Abstract
Early gastrointestinal cancers are defined as lesions limited to the mucosa or submucosa without invading the muscularis propria, regardless of the presence of lymph node metastases. Although the natural history of these diseases is basically alike worldwide, its management is quite different between the East and West; aggressive surgery is frequently adopted by Western surgeons, while less invasive techniques are adopted by Asian colleagues. These techniques include endoscopic mucosal resection and endoscopic submucosal dissection which are now accepted as treatments for early gastrointestinal cancers in selected cases. Recent advances in endoscopic detection and treatment techniques, especially in Japan and Korea, have prompted Western endoscopists to learn these techniques. This review addresses recent advances regarding endoscopic resections of early gastrointestinal cancers, which promoted its use in Western countries. In addition, prospective studies on endoscopic resection in Western countries are also described.
Keywords: Endoscopic resection, Gastrointestinal cancer, West
INTRODUCTION
Therapeutic endoscopy plays a major role in the management of gastrointestinal (GI) neoplasia. Its indications can be generalized into four broad categories; (i) to remove or obliterate neoplastic lesion, (ii) to palliate malignant obstruction, (iii) to treat bleeding, and (iv) the others.1,2 Only endoscopic resection allows complete histological staging of the cancer, which is critical as it allows stratification and refinement for further treatment. Although other endoscopic techniques such as ablation therapy may also cure early GI cancer, they can not provide a definitive pathological specimen.
Early stage lesions reveal low frequency of lymph node metastasis which allows for less invasive treatments and thereby improving the quality of life when compared to surgery.3 Endoscopic mucosal resection (EMR) and submucosal dissection (ESD) are now accepted worldwide as treatment modalities for early cancers of the GI tract.1-3 Recent advances in endoscopic detection and treatment techniques in Japan and Korea have prompted Western endoscopists to use EMR and ESD as elective treatment modalities in selected cases. However, they are still challenging techniques and require considerable experience.
Following possible explanations are proposed why EMR and ESD are less often performed in West; (i) the screening system for these diseases, (ii) the relative infrequency of gastric and esophageal cancers and their premalignant precursors, (iii) the differences in the judging criteria between Japanese and Western pathologists, and (iv) the risk perception of the procedures.4 The purpose of this review was to examine the recent advances in the endoscopic resection of early GI cancers in Western countries.
DETECTION OF EARLY GASTROINTESTINAL CANCERS
Early GI cancers (except the esophagus) are defined as being limited to the mucosa or submucosa but not invading the muscularis propria, regardless of the presence of lymph node metastases. A macroscopic classification of these lesions was first established by Japanese endoscopists (Table 1). It was ratified at the Paris workshop in 2002 and has now been accepted worldwide.5 Usually, early cancers are detected as 0-I and 0-III types in most of the world, but widespread use of chromoendoscopy, anti-foaming agents, and newer imaging methods have enabled identification of superficial lesions such as faint mucosal irregularity or discoloration, which had previously overlooked. Over 50% of cancers diagnosed in Japan are early cancers while its frequency is only 10% in the West.6
Table 1.
The Endoscopic Classification of Early Gastrointestinal Cancers
1. Endoscopic detection
Detection and diagnosis of early GI cancer can be difficult because of the less well defined subtle findings. These may include slight differences in color (i.e., more red or pale), loss of vascularity, nodularity, thickening, or depression.6 Thus, apart from searching for protrusion, erosion, ulceration, and depression, examination for early GI cancer requires continuous search for mucosa with features distinct from those of its surrounding.5 Therefore, the subtleties of endoscopic findings of early GI cancer require patient preparation to be most optimal. Effective methods to increase visualization of the mucosa of the upper gastrointestinal tract (UGI) include the usage of pronase, a proteolytic enzyme solution, to remove mucus.
Chromoendoscopy is an important adjunct technique to enhance visualization of superficial early cancers and to define their borders. Indigo carmine solution (0.2%), a contrast dye, is commonly used in the stomach to highlight the contours and topography of the lesion by entering mucosal depressions and crevices thus enabling the biopsy of the minute lesions. Recently, narrow band image (NBI) and autofluorescence images (AFI) are introduced as a virtual chromoendoscopy.
2. Histological diagnosis
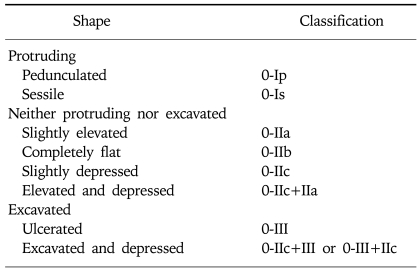
Apart from the diagnostic difficulty of biopsy as a reason for the low incidence of early GI cancer in the West, different histological criteria between the West and Japan would be another reason.7 For example, most mucosal cancer of the intestinal type in Japan is not diagnosed as cancer in the West (Fig. 1). There are large discrepancies in the diagnostic criterion between the Western and Japanese pathologist. These differences have caused considerable problems in the interpretation of Japanese cancer research by Western clinicians and researchers. Carcinoma is defined as invasion with submucosal layer, muscularis mucosa, or at least lamia propria in the West. However, in Japan, cellular atypia and structural atypia is also considered as GI neoplasia regardless of invasive findings.
Fig. 1.
Histological diagnosis of EGC. (A) A small depressed lesion is noticed on the posterior wall of upper gastric body. (B) Lesion without invasive finding is noticed on the biopsy, which is classified as dysplasia (Vienna classification 4.1.) in the West. In contrast, this lesion is classified as well differentiated adenocarcinoma (Vienna classification 4.2.) in Japan. (C) After the resection, submucosally invasive adenocarcinoma with lymphatic involvement (Vienna classification 5.2.) is diagnosed.
The differences between the Western and Japanese histological classifications of GI epithelial neoplastic lesions have been largely resolved by adopting the Vienna classification (Table 2).7 Non-invasive neoplasia include low- and high-grade dysplasia (LGD and HGD) in which the basement membrane is not infiltrated. Invasive lesions include intramucosal neoplasia infiltrating the submucosa. To quantify the risk of lymph node metastases, mucosal (m) and submucosal (sm) layers have each been divided into three sections: m1 (epithelium), m2 (lamina propria), and m3 (muscularis mucosae) and sm1, sm2, and sm3. In addition, sm1 is subclassified into a, b, and c according to the degree of horizontal extension. It is the depth that mainly determines the necessity of intervention.
Table 2.
Vienna Classification of Gastrointestinal Epithelial Neoplasia
ESD, endoscopic submucosal dissection.
The depth of invasion is measured microscopically and the risk of lymph node metastasis is known to be related with a defined micrometric cutoff. In squamous cell carcinoma (SCC) of the esophagus, when infiltration is less than 200 µm, the risk of nodal metastases is low.8 For early adenocarcinoma in Barrett's esophagus (BE) and early gastric cancer (EGC), a submucosal infiltration micrometric cutoff of 500 µm has been proposed, as the risk of nodal metastases appears low.9 In contrast, a 20-25% risk of node involvement with submucosal infiltration in Barrett's cancer has been reported in the West.10 In the colorectum, the risk of lymph node metastasis is negligible when the tumor invasion is less than 1,000 µm.11 Other factors, such as the degree of differentiation and the presence of vascular or lymphatic invasion are also related to the risk of lymph node metastasis.
ENDOSCOPIC TREATMENT OF EARLY GASTROINTESTINAL CANCERS IN THE WEST
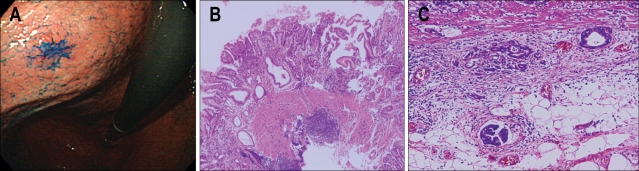
The treatment of early GI cancers in the West is still mainly an operation. However, many Western endoscopists are now learning from the Japanese experiences and increasingly adopting endoscopic resection (Fig. 2). In contrast to Japan and Korea, there has so far been only limited experience in Europe as well as in US. Here, we report the latest and largest prospective series so far in Western countries with early GI cancers, treated by EMR or ESD.
Fig. 2.
Standard EMR methods. (A) Snare polypectomy, (B) strip biopsy, (C) EMR with cap technique, and (D) EMR with ligation technique.
1. Esophagus
Multiple synchronous lesions as well as metachronous lesions have been reported up to 31% of patients in esophageal SCC.12 Five-year survival rate is up to 95% after EMR in patients with superficial SCC without lymph node metastasis in m1 and m2 SCC of the esophagus.13 Recently, an expanded indications for EMR in patients with superficial esophageal carcinoma m3 or sm1 has been proposed.14
A study from Germany, consisted of 12 HGD and 53 mucosal SCC of the esophagus, reported on the treatment using a ligation EMR technique.15 This was the first Western study which showed similar results to those of the Japan in early esophageal SCC. They reported that complete resection was achieved in 11/12 patients of HGD and 51/53 patients of mucosal SCC. Although recurrence was observed in 16 patients after EMR, the lesions were completely resected after further endoscopic treatment. Complications occurred in 15/65 patients all being esophageal strictures which were successfully managed by endoscopy. Seven year survival rate was 77%.
2. Barrett's esophagus
Endoscopic therapy aims to remove the dysplastic Barrett's epithelium allowing restoration of squamous epithelium. EMR could be a therapeutic alternative to surgical esophagectomy which carries substantial morbidity and mortality.16 When HGD is diagnosed in short segment BE (BE shorter than 30 mm in length), EMR would be considered to remove all the metaplastic epithelium. A study from US reported that visible areas of HGD in long segment BE (BE longer than 30 mm in length) may be removed by EMR, followed by photodynamic therapy to destroy invisible foci.17 Although strictures occurred in 30% of patients, 17 superficial esophageal cancers were removed by combining EMR and photodynamic therapy.
A study from Germany reported that circumferential EMR was carried out by using a simple snare technique without a cap in 12 patients with BE containing multifocal high-grade intraepithelial neoplasia or intramucosal cancer.18 Five had multifocal lesions while two developed strictures that required bougienage. There was no recurrence during the median follow-up of 9 months. Similar study from France reported on circumferential EMR performed in 21 patients with HGD or mucosal cancer.19 Three patients needed additional therapy such as surgery or chemotherapy due to residual disease after the endoscopic resection. In addition, two local recurrences were retreated by EMR.
A study from Netherlands, consisted of 77 esophagectomy specimens containing HGD or T1 adenocarcinoma, reported that lymph node metastasis occurred in 23% sm2 and 69% sm3 tumors, but not in m1, m2, m3, and sm1 lesions.20 They concluded that m1, m2, m3, and sm1 lesions could be treated endoscopically if the lesions are less than 30 mm, well differentiate type adenocarcinoma, and without lymphangitic invasion. However, care must be taken with the intitial diagnosis of endoscopic biopsy since significant changes in diagnosis occur after EMR such as downgrading from HGD to BE without dysplasia or being reclassified from benign to malignant diagnosis.21 A study from Germany reported that suck-and-cut EMR technique was performed in 100 consecutive patients with low-risk adenocarcinoma of the esophagus arising from BE, and complete local remission was achieved in 99/100 patients.22 During the mean follow-up period of 36.7 months, recurrent or metachronous carcinomas were found in 11% of the patients, but were successfully treated by repeated EMR. Five-year survival rate was 98%.
3. Stomach
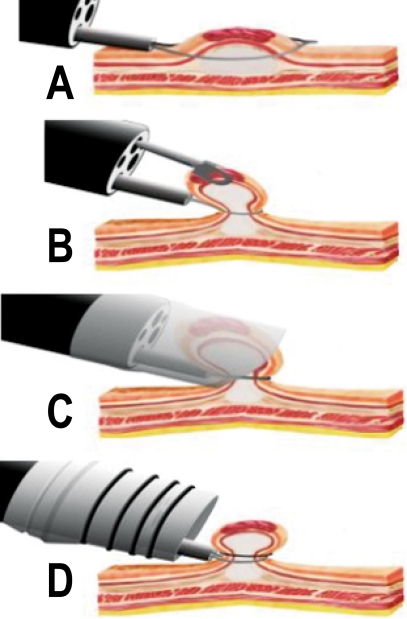
EMR and ESD are established as an alternative treatment to surgery for EGC in Japan.23 Criteria for endoscopic therapy are; (i) elevated type intramucosal cancer (0 to IIa) less than 20 mm, (ii) depressed type mucosal cancer without ulceration (0 to IIb, 0 to IIc) less than 10 mm, and (iii) well differentiated or moderately differentiated intestinal type adenocarcinoma. Recently, an extended indication for EMR in EGC has been proposed; (i) well differentiated cancers less than 30 mm without ulcer or ulcer scar, (ii) mucosal cancers less than 20 mm with ulcer or ulcer scar, (iii) sm1 cancer less than 20 mm without ulcer or ulcer scar, and (iv) poorly differentiated cancer less than 10 mm.24 The risk of node metastasis is about 0.4% for differentiated cancers while it is about 4% in undifferentiated mucosal cancers.25 In another report, poorly differentiated and signet ring cell carcinomas less than 5 mm in size would be considered for EMR or ESD.26 In addition, the risk of lymph node metastasis in additional groups of patients with EGC was defined in Japan by using a large database involving more than 5,000 patients who underwent gastrectomy with R2 level lymph node dissection.25 The results of this study led to an expanded list of candidates suitable for endoscopic resection including lesions larger than 21 mm and ulcerative lesions which were originally resected by surgery (Table 3). Although the need for gastrectomy in EGC can be reduced by expanding the criteria for EMR, it is difficult to resect large and ulcerative lesions by conventional EMR. Therefore, substantial experience has been gained and ESD using the IT knife (Fig. 3) became a standard practice in Japan for the treatment of EGC (Fig. 4).27
Table 3.
Indications for Endoscopic Mucosal Resection / Endoscopic Submucosal Dissection in Early Gastrointestinal Cancers
SCC, squamous cell carcinoma; AC, adenocarcinoma; HGD, high grade dysplasia; IMC, intramucosal cancer.
Fig. 3.
Endoscopic devices for ESD. (A) IT knife (KD-610L, Olympus), (B) modified IT knife with three-pointed star blade (Olympus), (C) Needle knife (KD-1L-1, Olympus), (D) Hook knife (KD-620LR, Olympus), (E) Flex knife (KD-630L, Olympus), (F) Triangle-tip knife (Olympus), (G) Flash knives with several length of needle (Fujinon Toshiba ES systems), (H) Mucosectom (DP-2518, Pentax), and (I) Small caliber tip transparent (ST) hood (DH-15GR, 15CR, Fujinon Toshiba ES systems).
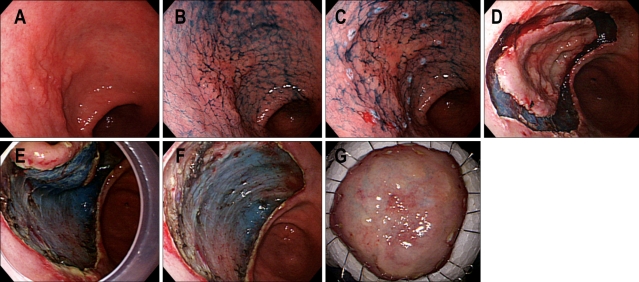
Fig. 4.
ESD procedures. (A) A depressed type EGC is noticed on the anterior wall of the antrum. (B) Indigo carmine dye is sprayed to detect the tumor boarder. (C) Markings are done by needle knife with coagulation current. (D) Mucosal cutting is done with IT knife using ENDO-CUT mode. (E) Dissecting submucosal layer is done by the aid of IT knife with ENDO CUT mode. Attachment cap is applied to stretch submucosal tissue. (F) A large ESD defect is noticed after complete one piece resection without perforation. (G) Flattened ESD specimen is fixed with thin needles on plate.
In Europe, there is a report from Germany on ESD using a new double-channel endoscope (the R-scope).28 After the resection, surgery was performed in 4/10 patients because of perforation, incomplete resection or deep submucosal tumor infiltration.
4. Duodenum
EMR has been used for ampullary and peri-ampullary neoplasias and sub-epithelial lesions including stromal cell tumors, cysts, and neuroendocrine tumors. Although endoscopic resection can provide a wide tumor resection with a negative resection margin, it is not yet recommended as a curative therapy for early stage ampulla of Vater cancer because of the high lymphovascular invasion rate.29 A study from US reported a higher risk of bleeding (33%) among 27 duodenal EMR afer complete resection.30
5. Submucosal tumor
EMR technique may also be applied to submucosal tumors in order to achieve histologic diagnosis and to achieve complete removal. In Germany, complete resection was achieved in 19/20 patients with submucosal esophageal tumors using a rubber band or a simple snare.31 Bleeding occurred in 40% of the cases and was successfully managed by endoscopic hemostasis. Rosch et al attempted endoscopic en bloc resection of mucosal and submucosal tumors of the UGI tract, using the IT knife.32 In this pilot series, complete removal was achievied in 25% of the mucosal and 36% of the submucosal lesions of 37 lesions; 13 in esophagus, 24 in stomach and 1 in duodenum. Perforation occurred in one case, and was managed conservatively with endoscopic clipping.
In US, variously located superficial neoplastic or submucosal lesions were treated by EMR using strip biopsy or suction cap techniques after the evaluation with high-frequency (20 MHz) ultrasonography.33 Wide variety of tumors including flat colonic adenomas, gastric and rectal neoplasms, ampullary adenomas, duodenal carcinoids, BE, and esophageal cancers including 24 epithelial lesions and 8 submucosal lesions were treated successfully. All lesions were completely resected except in one duodenal bulb carcinoid tumor. Minor complications such as pain and mild pancreatitis occurred in three cases. Six out of seven carcinomas revealed no recurrence during the mean follow-up of 12 months.
6. Colorectum
EMR and ESD are being successfully used for early-stage colon cancers, flat adenomas, large superficial colorectal tumors, and rectal carcinoids.34 Lymph node metastasis in T1 colorectal carcinoma occurs only after infiltrating submucosa and is correlated to the depth of submucosal penetration by the tumor.35 This supports the therapeutic effectiveness of endoscopic removal of polyps and flat lesions that are confined to the mucosa, regardless of their size. On the other hand, colorectal laterally spreading tumor (LST) classified as granular (LST-G) and non granular type (LST-NG), are defined as lesions larger than 10 mm in diameter, with a low vertical axis, extending along the luminal wall.36 For en bloc resection of flat lesions larger than 20 mm, conventional EMR is inadequate because of incomplete removal and frequent local recurrence. When analyzing the endoscopic features of 257 LSTs in order to assess which features correlated with the depth of invasion, unevenness of nodules, presence of large nodules, size, histological type, and presence of depression in the tumor were significantly associated with the depth of invasion.36 In addition, LST-NG showed a higher frequency of sm invasion than LST-G (14% vs. 7%).37 Presence of a large nodule in LST-G type was associated with higher sm invasion while pit pattern, sclerous wall change, and larger size were significantly associated with higher sm invasion in LST-NG type. Therefore, it is advisable to perform endoscopic piecemeal resection for LST-G type with the area including the large nodule resected first. Besides, LST-NG type should be removed by en bloc resection because of the higher potential of sm invasion when compared to that of the LST-G type.37
In aspect of Western experiences, a study from Germany reported that complications occurred in two patients of 57 patients after EMR in large colorectal neoplasia between 10 mm and 50 mm.38 Recurrence rate following EMR range from 0% to 40% which could be reduced when combined by argon plasma coagulation.39,40 However, a recent study from Poland revealed that argon plasma coagulation did not reduce the recurrence rate compared to polypectomy alone.41 Besides, a study from Italy reported that EMR was performed for 139 SP in 136 patients by snare polypectomy, and invasive carcinoma was found in 17 cases.42 After 12 months of follow up after EMR, no local recurrence was detected in 7 patients with invasive carcinoma without surgery. Another study from UK reported on 30 large colorectal polyps which were treated by en bloc resection in 22 cases and by piecemeal resection in 8 cases.43 Histologically, the lesions were predominantly adenomatous polyps, but 7 cases revealed incidental focus of adenocarcinoma. Although bleeding occurred in two cases, there was no bowel perforation. There was no evidence of recurrence during the median follow-up of 21 months.
In a prospective cohort study in Italy, IT knife was used for EMR of large colorectal polyps larger than 3 cm which are unsuitable for standard polypectomy.44 The results showed the likelihood of complete en bloc resection of mucosal lesions improved by new approach with IT knife when compared with previous studies on colonic EMR, even for lesions located in difficult positions or larger than 30 mm. En bloc resection was achieved only in 55.1% of the lesions and piecemeal resection was performed in the rest of the cases. Although complete tumor removal was achieved in 19 patients, 13 had LGD, 15 had HGD, and one had a tumor invading the submucosa. Complications occurred in four patients which were all managed conservatively. Local recurrences were detected in five patients and were treated by argon plasma coagulation and snare polypectomy. There was no recurrence during the median follow-up period of 15.7 months.44
MANAGEMENT AFTER ENDOSCOPIC RESECTION
After the retrieval, resected specimen is flattened and fixed on the board (Fig. 4). Additional resections and reconstructions are seldom necessary as long as the marking dots are completely included in en bloc resection. Accurate evaluation of the resected specimen should be achieved immediately after the removal before being immersed in formaldehyde. Orientation of the specimen is accomplished by fixing its periphery with thin needles inserted into an underlying plate of rubber or wood. The submucosa side of the specimen is apposed to the plate.
After the fixation, the specimen should be sectioned serially in 2 mm intervals parallel to a line that includes the closest resection margin of the specimen so that both lateral and vertical margins could be assessed. The depth of tumor invasion (T) is then evaluated along with the degree of differentiation and lymphatic or vascular involvement.
1. Complications
The complications of endoscopic resection for EGC include pain, bleeding, and perforation.45 Pain after resection is typically mild. Standard dose of proton-pump inhibitor is administrated for 4-8 weeks to prevent postoperative bleeding and to promote ulcer healing.46 For the first 24 hours after the procedure, the patient is kept nil by mouth, followed by clear liquid on the second day, and soft diet for another three days. ESD-induced ulcer is reported to heal within 8 weeks after resection under antacid treatment.47
Bleeding is the most common complication occurring in up to 7-8% of patients undergoing standard EMR or ESD.48 Immediate bleeding appears more common with resections of tumors located on the upper third of the stomach. During ESD, immediate minor bleeding is not uncommon, but could be successfully treated by grasping the bleeding vessels using a hot biopsy forcep with 80W soft mode coagulation of ICC 200 (ERBE, Tubingen, Germany) (Fig. 5A, B). Endoclips are also often deployed for more brisk bleeding. Delayed bleeding, manifested by hematemesis or melena at 0-30 days after the procedure, is treated by emergent endoscopy using similar techniques. Although most bleeding (75%) occurs within 12 hours after the procedure, delayed bleeding after ESD occurs in 4-7% which is also strongly related with the location and size of the tumor.49
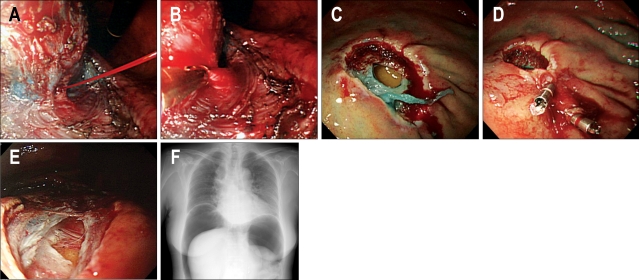
Fig. 5.
Complications during ESD. (A) Arterial bleeding is noticed from the exfoliated submucosal layer. (B) Endoscopic hemostasis is done by capturing the bleeding vessel. (C) Perforation is noticed on the greater curvature of upper body. (D) Endoscopic closure is done by using endoclips. (E) Small perforation is noticed. (F) Pneumoperitoneum is noticed.
Perforation is rare in EMR, but is relatively more common in ESD. The risks of perforation during ESD are about 4%.48 Perforations are related with tumor location and ulcer findings, but no relationship was found between perforation and tumor size. Gastric perforation during endoscopic resection can be conservatively treated by complete endoscopic closure with endoclips (HX-600-090; Olympus Co., Tokyo, Japan) when there is no peritoneal dissemination.50 This was attempted because the stomach is comparatively clean during ESD due to the fasting nature and because of the antibacterial effect of gastric acid (Fig. 5C, D). If abdominal fullness due to air leakage from the perforated lesion is severe (Fig. 5E, F), decompression of the pneumoperitoneum should be performed by 14G puncture needle with side slits after the testing using 23G needle syringe filled with saline under the confirmation by transabdominal ultrasonography. Nasogastric suction should be applied for 12 hours with broad-spectrum antibiotic given for 2 days. A diet should be advanced 3-4 days later.
Recently, to prevent gastric perforation, glyceol or sodium hyaluronate are used as injection agents.51 These makes the ESD procedure easier and safer because these agent stay longer in the submucosa and produce clear dissection layer. Regarding tissue damage due to the injected solution during ESD, sufficient one should be used for successful ESD. A combination of hyaluronic acid and glycerin solution is the most favorable submucosal injection solution considering tissue damage and sufficient lesion-lifting ability.52
CONCLUSION
In the West, EMR and ESD are not yet widely performed due to the different screening system for early GI cancers, relative infrequency of UGI neoplasia, discrepancy with Japanese pathological diagnosis, and insufficient perception of the procedures. For all GI cancers, prognosis correlates with stage of the disease at diagnosis. With the discovery of early GI lesions, EMR and ESD should be attempted considering their relatively low mortality and morbidity compared with surgery.
EMR and ESD techiques should be considered in the West as elective treatment modality for early GI cancers as long as it is performed under the right indications by an expertise. More experiences are needed to strenghten the performance capacity and foster the cooperation among skilled endoscopists in Japan and Korea.
References
- 1.Rembacken BJ, Gotoda T, Fujii T, Axon AT. Endoscopic mucosal resection. Endoscopy. 2001;33:709–718. doi: 10.1055/s-2001-16224. [DOI] [PubMed] [Google Scholar]
- 2.Soetikno RM, Gotoda T, Nakanishi Y, Soehendra N. Endoscopic mucosal resection. Gastrointest Endosc. 2003;57:567–579. doi: 10.1067/mge.2003.130. [DOI] [PubMed] [Google Scholar]
- 3.Conio M, Ponchon T, Blanchi S, Filiberti R. Endoscopic mucosal resction. Am J Gastroenterol. 2006;101:653–663. doi: 10.1111/j.1572-0241.2006.00424.x. [DOI] [PubMed] [Google Scholar]
- 4.Fleischer D. Endoscopic mucosal resection: (not) made in the USA (so commonly). A dissection of the definition, technique, use, and controversies. Gastrointest Endosc. 2000;52:440–444. doi: 10.1067/mge.2000.108482. [DOI] [PubMed] [Google Scholar]
- 5.Endoscopic Classification Review Group. Update on the Paris Classification of superficial neoplastic lesions in the digestive tract. Endoscopy. 2005;37:570–578. doi: 10.1055/s-2005-861352. [DOI] [PubMed] [Google Scholar]
- 6.Axon A. Symptoms and diagnosis of gastric cancer at early curable stage. Best Practice & Research Clinical Gastroenterology. 2006;20:697–708. doi: 10.1016/j.bpg.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Stolte M. The new Vienna classification of epithelia neoplasia of gastrointestinal tract: advantages and disadvantages. Virchows Arch. 2003;442:99–106. doi: 10.1007/s00428-002-0680-3. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe M, Komukai S, Ajioka Y, Nisikura K, Hashidate H, Kijima H. Histopathology of m3 and sm1 invasive squamous cell carcinoma of the esophagus with special reference to endoscopic resection. Stomach and Intestine. 1998;33:985–992. [Google Scholar]
- 9.Kashimura H, Ajioka Y, Watanabe H, et al. Risk factors for nodal micrometastasis of submucosal gastric carcinoma: Assessment of indications for endoscopic treatment. Gastric Cancer. 1999;2:33–39. doi: 10.1007/s101200050018. [DOI] [PubMed] [Google Scholar]
- 10.Stein HJ, Feith M, Mueller J, et al. Limited resection for early adenocarcinoma in Barrett's esophagus. Ann Surg. 2000;232:733–742. doi: 10.1097/00000658-200012000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokoyama J, Ajioka Y, Watanabe H, et al. Lymh node metastatic and micrometastasis of submucosal invasive colorectal carcinoma: An indicator of the curative potential of endoscopic treatment. Acta Med Biol. 2002;50:1–8. [Google Scholar]
- 12.Pesko P, Rakic S, Milicevic M, Bulajic P, Gerzic Z. Prevalence and clinicopathologic features of multiple squamous cell carcinoma of the esophagus. Cancer. 1994;73:2687–2690. doi: 10.1002/1097-0142(19940601)73:11<2687::aid-cncr2820731106>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 13.Japanese Society for Esophageal Disease. Guidelines for the clinical and pathologic studies for carcinoma of the esophagus. Jpn J Surg. 1976;6:79–86. doi: 10.1007/BF02468890. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi K, Tanabe S, Koizumi W, et al. Expansion of the indications for endoscopic mucosal resection in patients with superficial esophageal carcinoma. Endoscopy. 2007;39:36–40. doi: 10.1055/s-2006-945148. [DOI] [PubMed] [Google Scholar]
- 15.Pech O, May A, Gossner L, et al. Curative endoscopic therapy in patients with early esophageal squamous-cell carcinoma or high-grade intraepithelial neoplasia. Endoscopy. 2007;39:30–35. doi: 10.1055/s-2006-945040. [DOI] [PubMed] [Google Scholar]
- 16.May A, Gossner L, Pech O, et al. Local endoscopic therapy for intraepithelial high-grade neoplasia and early adenocarcinoma in Barrett's oesophagus: acute-phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol. 2002;14:1085–1091. doi: 10.1097/00042737-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Buttar NS, Wang KK, Lutzke LS, Krishnadath KK, Anderson MA. Combined endoscopic mucosal resection and photodynamic therapy for esophageal neoplasia within Barrett's esophagus. Gastrointest Endosc. 2001;54:682–688. doi: 10.1067/gien.2001.0003. [DOI] [PubMed] [Google Scholar]
- 18.Seewald S, Akaraviputh T, Seitz U, et al. Circumferential EMR and complete removal of Barrett's epithelium: a new approach to management of Barrett's esophagus containing high-grade intraepithelial neoplasia and intramucosal carcinoma. Gastrointest Endosc. 2003;57:854–859. doi: 10.1016/s0016-5107(03)70020-0. [DOI] [PubMed] [Google Scholar]
- 19.Giovannini M, Bories E, Pesenti C, et al. Circumferential endoscopic mucosal resection in Barrett's esophagus with high-grade intraepithelial neoplasia or mucosal cancer. Preliminary results in 21 patients. Endoscopy. 2004;36:782–787. doi: 10.1055/s-2004-825813. [DOI] [PubMed] [Google Scholar]
- 20.Buskens CJ, Westerterp M, Lagarde SM, Bergman JJ, ten Kate FJ, van Lanschot JJ. Prediction of appropriateness of local endoscopic treatment for high-grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc. 2004;60:703–710. doi: 10.1016/s0016-5107(04)02017-6. [DOI] [PubMed] [Google Scholar]
- 21.Nijhawan PK, Wang KK. Endoscopic mucosal resection for lesions with endoscopic features suggestive of malignancy and high-grade dysplasia within Barrett's esophagus. Gastrointest Endosc. 2000;52:328–332. doi: 10.1067/mge.2000.105777. [DOI] [PubMed] [Google Scholar]
- 22.Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett's cancer) Gastrointest Endosc. 2007;65:3–10. doi: 10.1016/j.gie.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 23.Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1–11. doi: 10.1007/s10120-006-0408-1. [DOI] [PubMed] [Google Scholar]
- 24.Amano Y, Ishihara S, Amano K, et al. An assessment of local curability of endoscopic surgery in early gastric cancer without satisfaction of current therapeutic indications. Endoscopy. 1998;30:548–552. doi: 10.1055/s-2007-1001342. [DOI] [PubMed] [Google Scholar]
- 25.Gotoda T, Yanagisawa A, Sasako M, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225. doi: 10.1007/pl00011720. [DOI] [PubMed] [Google Scholar]
- 26.Makuuchi H, Kise Y, Shimada H, Chino O, Tanaka H. Endoscopic mucosal resection for early gastric cancer. Semin. Surg Oncol. 1999;17:108–116. doi: 10.1002/(sici)1098-2388(199909)17:2<108::aid-ssu5>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Gotoda T, Kondo H, Ono H, et al. A new endoscopic mucosal resection procedure using an insulation-tipped elecctrosurgical knife for rectal flat lesions. Gastrointest Endosc. 1999;50:560–563. doi: 10.1016/s0016-5107(99)70084-2. [DOI] [PubMed] [Google Scholar]
- 28.Neuhaus H, Costamagna G, Deviere J, Fockens P, Ponchon T, Rosch T ARCADE Group. Endoscopic submucosal dissection (ESD) of early neoplastic gastric lesions using a new double-channel endoscope (the "R-scope") Endoscopy. 2006;38:1016–1023. doi: 10.1055/s-2006-944830. [DOI] [PubMed] [Google Scholar]
- 29.Lee SY, Jang KT, Lee KT, et al. Can endoscopic resection be applied for early stage ampulla of Vater cancer? Gastrointest Endosc. 2006;63:783–788. doi: 10.1016/j.gie.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 30.Ahmad NA, Kochman ML, Long WB, Furth EE, Ginsberg GG. Efficacy, safety, and clinical outcomes of endoscopic mucosal resection: a study of 101 cases. Gastrointest Endosc. 2002;55:390–396. doi: 10.1067/mge.2002.121881. [DOI] [PubMed] [Google Scholar]
- 31.Wehrmann T, Martchenko K, Nakamura M, Riphaus A, Stergiou N. Endoscopic resection of submucosal esophageal tumors: a prospective case series. Endoscopy. 2004;36:802–807. doi: 10.1055/s-2004-825814. [DOI] [PubMed] [Google Scholar]
- 32.Rosch T, Sarbia M, Schumacher B, et al. Attempted endoscopic en bloc resection of mucosal and submucosal tumors using insulated-tip knives: a pilot series. Endoscopy. 2004;36:788–801. doi: 10.1055/s-2004-825838. [DOI] [PubMed] [Google Scholar]
- 33.Waxman I, Saitoh Y. Clinical outcome of endoscopic mucosal resection for superficial GI lesions and the role of high-frequency US probe sonography in an American population. Gastrointest Endosc. 2000;52:322–327. doi: 10.1067/mge.2000.105723. [DOI] [PubMed] [Google Scholar]
- 34.Saito Y, Uraoka T, Matsuda T, et al. Endoscopic treatment of large superficial colorectal tumors: a case series of 200 endoscopic submucosal dissections (with video) Gastrointest Endosc. 2007 doi: 10.1016/j.gie.2007.02.053. in press. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto S, Watanabe M, Hasegawa H, et al. The risk of lymph node metastasis in T1 colorectal carcinoma. Hepatogastroenterology. 2004;51:998–1000. [PubMed] [Google Scholar]
- 36.Saito Y, Fujii T, Kondo H, et al. Endoscopic treatment for laterally spreading tumors in the colon. Endoscopy. 2001;33:682–686. doi: 10.1055/s-2001-16213. [DOI] [PubMed] [Google Scholar]
- 37.Uraoka T, Saito Y, Matsuda T, et al. Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut. 2006;55:1592–1597. doi: 10.1136/gut.2005.087452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bergmann U, Beger HG. Endoscopic mucosal resection for advanced non-polypoid colorectal adenoma and early stage carcinoma. Surg Endosc. 2003;17:475–479. doi: 10.1007/s00464-002-8931-6. [DOI] [PubMed] [Google Scholar]
- 39.Zlatanic J, Waye JD, Kim PS, Baiocco PJ, Gleim GW. Large sessile colonic adenomas: use of argon plasma coagulator to supplement piecemeal snare polypectomy. Gastrointest Endosc. 1999;49:731–735. doi: 10.1016/s0016-5107(99)70291-9. [DOI] [PubMed] [Google Scholar]
- 40.Brooker JC, Saunders BP, Shah SG, Thapar CJ, Suzuki N, Williams CB. Treatment with argon plasma coagulation reduces recurrence after piecemeal resection of large sessile colonic polyps: a randomized trial and recommendations. Gastrointest Endosc. 2002;55:371–375. doi: 10.1067/mge.2002.121597. [DOI] [PubMed] [Google Scholar]
- 41.Regula J, Wronska E, Polkowski M, et al. Argon plasma coagulation after piecemeal polypectomy of sessile colorectal adenomas: long-term follow-up study. Endoscopy. 2003;35:212–218. doi: 10.1055/s-2003-37254. [DOI] [PubMed] [Google Scholar]
- 42.Conio M, Repici A, Demarquay JF, Blanchi S, Dumas R, Filiberti R. EMR of large sessile colorectal polyps. Gastrointest Endosc. 2004;60:234–241. doi: 10.1016/s0016-5107(04)01567-6. [DOI] [PubMed] [Google Scholar]
- 43.Jameel JK, Pillinger SH, Moncur P, Tsai HH, Duthie GS. Endoscopic mucosal resection (EMR) in the management of large colorectal polyps. Colorectal Dis. 2006;8:497–500. doi: 10.1111/j.1463-1318.2006.00966.x. [DOI] [PubMed] [Google Scholar]
- 44.Repici A, Conio M, De Angelis C, et al. Insulated-tip knife endoscopic mucosal resection of large colorectal polyps unsuitable for standard polypectomy. Am J Gastroenterol. 2007 doi: 10.1111/j.1572-0241.2007.01198.x. in press. [DOI] [PubMed] [Google Scholar]
- 45.Kaneko E, Hanada H, Kasugai T, Ogoshi K, Niwa K. The survey of gastrointestinal endoscopic complications in Japan. Gastroenterol Endosc (In Japanese) 2000;42:308–313. [Google Scholar]
- 46.Lee SY, Kim JJ, Lee JH, et al. Healing rate of EMR-induced ulcer in relation to the duration of treatment with omeprazole. Gastrointest Endosc. 2004;60:213–217. doi: 10.1016/s0016-5107(04)01683-9. [DOI] [PubMed] [Google Scholar]
- 47.Kakushima N, Yahagi N, Fujishiro M, et al. The healing process of gastric artificial ulcers after endoscopic submucosal dissection. Dig Endosc. 2004;16:327–331. [Google Scholar]
- 48.Oda I, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection for early gastric cancer: Technical feasibility, operation time and complications from large consecutive cases. Dig Endosc. 2005;17:54–58. [Google Scholar]
- 49.Shiba M, Higuchi K, Kadouchi K, et al. Risk factors for bleeding after endoscopic mucosal resection. World J Gastroenterol. 2005;14:7335–7339. doi: 10.3748/wjg.v11.i46.7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Minami S, Gotoda T, Ono H, Oda I, Hamanaka H. Complete endoscopic closure using endoclips for gastric perforation during endoscopic resection for early gastric cancer can avoid emergent surgery. Gastrointest Endosc. 2006;63:596–601. doi: 10.1016/j.gie.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 51.Fujishiro M, Yahagi N, Nakamura M, et al. Successful outcomes of a novel endoscopic treatment for GI tumors: endoscopic submucosal dissection with a mixture of high-molecular-weight hyaluronic acid, glycerin, and sugar. Gastrointest Endosc. 2006;63:243–249. doi: 10.1016/j.gie.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Fujishiro M, Yahagi N, Kashimura K, et al. Tissue damage of different submucosal injection solutions for EMR. Gastrointest Endosc. 2005;62:933–942. doi: 10.1016/j.gie.2005.07.052. [DOI] [PubMed] [Google Scholar]