancer was the initial disease in all the patients with residual and recurrent cancer, whereas 60.8% (14/23) of patients with newly developed cancer revealed peptic ulcer diseases as their primary disease. The proportion of EGC was 63.6% (7/11) for the residual cancer patients and 33.3% (7/21) for the recurrent cancer patients. However, only 8.7% (2/23) of the newly developed cancers were diagnosed as EGC. Under the Japanese classification system for EGC, 71% (5/7) of the residual cancers were elevated type (Fig. 1), whereas 86% (6/7) of the recurrent cancers were depressed type (Fig. 2) (p=0.00). According to the Bormann's classification of advanced RGC, 66.7% (14/21) of the newly developed cancers were ulcerative type, whereas 78.6% (11/14) of recurrent cancers were ulceroinfiltrative type (p=0.00) (Table 3).
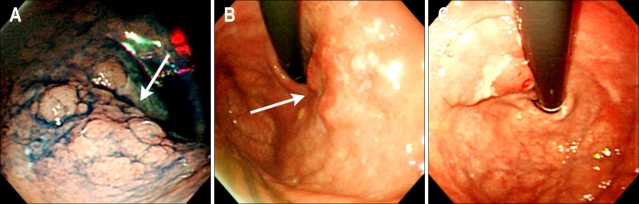
Fig. 1.
Endoscopic findings. Early gastric cancer (EGC) is noticed on the greater curvature side of the high body (A) and cardia (B and C) not close to the anastomosis site (residual cancer). Under the Japanese classification of EGC, 71% (5/7) of residual cancers are elevated types.
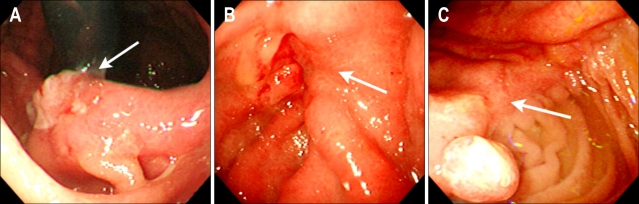
Fig. 2.
Early gastric cancer (EGC) on the anastomosis site (recurrent cancer). Contrary to residual cancer, 86% (6/7) of the recurrent cancers are depressed types according to the Japanese classification of EGC (p=0.00).
Table 3.
Endoscopic Features of Residual, Recurrent and Newly Developed Cancer of the Remnant Stomach
EGC, early gastric cancer; AGC, advanced gastric cancer.
DISCUSSION
In this study, RGCs that developed less than 10 years after surgery, most of the EGCs at the anastomosis site were depressed type cancers according to the Japanese classification system. However, elevated type EGCs were predominant in cancers on the anastomosis site.
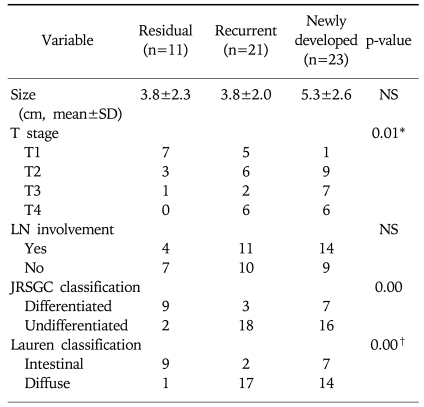
These endoscopic characteristics could be partly explained by the pathologic differences (Table 4). According to the UICC (International Union Against Cancer) TNM criteria,7 there were statistically significant differences in the T staging among three groups; residual cancers were less invasive than the recurrent and newly developed cancers. Furthermore, based on the JRSGC classification,8 81.8% (9/11) of the residual cancers were differentiated, whereas 14.3% (3/21) of the recurrent cancers and 30.4% (7/23) of the newly developed cancers were differentiated type adenocarcinoma. By Lauren's classification,9 90.0% (9/10) of residual cancers were intestinal type, while 89.5% (17/19) of the recurrent cancers and 66.7% (14/21) of the newly developed cancers were diffuse type. These findings for the residual cancers (well differentiated and elevated type EGC) are very similar to the characteristics of synchronous multiple gastric cancers.10
Table 4.
Pathologic Features of Residual, Recurrent and Newly Developed Cancer of the Remnant Stomach
*†In two cases of recurrent cancers, total gastrectomy revealed no residual tumor, although cancers were proven by biopsy. One case of a residual cancer was of the mixed type. Two cases of newly developed cancer were of the mixed type.
LN, lymph node.
JRSGS, Japanese Research Society for Gastric Cancer.
The prevalence of multiple gastric cancers is between 3.7-6.7%.11,12 A study reported that multiple carcinomas were associated significantly more often with adenomas, atrophic gastritis or intestinal metaplasia than that of the solitary carcinomas.10 They suggested that multiple and solitary carcinomas represent different developmental stages of a fundamentally identical process, with the cancer phenotype being dependent on the speed of progression. In other words, slow progression results in multiple tumors and rapid progression results in solitary tumors. Therefore, the residual cancers might have originated from those multiple gastric cancers that were not detected during the initial workup, whereas almost all the EGCs of the recurrent cancers were depressed type. Advanced RGCs were more easily detected in recurrent cancers than in residual cancers. These lesions might have resulted from the downward growing nature and undifferentiated pathology of the recurrent cancer.
Remnant stomach after benign gastric surgery is considered as a risk factor for the development of gastric cancer.13-15 Although exact mechanism is unclear, achlorhydria, atrophic gastritis, previous gastric surgery, an N-nitroso compound, persistent bile reflux, denervation of the gastric mucosa and bacterial invasion of the gastric stump have been regarded as possible etiologies.16-18 Among them, the combination of persistent bile reflux and denervation are assumed to enhance tumorigenesis at the anastomosis site.18 Therefore, those patients who had undergone Billroth II reconstruction at the initial surgery for benign lesions tended to have the development of cancer at stump site, whereas those patients who undergone Billroth I tended to have cancer at the non-stump site.19 Also in our study, almost all the RGC patients (13/14) with initially benign disease developed gastric cancer on the anastomosis site, except in one case. In contrast, only 65% (5/9) of newly developed cancer patients with initially malignant diseases developed cancer at the stump site. This result shows that the gastric mucosa of the patients who underwent malignant gastric surgery tends to develop cancer more easily than those patients with initially benign disease.
According to Kaminishi et al,4 a cut-off point of 10 year was proposed as a time limit that differentiates recurrent cancer from primary cancers. However, a 10 year time limit for primary gastric cancer is controversial. RGC was initially defined as that gastric cancer detected more than five years after benign surgery,3 and many investigators agree on this definition.11,16 In a recent study, most patients (29/32, 91%) with residual or recurrent cancer developed their tumors within five years after their surgery. However, even if the definition of the time interval was changed to five years, the results of our study would not been changed.
The time interval between the first operation and the development of a newly developed cancer was significantly different according to the nature of the initial disease (benign disease: 25.9±10.4 years, malignant disease: 17.0±6.1 years; mean±SD). This finding is consistent with previous report.19 A possible explanation for this result may derive from the differences in the patients' ages at the peak of incidence. In this study, the mean age of the patients when they underwent subtotal gastrectomy for benign disease was 32.3±10.9 years, whereas that for malignant disease was 44.7±10.2 years. These findings are consistent with previous studies that the patient age at the peak incidence of peptic ulcer disease is lower than that for gastric cancer.20,21 In contrast to that, the age of tumor recurrence in two groups did not differ. Therefore, the differences in the patients' ages at the peak incidence may account for the difference in the time interval between the first operation and the development of a newly developed cancer. A second possibility is that those patients who had undergone surgery for gastric malignancy exhibited precancerous lesions such as atrophic gastritis and intestinal metaplasia, and the possibility of developing cancer might be higher for them than for the patients who had undergone benign gastric surgery. Another possibility may include a higher probability of finding lesions in patients who have undergone malignant surgery compared to those patients who have undergone benign gastric surgery because the first group underwent aggressive and frequent follow-up endoscopies.
It has been reported that periodic surveillance endoscopy procedures are recommended at intervals of 2-3 years to detect early-stage RGC.22 However, in our patients with recurrent cancer, even annual endoscopic surveillance could not detect early gastric cancer because of the difficulty in finding depressed lesions on the deformed anastomosis site. Therefore, a more frequent surveillance program (at intervals of six months to one year) with aggressive biopsy of suspicious lesions should be instituted. Furthermore, an important reason for a few newly developed EGCs is the failure to continue regular surveillance endoscopies 10 years after the patients' subtotal gastrectomy. These results emphasize the importance of surveillance endoscopies even at 10 years after subtotal gastrectomy.
In conclusion, frequent endoscopic screening with active biopsy of the suspicious lesions should be done during the first 5 years after subtotal gastrectomy. Endoscopists should mainly try to find the depressed lesions on the anastomosis site and also the elevated lesions on the non-anastomosis site. In addition, patients should undergo periodic screening endoscopy 10 years after their subtotal gastrectomy in order to find EGC earlier which should especially be done for discovering any initial malignant disease.
References
- 1.Johannesson KA, Hammar E, Stael von Holstein C. Mucosal changes in the gastric remnant: long-term effects of bile reflux diversion and Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2003;15:35–40. doi: 10.1097/00042737-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Helsingen N, Hillestad L. Cancer development in the gastric stump after partial gastrectomy for ulcer. Ann Surg. 1956;143:173–179. doi: 10.1097/00000658-195614320-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balfour DC. Factors influencing the life expectancy of patients operated on for gastric ulcer. Ann Surg. 1922;76:405–408. doi: 10.1097/00000658-192209000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaminishi M, Shimoyama S, Yamaguchi H, et al. Classification and carcinogenesis of gastric stump cancer. Gastroenterol Surg. 1993;16:1253–1265. [Google Scholar]
- 5.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. 2nd English ed. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 6.Borrmann R. Geshwulste des magens und duodenums. In: Henke F, Lubarsch O, editors. Handbuch der Speziellen Pathologischen Anatomieund Histologis. Berlin, Germany: Springer-Verlag; 1926. p. 865. [Google Scholar]
- 7.Sobin LH, Fleming ID. TNM Classification of Malignant Tumors, fifth edition. Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer. 1997;80:1803–1804. doi: 10.1002/(sici)1097-0142(19971101)80:9<1803::aid-cncr16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Japanese Research Society of Gastric Cancer. Japanese classification of gastric cancer. Tokyo: Kanehara Co. Ltd.; 1995. [Google Scholar]
- 9.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microb Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 10.Wittekind C, Klimpfinger M, Hermanek P, et al. Multiple simultaneous gastric carcinomas. Br J cancer. 1997;76:1604–1609. doi: 10.1038/bjc.1997.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greene FL. Management of Gastric remnant carcinoma based on the results of a 15-year endoscopic screening program. Ann Surg. 1996;223:701–708. doi: 10.1097/00000658-199606000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kodera Y, Yamamura Y, Torii A, et al. Incidence, diagnosis and significance of multiple gastric cancer. Br J Surg. 1995;82:1540–1543. doi: 10.1002/bjs.1800821127. [DOI] [PubMed] [Google Scholar]
- 13.Fisher SG, Davis F, Nelson R, et al. A cohort study of stomach cancer risk in men after gastric surgery for benign disease. J Natl Cancer Inst. 1993;85:1303–1310. doi: 10.1093/jnci/85.16.1303. [DOI] [PubMed] [Google Scholar]
- 14.Lundegardh G, Hans-Olov A, Helmick C, et al. Stomach cancer after partial gastrectomy for benign ulcer disease. New Engl J Med. 1988;319:195–200. doi: 10.1056/NEJM198807283190402. [DOI] [PubMed] [Google Scholar]
- 15.Tersmette AC, Offerhaus GJA, Tersmette KWF, et al. Meta analysis of the risk of gastric stump cancer: detection of high risk patient subsets for stomach cancer after remote partial gastrectomy for benign conditions. Cancer Res. 1990;50:6486–6489. [PubMed] [Google Scholar]
- 16.Pointner R, Schwab G, Konigsrainer A, et al. Gastric stump cancer: etiopathological and clinical aspects. Endoscopy. 1989;21:115–119. doi: 10.1055/s-2007-1012917. [DOI] [PubMed] [Google Scholar]
- 17.Northfield TC, Hall CN. Carcinoma of the gastric stump: risk and pathogenesis. Gut. 1990;31:1217–1219. doi: 10.1136/gut.31.11.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaminishi M, Shimizu N, Shimoyama S, et al. Etiology of gastric remnant cancer with special reference to the effects of denervation of the gastric mucosa. Cancer Res. 1995;75:1490–1496. doi: 10.1002/1097-0142(19950315)75:6+<1490::aid-cncr2820751518>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Tanigawa N, Nomura E, Niki M, et al. Clinical study to identify specific characteristics of cancer newly developed in the remnant stomach. Gastric Cancer. 2002;5:23–28. doi: 10.1007/s101200200003. [DOI] [PubMed] [Google Scholar]
- 20.Rhee JK, Cha KM. A clinical analysis of peptic ulcer required surgical treatment. J Korean Gastroenterol. 1987;19:49–55. [Google Scholar]
- 21.Suk DS. Epidemiological comparative study between Korean and Japanese Gastric carcinoma. J Korean Gastroenterol. 1990;22:32–35. [Google Scholar]
- 22.Hosokawa Y, Kaizaki K, Watanabe H, et al. Endoscopic surveillance for gastric remnant cancer after early cancer surgery. Endoscopy. 2002;34:469–473. doi: 10.1055/s-2002-32007. [DOI] [PubMed] [Google Scholar]