Abstract
Both genetic and epigenetic events have been implicated in the stepwise histological progression involving adenoma-carcinoma and hyperplastic polyp/serrated adenoma-carcinoma sequences in the development of colorectal cancer. Genetic changes have been observed at each step in the initiation and progression of polyps to adenocarcinomas. Epigenetic changes also occur at each step in the pathogenesis of colorectal cancers and include CpG island DNA hypermethylation in the promoter region of genes resulting in transcriptional silencing through associated changes in chromatin structure and effects on binding of transcription factors, and DNA global hypomethylation which leads to chromosomal instability. Recent studies on MLH1 and APC genes indicate that epigenetic and genetic changes cooperate to facilitate tumor initiation and progression. Since aberrant CGI DNA promoter hypermethylation can be detected not only in colorectal polyps and cancers, but also in sera and stool, hypermethylated genes may serve as molecular markers for early detection, risk assessment and diagnosis. In addition, silenced genes caused by CGI DNA promoter hypermethylation can be reactivated by demethylating agents and also by both the inhibitors of DNA methyltransferases and histone deacetylases. Therefore, these epigenetically acting drugs should be evaluated for their chemopreventive and therapeutic potential for colorectal cancers.
Keywords: Epigenetic changes, DNA methylation, DNA hypomethylation, CpG island, Colorectal polyps, Colorectal cancer
INTRODUCTION
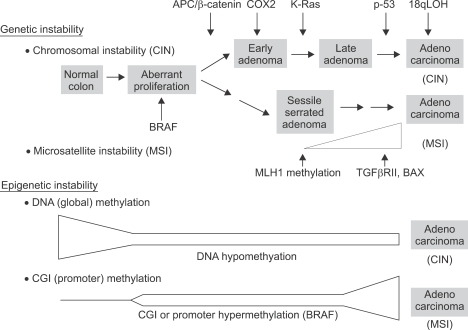
Colorectal cancer develops as a result of progressive accumulation of genetic and epigenetic alterations which leads to genetic instability resulting in malignant transformation.1,2 There are at least two major genetic instability pathways involved in colorectal carcinogenesis, chromosomal instability (CIN) and microsatellite instability (MSI) (Fig. 1).2,3 The chromosomal instability pathway is found in about 80% of colorectal cancers. This pathway involves chromosomal aberrations such as loss of heterozygosity of 5q, 17p, and 18q, with inactivation of Adenomatous polyposis coli (APC), p53, and (Deleted in colon cancer) DCC genes. MSI, a second form of genetic instability, is found in most cases of hereditary non-polyposis colorectal cancer (HNPCC) and in about 15% of sporadic colorectal cancers. This pathway involves inactivation of DNA mismatch repair genes followed by mutations in the microsatellite repeat sequences in the genes that are important in tumor progression and as TGF RII and BAX, which leads to uncontrolled cellular growth and decreased apoptosis. Chromosomal aberrations are rare in microsatellite unstable tumors.
Fig. 1.
Overall scheme of key genetic and epigenetic events in colorectal tumorigenesis. Both genetic and epigenetic changes play important roles in colorectal tumorigenesis. Genetic changes may be broadly classified into two categories; chromosomal instability (CIN) and microsatellite instability (MSI). Although about 80% of tumors exhibit chromosomal instability, stability of microsatellite DNA and involves chromosomal aberrations such as loss of heterozygosity. The typical genetic events associated with tumors in this pathway are shown. MSI is found in most cases of hereditary non-polyposis colorectal cancer and in 15% of sporadic tumors. This pathway involves inactivation of DNA mismatch repair genes caused by promoter hypermethylation of hMLH1 followed by mutations in the microsatellite sequences of the genes important in tumor progression such as TGFβ RII and BAX. MSI is a late event in colorectal tumorigenesis and chromosomal aberrations are rare in MSI tumors. There are two major epigenetic aberrations that occur during colorectal tumorigenesis, global DNA hypomethylation and region specific or promoter hypermethylation of CpG islands (CGI). Global DNA hypomethylation occurs early in colorectal tumorigenesis (aberrant crypt foci/hyperplastic polyps) and remains at constant levels during tumor progression. It is frequently associated with chromosomal instability. CGI promoter hypermethylation also occurs early in colorectal tumorigenesis, but the propensity of methylation increases with tumor progression. In addition, different frequencies and patterns of promoter methylation of specific genes are observed during tumor initiation and progression. CGI promoter hypermethylation is frequently associated with BRAF mutation and MSI.
Recently, additional pathways involving epigenetic alterations have been described (Fig. 1).4,5-7 Epigenetic alterations lead to altered gene expression that are not caused by changes in the primary DNA sequence. Both global DNA hypomethylation and region specific or promoter hypermethylation of CpG islands in specific regions of gene promoters are common epigenetic events which takes place in the mammalian genome leading to oncogenesis.7 Hypomethylation and hypermethylation of DNA are relative terms and indicate less or more methylation than in normal tissue. Recent studies indicate that promoter hypermethylation leads to transcriptional silencing of the genes involved in tumor suppression, cell cycle control, DNA repair, apoptosis and invasion, whereas global DNA hypomethylation can lead to chromosomal instability.4,8-11 These genetic and epigenetic alterations work in concert and are key pathogenic events in initiation and progression of colorectal polyps to invasive cancer. Furthermore, DNA methylation patterns show promise as useful biomarkers of tumor type, risk assessment, prognosis, and also as targets for prevention and therapy of colorectal cancer.
DNA PROMOTER HYPERMETHYLATION IN COLORECTAL CANCER
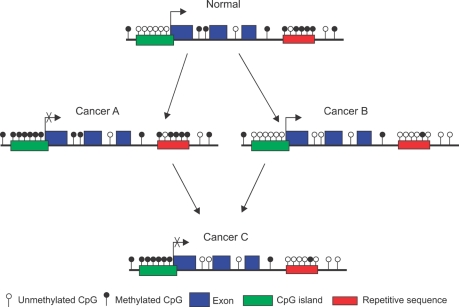
DNA methylation refers to the methylation of cytosine residues (5-methylcytosine) that precede a guanine in the DNA sequence (CpG dinucleotide) throughout the genome and is maintained in relatively stable patterns that are established during development. In the majority of the genome, about 70% of CpG dinucleotides are heavily methylated.8 The CpG dinucleotide is underrepresented in the human genome and account for about 1% of genome, presumably due to the propensity of methylated cytosine to mutate to thymine. However, in half of all genes, these dinucleotides can be clustered in small stretches of DNA (0.3-2 Kb) called CpG islands (CGI) often in the 5' promoter regions where gene transcription begins.5,12-14 The methylation of CpG sites in the human genome is maintained by a number of DNA methyltransferases (DNMTs).8,15 The CGIs are normally unmethylated, but many of CGIs become methylated in cancer (Fig. 2). The mechanisms involved in aberrant regulation of DNA methylation in cancers are not well understood, although an altered level of DNMTs have been implicated.16,17 The aberrantly methylated CGIs in the gene promoters cause transcriptional repression of genes involved in the initiation and progression of cancer.18
Fig. 2.
Common DNA methylation changes observed in cancer. In normal tissues, the majority of CpG islands in the promoters of tumor suppressor genes and genes regulating cell cycle and apoptosis are methylation-free and are expressed normally. However, repetitive sequences and interspersed CpG dinucleotides are heavily methylated. The genome of cancer cells is characterized by regional or promoter hypermethylation of CpG islands and/or reduction in the number of methylated CpG dinucleotides in the repetitive sequences and interspersed regions, so-called global DNA hypomethylation. In cancer cells, 3 types of combination of these two events may occur. Cancer A with promoter hypermethylation and global DNA methylation, Cancer B with unmethylated promoters with global DNA hypomethylation and Cancer C with promoter hypermethylation and global DNA hypomethylation. Cancer A and cancer B occur more frequently than cancer C.
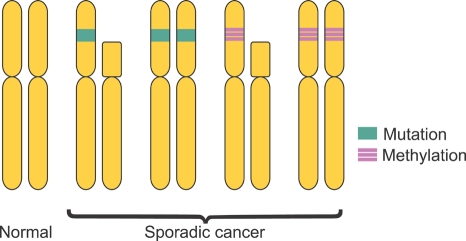
According to Knudson's Two-Hit hypothesis, the disruption of function of gene requires a complete loss of function of both copies of the gene (Fig. 3).19 In sporadic cancers, the first hit is usually a somatic mutation within the coding region of one copy of the gene. The second hit usually involves somatic loss of the chromosomal region containing the other copy of the gene, or loss of heterozygosity. Aberrant promoter methylation and associated silencing of gene transcription can constitute the first hit in sporadic cancers and chromosomal deletions in the second gene copy may be the second hit. Promoter hypermethylation of both copies of a tumor suppressor gene may also occur, resulting in disruption of gene function.
Fig. 3.
Genetic and epigenetic changes that inactivate tumor suppressor genes according to Knudson's two-hit hypothesis. When caused only by genetic changes, the first hit may be somatic mutation within the coding region of one copy of the gene. The second hit generally involves loss of the chromosomal region of the other copy of the gene or loss of heterozygosity. The loss of both alleles of a tumor suppressor gene leads to a series of events that causes malignant transformation. Aberrant promoter hypermethylation can have the same effect as a coding region mutation in one copy of the gene (the first hit) and is frequently associated with the loss of other copy of the gene by either somatic loss of the chromosomal region or by aberrant promoter hypermethylation of the other copy (biallelic methylation) which represents the second hit.
IS DNA (PROMOTER) HYPERMETHYLATION A CAUSE OR AN EFFECT OF GENETIC EVENTS?
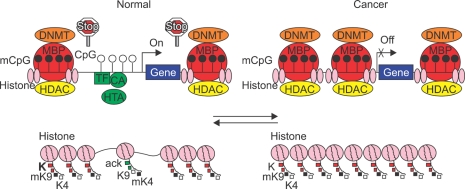
Whether DNA hypermethylation is a cause or an effect of genetic events involved in the pathogenesis of colorectal cancer is still controversial, but recent studies suggest important roles for DNA hypermethylation in tumor initiation and progression. Once DNA methylation is established in the promoter (de novo methylation), it tends to spread towards the transcription start site, but normally specific mechanisms appear to exist to block this spreading of methylation.20,21 However, in neoplasia, this barrier is removed and spreading occurs (Fig. 4).21-24 The observation that promoter methylation of MLH1, a DNA repair enzyme, and loss of MLH1 protein expression occurs frequently in sporadic colorectal cancers with MSI indicated that DNA hypermethylation has potential pathogenic consequences in sporadic colorectal cancer.25-28 Since treatment with DNA demethylating agent such as 5-aza-2'-deoxycytidine frequently restores MLH1 gene expression in cancer cells concomitant with the appearance of unmethylated MLH1 promoter, DNA methylation appears to play a dominant role in silencing of the gene.26-28 As inactivation of MLH1 resulting from promoter methylation leads to progression of carcinogenetic process of MSI colorectal cancers through an increased mutation rate in the repetitive sequences of the genes involved in tumor progression such as TGF-β RII and BAX, DNA methylation appears to be a cause rather than a consequence of colorectal carcinogenesis (Fig. 1). Detailed analysis of the methylation status of specific CpG sites in the MLH1 promoter indicated that the methylation status of a small cluster of CpGs in a specific region in the proximal region of the MLH1 promoter (C region) regulate the gene transcription.28 In sporadic colorectal cancers with MSI and methylated MLH1, BRAF mutation was observed, but not in hereditary nonpolyposis colorectal cancer.29,30
Fig. 4.
CpG island methylation status in the promoter and the related chromatin structures in transcriptional activation and silencing. In normal cells, the CpG sites adjacent to transcription start site in the promoter of the genes are frequently unmethylated. The transcriptional machinery is activated by the binding of transcriptional factors (TFs), co-acting factors (CAs) and histone acetyltransferases (HATs) in this region. Thus, the gene promoter shown on the upper left is transcriptionally active. Upstream and downstream of this region, CpG islands are methylated by DNA methyltransferases (DNMTs). In these regions methylcytosine-binding proteins (MBPs) that bind to methylated CpG sites recruit histone deacetylases (HDACs) and histone methyltransferases to form a complex. Stop signs (barriers) indicate the mechanisms involved in preventing the spreading of CpG island methylation to unmethylated transcriptionally active region of the promoter. Left bottom shows the related chromatin structure around the transcriptionally active, unmethylated promoter occupied by nucleosomes composed of histone complexes. The lysine residues in the tails of histone H3 are acetylated (acK). Lysine 4 is methylated (mK4) and lysine 9 is unmethylated (K9). These changes contribute to open and relaxed conformation of the chromatin allowing key components of the gene transcription apparatus accessible to this region of the promoter. In the regions, upstream and downstream of transcriptionally active promoter regions, the lysine residues are deacetylated (K), and methylated (mK9) respectively and the chromatin structures have closed and dense conformation. In cancer cells depicted in the upper right, the barriers for the spreading of CpG island methylation are removed. Methylation spreads toward the promoter region near the transcription start site resulting in transcriptional silencing. Thus, the gene promoter shown on the upper right is transcriptionally inactive. These events result in closed and dense chromatin conformation making it difficult for the key components of gene transcription apparatus to bind to the promoter contributing further to transcriptional repression.
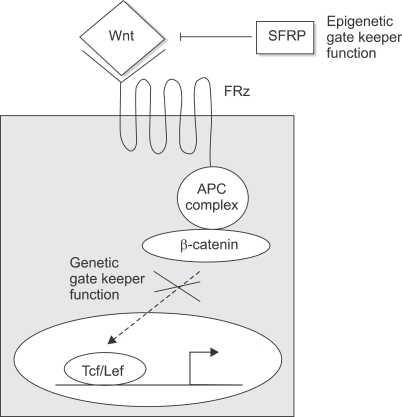
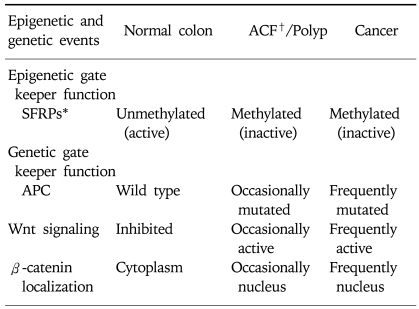
Loss of APC function with activation of Wnt pathway is observed in most familial adenomatosis polyposis-associated and sporadic colorectal cancer.31,32 While inactivation of APC gene is caused frequently by DNA mutations, hypermethylation of CpG sites in the promoter region of APC gene has been reported in 18% of colorectal cancer.33 Recently, Deng et al analyzed the methylation status of APC promoter in colorectal cancer cell lines with different APC expression status and identified two small regions of clusters of CpGs in the 5' region of APC promoter and have shown that methylation status of CpGs in these regions dictate the transcriptional state of the gene.34 Thus, abnormal activation of Wnt pathway resulting in the expansion of stem cell and progenitor-cell populations can be caused by either mutation or promoter hypermethylation of APC genes or mutation of β-catenin. Recently, abnormal methylation of promoter regions of genes encoding secreted frizzled related proteins (SFRPs) has been reported in colorectal neoplasms.9,35 In normal colon epithelial cells, SFRPs function as antagonists of Wnt signaling by competing with Wnt proteins for binding to their receptors frizzled (FRZ) on the cell surface (Fig. 5).36,37 Thus, SFRPs serve as constitutive inhibitors of Wnt signaling in normal colon epithelial cells. When SFRP expression is lost through promoter methylation of SFRP genes, Wnt signaling becomes activated through the receptor FRZ resulting in the expansion of colon epithelial stem cells and progenitor cells. Persistent activation of the Wnt pathway by methylated SFRP genes leads to mutations in the downstream components of the pathway such as APC (Table 1).38 From these studies it appears that epigenetic and genetic changes cooperate to facilitate tumor initiation and progression. Many additional genes have been found to have hypermethylated promoters with the silencing of genes. This includes 6OMGMT, p16INK4a, HIC1, and RASSF1.5,9,14
Fig. 5.
Wnt signaling pathway is regulated by SFRPs, epigenetic gatekeepers and APC; a genetic gatekeeper. In normal colon epithelial cells, secreted frizzled related proteins (SFRPs) inhibit Wnt signaling by competing with Wnt ligand for binding to their receptor, Frizzled (FRZ). In cancer cells, however, methylation of CpG islands of the promoters of SFRPs inhibits their transcription causing the Wnt signaling pathway to become active. Thus, SFRPs function as an epigenetic gatekeeper. When Wnt signaling is inactive in normal colon epithelial cells, the APC complex phosphorylates β-catenin leading to its degradation preventing the nuclear accumulation of β-catenin and its binding to Lef/Tcf HMG box transcription factors. These events result in the differentiation and homeostasis of colonic epithelial cells. In cancer cells, mutated APC allow unphosphorylated β-catenin to translocate to nucleus and activate transcription of genes that promote cell proliferation and survival. Therefore, APC expression functions as a genetic gatekeeper.
Table 1.
Epigenetic and Genetic Regulation of Wnt Signaling Pathway in Normal and Neoplastic Colon
*SFRPs, secreted frizzled related proteins.
†ACF, aberrant crypt foci.
MECHANISMS INVOLVED IN DNA PROMOTER HYPERMETHYLATION INDUCED GENE SILENCING
At least two mechanisms have been suggested to be involved in the inactivation of gene transcription by DNA methylation. DNA methylation may inhibit gene transcription by inhibiting direct interaction between methylated promoters and transcription factors such as AP2, CREB, E2F, and NF-κB and c-MYC.5,39 DNA methylation may also mediate transcriptional silencing by recruiting methylated DNA binding proteins such as MeCP2, MBD1, and MBD25,40,41 that recognize methylated DNA and recruit histone deacetylases (HDACs) and histone methyltransferases to gene promoters. The HDACs induce deacetylation of normally acetylated histone leading to the formation of a closed chromatin structure that prevents the binding of transcriptional factors to the promoter. Histones are the target of several post translational modifications such as methylation, acetylation, and phosphorylation.8,42,43 Acetylated histones are associated with open or relaxed transcriptionally active chromatin regions, whereas hypoacetylated histones are associated with transcriptionally silent regions characterized by closed or dense chromatin structures (Fig. 4).8 The effect of a specific series of post translational modifications of histones on gene expression is termed the "histone code" and is thought to play an important role in DNA methylation and gene transcription.44
CpG ISLAND METHYLATOR PHENOTYPE (CIMP)
CGIs of multiple gene promoters are methylated in some cancers. It has also been observed that many gene promoters are methylated in normal colonic mucosa as a function of age and undergo more extensive methylation in cancer. These changes are termed age-related methylation.4,45 However, promoter methylation of several genes has been found to be limited to colorectal cancers. This category of methylated genes includes tumor suppressor genes (p16, p14, THBS1), and DNA repair enzymes (MLH1 and MGMT).4,5,15 When a large number of colorectal cancers were examined, some were found to accumulate high rates of aberrant DNA promoter methylation in multiple genes and this subset of cancers were classified as having the CpG island methylator phenotype (CIMP) (20-30%).15,46,47 Subsequent studies indicated that CIMP-H (high) colorectal cancers have distinct clinicopathological features including older age, proximal location in the colon, poor differentiation, mucinous histology, high rates of BRAF mutations, and low rates of p53 mutations. In addition, CIMP positive cancers were frequently associated with MSI resulting from MLH1 promoter methylation.5,15,47 When mucinous colorectal cancers were compared with non-mucinous cancers, more frequent occurrences of CIMP-H (38% vs. 18%), MSI (27% vs. 12%) and BRAF mutation (46% vs. 16%) were observed indicating that pathogenesis of mucinous colorectal cancer may involve distinct genetic and epigenetic changes.48,50 Genetically, CIMP-H colorectal cancers can be broadly divided into two groups: one with MSI and BRAF mutation and another with MSS and K-RAS mutations.48 A high frequency of K-RAS mutation may in part be explained by the methylation of a DNA repair enzyme, MGMT, resulting in increased frequency of G to A mutations.51 However, the concept of CIMP has been disputed by some investigators. One group proposed that all methylation events in colorectal cancers were related to aging rather than neoplastic process.52 Other groups reported that although they observed a significant association between promoter methylation of multiple genes and the proximal location of the tumors, the significance disappeared when tumors with MLH1 methylation or with MSI were excluded from the analysis.53,54
In order for the significance of CIMP to be fully evaluated it will be necessary to reach a consensus on better definition of a panel of marker genes, criteria for CIMP, standardization of methodologies to analyze methylation status quantitatively and better delineation of region specific promoter methylation profiles of multiple genes (proximal vs. distal).
DNA PROMOTER HYPERMETHYLATION IN COLORECTAL POLYPS
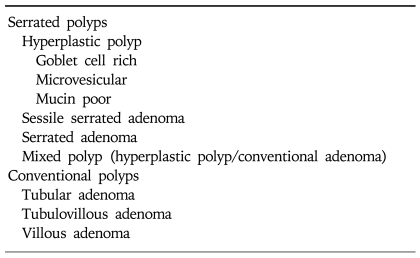
Colorectal cancers develop as a result of the transformation of normal colonic mucosal epithelium to cancer through a series of precursor lesions with genetic and epigenetic changes termed the adenoma to carcinoma sequence and the hyperplastic polyp-serrated adenoma to carcinoma sequence.55-57 In addition to genetic changes such as APC and KRAS mutations, aberrant CGI methylation of tumor suppressor genes occurs in these precursor lesions. Aberrant CGI methylation has been found in aberrant crypt foci (ACF), the earliest precursor lesions and in adenomas.58-60 In adenomas aberrant CGI methylation of genes was observed less frequently than in adenocarcinoma but increased during the progression of early adenomas to advanced adenomas (Fig. 1).58 The frequency of specific gene methylation at the different steps of the adenoma-carcinoma progression sequence varies in a gene specific fashion. Three genes (MGMT, p16INK4a and HLTF) showed the largest increase between the early and advanced adenomas whereas the proportion of tumors with methylated MLH1 and TIMP3 was highest in adenocarcinoma. Thus, the inactivation of tumor suppressor genes caused by aberrant DNA methylation appears to affect tumor initiation or progression process depending on the genes. Recently, colorectal polyps have been classified into two major groups, serrated polyps and conventional adenomas (Table 2).61,62 In serrated polyps that include hyperplastic polyps, sessile serrated adenomas, sessile adenomas and mixed polyps, higher frequency of CGI methylation and BRAF mutations were observed compared with tubular adenomas.63-66 BRAF mutations occur rarely in tubular adenomas. Most hyperplastic polyps are diminutive lesions with a negligible risk of developing into cancer whereas sessile serrated adenomas, serrated adenomas and mixed polyps may progress to cancer. Recent prospective study indicated that the prevalence of sessile serrated adenomas is about 9% in patients undergoing colonoscopy compared with the prevalence for sessile adenomas (0.7%) and mixed polyps (1.7%).61 Sessile serrated adenomas were associated with BRAF mutations, proximal location, female gender and presence of multiple polyps. In other studies, serrated adenomas were also frequently associated with CIMP and MSI-H cancers and tended to show near diploid DNA indices, more frequent allelic imbalance at 18q and less frequent allelic imbalance at 5q or KRAS mutations.67,68 There are also differences in the proportions of specific methylated genes between serrated polyps and tubular adenomas.59,64 These studies support the hypothesis that alternative genetic and epigenetic events may be involved in hyperplastic polyp-serrated adenoma to carcinoma sequence.
Table 2.
Histological Classification of Colorectal Polyps
DNA GLOBAL HYPOMETHYLATION IN COLORECTAL CANCER
Another major epigenetic change that occurs in cancer is DNA hypomethylation. Over 40% of human DNA consists of frequent repetitive elements such as short interspersed transposable element (SINE or Alu elements) or long interspersed transposable element (LINE). These repetitive elements are normally methylated but become hypomethylated with aging and in cancer. This is termed genome wide- or global hypomethylation or DNA hypomethylation (Fig. 2).69 Two decades ago, Feinberg and Vogelstein and their coworkers reported that DNA hypomethylation was a common epigenetic event in colorectal cancers and polyps compared with normal colonic mucosa.70 Recent studies showing that global DNA methylation levels are lower (1/4th) in benign or malignant colonic neoplasms compared with normal colonic mucosa support the earlier observation. Furthermore, no difference in the level of hypomethylation was observed between benign (hyperplastic polyp, small and large adenomas) and malignant lesions of the colon.71 However, the biological significance of DNA hypomethylation as well as its relationship with promoter methylation in colorectal cancer remains unclear. Recently, in Apc Min/+ mouse model, DNA hypomethylation was shown to promote early stage tumor formation but strongly suppresses overall tumorigenesis in the intestine.72 These results suggest that DNA hypomethylation can promote early events in colorectal tumorigenesis while blocking progression. Studies on cell lines indicate that DNA hypomethylation of repetitive sequences may predispose cells to chromosomal defects and rearrangements that result in genetic instability.73 Recently, Deng et al studied promoter hypermethylation and DNA hypomethylation in colorectal cancer tissues and cell lines and observed that most cancers showed only one kind of epigenetic alterations (i.e. either promoter hypermethylation or DNA hypomethylation), indicating that these are independent and alternative events in most cases.74 They also observed strong correlation between promoter hypermethylation and microsatellite instability and between DNA hypomethylation and chromosomal instability (Fig. 1). Furthermore, cancer cells with chromosomal instability showed open chromatin conformation and enriched histone acetylation (capable of inducing active gene transcription), whereas cells with MSI had closed chromatin conformation and low level of histone acetylation (causing silencing of gene transcription). From these data they hypothesized that in colorectal cancer, promoter hypermethylation and DNA hypomethylation are associated with altered chromatin conformation and histone acetylation causing MSI and chromosomal instability respectively.
CLINICAL IMPLICATION OF THE EPIGENETIC CHANGES IN COLORECTAL CANCER
Aberrantly methylated genes may serve as effective molecular markers for early detection, risk assessment and diagnosis as well as molecular targets for prevention and therapy of colorectal neoplasia. Aberrant CGI methylation occurs early during colorectal carcinogenesis with increase in frequency and extent during tumor progression. Recently, aberrantly methylated CGI of three genes, p16INK4a, MGMT and MLH1 were detected in fecal DNA from 31%, 48% and 0% of patients with adenomas and in 16%, 27% and 10% of individuals with no detectable polyps.58 Aberrant CGI methylation in noncancerous colonic epithelium have been previously detected in older people (age-related CGI methylation) and in patients with ulcerative colitis.45,75,76 Therefore, identification of tumor specific pattern of CGI methylation of multiple genes distinct from age and inflammation related pattern of CGI methylation may provide more specific molecular markers for early detection and risk assessment of colorectal cancer. Methylated MLH1 DNA in the serum of individuals with colorectal cancers with methylated DNA has been detected in 30% of cases with 100% specificity.77 Thus, the potential utility of aberrantly methylated genes as markers in early detection, risk assessment and the diagnosis of colorectal cancer appear promising and warrant further investigation. A major difference between mutations and epigenetic gene silencing caused by aberrant CGI methylation is the irreversibility of the former and potential reversibility of the latter. The potential reversibility of epigenetic changes in colorectal neoplasms provides the possibility of making the cancer cells behave more like normal cells by inducing differentiation and apoptosis. In a mouse model of multiple intestinal neoplasia, polyp formation was suppressed by decreasing DNA promoter methylation by both genetic and pharmacological methods. At least three approaches are available to demethylate the aberrantly methylated genes in neoplasm. These are demethylating agents such as 5-azacytidine and 5-aza-2'-deoxycytidine, inhibitors of histone deacetylase such as trichostatin and sodium or phenyl butyrate and inhibitors of DNMTs (DNA methyltransferases).80,82 5-aza-2'-deoxycytidine was found to have some therapeutic effects in hematopoietic neoplasms but its efficacy in solid tumors has not yet been demonstrated. Inhibition of histone deacetylase (HDAC), an important component of DNA methylation associated gene silencing was shown not to reactivate genes in cancer cells when used alone but has additive or synergistic effect when some demethylation is first achieved by low doses of 5-aza-2'-deoxycytidine.80 However, recently HDAC inhibitors have been reported to show significant activity against a variety of hematological and solid tumors, both in monotherapy as well as in combination therapy with other drugs.81 In addition, DNA methylating enzymes, DNMT1 and DNMT3b, had to be simultaneously disrupted to reverse epigenetic gene silencing in cancer cells.82 These approaches are currently being investigated in the development of epigenetic therapy and chemoprevention for solid tumors.81,83
SUMMARY
Colorectal cancers arise from precursor lesions that progress to adenocarcinomas through a step wise histological progression involving adenoma-carcinoma and hyperplastic polyp/serrated adenoma-carcinoma sequences. Pathological changes observed in polyps to adenocarcinomas sequences are characterized by specific genetic alterations that occur at each step in the initiation and progression of polyps to adenocarcinomas. Recently, epigenetic changes have also been shown to occur in colorectal polyps and adenocarcinomas. Epigenetic changes include CGI DNA promoter hypermethylation and DNA global hypomehylation. Aberrant CGI DNA methylation affects the promoter region of genes involved in DNA repair, cell cycle control, tumor suppression and apoptosis. Furthermore, it induces transcriptional silencing of the genes by inhibiting the binding of transcriptional factors to the promoters and also by changes in chromatin structure resulting from histone modifications. Recent studies on MLH1 and APC indicate that epigenetic and genetic changes cooperate to facilitate tumor initiation and progression. The repetitive elements in human DNA are normally methylated but become hypomethylated in colorectal neoplasms. Recent studies indicate that aberrant CGI DNA promoter methylation and DNA global hypomethylation in colorectal cancers are independent events in most cases and are frequently associated with MSI and chromosomal instability respectively. Since aberrant CGI DNA promoter methylation can be readily detected not only in neoplastic tissues but also in the sera and stools, hypermethylated genes may serve as molecular markers for early detection, risk assessment and diagnosis. Furthermore, silenced genes caused by CGI DNA hypermethylation can be reactivated by treatment with demethylating agents and inhibitors of DNA methyltransferases and histone deacetylases. Therefore, these epigenetically acting drugs should be evaluated for their chemopreventive and/or therapeutic potential for colorectal cancers.
ACKNOWLEDGEMENTS
We would like to thank Drs. James R. Gum, Jr. and Sanjay Kakar for helpful discussion and suggestions and Ms. Rita Burns and Lisa Cun for the preparation of this manuscript. This work was supported by the Department of Veterans Affairs Medical Research Service, the Oberkotter Foundation Grant and the Theodora Betz Foundation grant.
References
- 1.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 2.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 3.Chung DC. The genetic basis of colorectal cancer: insights into critical pathways of tumorigenesis. Gastroenterology. 2000;119:854–865. doi: 10.1053/gast.2000.16507. [DOI] [PubMed] [Google Scholar]
- 4.Rashid A, Issa JP. CpG island methylation in gastroenterologic neoplasia: a maturing field. Gastroenterology. 2004;127:1578–1588. doi: 10.1053/j.gastro.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Kondo Y, Issa JP. Epigenetic changes in colorectal cancer. Cancer and Metastasis. 2004;23:29–39. doi: 10.1023/a:1025806911782. [DOI] [PubMed] [Google Scholar]
- 6.Grady WM. Epigenetic events in the colorectum and in colon cancer. Biochem Soc Trans. 2005;33:684–688. doi: 10.1042/BST0330684. [DOI] [PubMed] [Google Scholar]
- 7.Callinan PA, Feinberg AP. Emerging science of epigenomics. Hum Mol Genet. 2006;15:95–101. doi: 10.1093/hmg/ddl095. [DOI] [PubMed] [Google Scholar]
- 8.Lengauer C. Cancer. An unstable liaison. Science. 2003;300:442–443. doi: 10.1126/science.1084468. [DOI] [PubMed] [Google Scholar]
- 9.Herman JG, Baylin SB. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med. 2003;349:2042–2054. doi: 10.1056/NEJMra023075. [DOI] [PubMed] [Google Scholar]
- 10.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer- a mechanism for early oncogenic pathway addiction. Nat Rev Cancer. 2006;6:107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 11.Ehrlich M. DNA methylation in cancer: too much, but also too little. Oncogene. 2002;21:5400–5413. doi: 10.1038/sj.onc.1205651. [DOI] [PubMed] [Google Scholar]
- 12.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4:143–152. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 13.Cross SH, Bird AP. CpG islands and genes. Curr Opin Genetic Dev. 1995;5:309–314. doi: 10.1016/0959-437x(95)80044-1. [DOI] [PubMed] [Google Scholar]
- 14.Bird AP. CpG-rich islands and the function of DNA methylation. Nature. 1986;321:209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- 15.Issa JP. CpG island methylator phenotype in cancer. Nat Rev Cancer. 2004;4:988–993. doi: 10.1038/nrc1507. [DOI] [PubMed] [Google Scholar]
- 16.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 17.Laird PW, Jackson-Grusby L, Faxeli A, et al. Suppression of intestinal neoplasia by DNA hypomethylation. Cell. 1995;81:197–205. doi: 10.1016/0092-8674(95)90329-1. [DOI] [PubMed] [Google Scholar]
- 18.Eads CA, Nickel AE, Laird PW. Complete genetic suppression of polyp formation and reduction of CpG island hypermethylation in Apc (Min/+) Dnmt1-hypomorphic mice. Cancer Res. 2002;62:1296–1299. [PubMed] [Google Scholar]
- 19.Knudson AG. Two genetic hits (more or less) to cancer. Nat Rev Cancer. 2001;1:157–162. doi: 10.1038/35101031. [DOI] [PubMed] [Google Scholar]
- 20.Turker MS. Gene silencing in mammalian cells and the spread of DNA methylation. Oncogene. 2002;21:5388–5392. doi: 10.1038/sj.onc.1205599. [DOI] [PubMed] [Google Scholar]
- 21.Song JZ, Stirzaker C, Harrison J, Melki JR, Clark SJ. Hypermethylation trigger of the glutathione-S-transferase gene (GSTP1) in prostate cancer cells. Oncogene. 2002;21:1048–1061. doi: 10.1038/sj.onc.1205153. [DOI] [PubMed] [Google Scholar]
- 22.Stirzaker C, Song JZ, Davidson B, Clark SF. Transcriptional gene silencing promotes DNA hypermethylation through a sequential change in chromatin modifications in cancer cells. Cancer Res. 2004;64:3871–3877. doi: 10.1158/0008-5472.CAN-03-3690. [DOI] [PubMed] [Google Scholar]
- 23.Velicescu M, Weisenberger DJ, gonzales FA, Tsai YC, Nguyen CT, Jones PA. Cell division is required for de novo methylation of CpG islands in bladder cancer cells. Cancer Res. 2002;62:2378–2384. [PubMed] [Google Scholar]
- 24.Ushijima T, Okichi-Takada E. Aberrant methylation in cancer cells: where do they come from? Cancer Sci. 2005;96:206–211. doi: 10.1111/j.1349-7006.2005.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kane MF, Loda M, Gaida GM, et al. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997;57:808–811. [PubMed] [Google Scholar]
- 26.Herman JG, Umar A, Polyak K, et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci USA. 1998;95:6870–6875. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veigl ML, Kasturi L, Olechnowicz J, et al. Biallelic inactivation of hMLH1 by epigenetic gene silencing, a novel mechanism causing human MSI cancers. Proc Natl Acad Sci USA. 1998;95:8698–8702. doi: 10.1073/pnas.95.15.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng G, Chan A, Hong J, Chae HS, Kim YS. Methylation of CpG in a small region of the hMLH1 promoter invariably correlates with the absence of gene expression. Cancer Res. 1999;59:2029–2033. [PubMed] [Google Scholar]
- 29.Li WQ, Kawakami K, Ruszkiewicz A, Bennett G, Moore J, Iacopetta B. BRAF mutations are associated with distinctive clinical, pathological and molecular features of colorectal cancer independently of microsatellite instability status. Mol Cancer. 2006;5:2. doi: 10.1186/1476-4598-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng G, Bell I, Crawley S, et al. BRAF mutation is frequently present in sporadic colorectal cancer with methylated hMLH1, but not in hereditary nonpolyposis colorectal cancer. Clin Cancer Res. 2004;10:191–195. doi: 10.1158/1078-0432.ccr-1118-3. [DOI] [PubMed] [Google Scholar]
- 31.Ilyas M, Staub J, Tomlinson IP, Bodmer WF. Genetic pathways in colorectal and other cancers. Eur J Cancer. 1999;35:1986–2002. doi: 10.1016/s0959-8049(99)00298-1. [DOI] [PubMed] [Google Scholar]
- 32.Powell SM, Zilz N, Beazer-Barclay Y, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–237. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 33.Esteller M, Corn PG, Baylin SB, Herman JG. A gene hypermethylation profile on human cancer. Cancer Res. 2001;61:3225–3229. [PubMed] [Google Scholar]
- 34.Deng G, Song G-A, Pong E, Sleisenger M, Kim YS. Promoter methylation inhibits APC gene expression by causing changes in chromatin conformation and interfering with the binding of transcription factor CCAAT-binding factor. Cancer Res. 2004;64:2692–2698. doi: 10.1158/0008-5472.can-03-3000. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki H, Watkins DN, Jair KW, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36:417–422. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 36.Finch PW, He X, Kelley MJ, et al. Purification and molecular cloning of a secreted, frizzled-related antagonist of Wnt action. Proc Natl Acad Sci USA. 1997;94:6770–6775. doi: 10.1073/pnas.94.13.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rattner A, Hsieh JC, Smallwood PM, et al. A family of secreted proteins contains homology to the cysteine-rich ligand-binding domain of frizzled receptors. Proc Natl Acad Sci USA. 1997;94:2859–2863. doi: 10.1073/pnas.94.7.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kinzler KW, Vogelstein B. Cancer susceptibility genes, gatekeepers and caretakers. Nature. 1997;386:761–763. doi: 10.1038/386761a0. [DOI] [PubMed] [Google Scholar]
- 39.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation - a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–196. [PubMed] [Google Scholar]
- 40.Jones PL, Veenstra GJ, Wade PA, et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- 41.Fateni M, Wade PA. MBD family proteins: reading the epigenetic code. J Cell Sci. 2006;119:3033–3037. doi: 10.1242/jcs.03099. [DOI] [PubMed] [Google Scholar]
- 42.Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 43.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 44.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 45.Ahuja N, Li Q, Mohan AL, Baylin SB, Issa JP. Aging and DNA methylation in colorectal mucosa and cancer. Cancer Res. 1998;58:5489–5494. [PubMed] [Google Scholar]
- 46.Toyota M, Ahuja N, Ohe-Toyota M, Herman JG, Baylin SB, Issa JP. CpG island methylator phenotype in colorectal cancer. Proc Natl Acad Sci USA. 1999;96:8681–8686. doi: 10.1073/pnas.96.15.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Issa JP, Shen L, Toyota M. CIMP, at last. Gastroenterology. 2005;129:1121–1124. doi: 10.1053/j.gastro.2005.07.040. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka H, Deng G, Matsuzaki K, et al. BRAF mutation, CpG island methylator phenotype and microsatellite instability occur more frequently and concordantly in mucinous than non-mucinous colorectal cancer. Int J Cancer. 2006;118:2765–2671. doi: 10.1002/ijc.21701. [DOI] [PubMed] [Google Scholar]
- 49.Song GA, Deng G, Bell I, Kakar S, Sleisenger MH, Kim YS. Mucinous carcinomas of the colorectum have distinct molecular genetic characteristics. Int J Oncol. 2005;26:745–750. [PubMed] [Google Scholar]
- 50.Park SY, Lee HS, Choe G, Chung JH, Kim WH. Clinicopathological characteristics, microsatellite instability, and expression of mucin core proteins and p53 in colorectal mucinous adenocarcinomas in relation to location. Virchows Arch. 2006;449:40–47. doi: 10.1007/s00428-006-0212-7. [DOI] [PubMed] [Google Scholar]
- 51.Esteller M, Toyota M, Sanchez-Cespedes M, et al. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltranserase by promoter hypermethylation is associated with G to A mutations in K-ras in colorectal tumorigenesis. Cancer Res. 2000;60:2368–2371. [PubMed] [Google Scholar]
- 52.Yamashita K, Dai T, Dai Y, Yamamoto F, Perucho M. Genetics supersedes epigenetics in colon cancer phenotype. Cancer Cell. 2003;4:121–131. doi: 10.1016/s1535-6108(03)00190-9. [DOI] [PubMed] [Google Scholar]
- 53.Hawkins N, Norrie M, Cheong K, et al. CpG island methylation in sproadic colorectal cancers and its relationship to microsatellite instability. Gastroenterology. 2002;122:1376–1387. doi: 10.1053/gast.2002.32997. [DOI] [PubMed] [Google Scholar]
- 54.Anacleto C, Leopoldino AM, Rossi B, et al. Colorectal cancer "methylator phenotype": fact or artifact? Neoplasia. 2005;7:331–335. doi: 10.1593/neo.04502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.IIino H, Jass JR, Simms LA, et al. DNA microsatellite instability in hyperplastic polyps, serrated adenomas, and mixed polyps: a mild mutator pathway for colorectal cancer? J Clin Pathol. 1999;52:5–9. doi: 10.1136/jcp.52.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hawkins NJ, Bariol C, Ward RL. The serrated neoplasia pathway. Pathology. 2002;34:548–555. [PubMed] [Google Scholar]
- 57.Snover DC, Jass JR, Fenoglio-Preiser C, Batts KP. Serrated polyps of the large intestine: a morphologic and molecular review of an evolving concept. Am J Clin Pathol. 2005;124:380–391. doi: 10.1309/V2EP-TPLJ-RB3F-GHJL. [DOI] [PubMed] [Google Scholar]
- 58.Petko Z, Ghiassi M, Shuber A, et al. Aberrantly methylated CDKN2A, MGMT, and MLH1 in colon polyps and in fecal DNA from patients with colorectal polyps. Clin Cancer Res. 2005;11:1203–1209. [PubMed] [Google Scholar]
- 59.Kim YH, Petko Z, Dzieciatkowski S, Lin L, et al. CpG island methylation of genes accumulates during the adenoma progression step of the multistep pathogenesis of colorectal cancer. Genes Chromosomes Cancer. 2006;45:781–789. doi: 10.1002/gcc.20341. [DOI] [PubMed] [Google Scholar]
- 60.Park S-J, Rashid A, Lee J-H, Kim SG, Hamilton SR, Wu T-T. Frequent CpG island methylation in serrated adenomas of the colorectum. Am J Pathol. 2003;162:815–822. doi: 10.1016/S0002-9440(10)63878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spring KJ, Zhao ZZ, Karamatic R, et al. High prevalence of sessile serrated adenomas with BRAF mutations: a prospective study of patients undergoing colonoscopy. Gastroenterology. 2006;131:1400–1407. doi: 10.1053/j.gastro.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 62.Makinen MJ. Colorectal serrated adenocarcinoma. Histopathology. 2007;50:131–150. doi: 10.1111/j.1365-2559.2006.02548.x. [DOI] [PubMed] [Google Scholar]
- 63.Kambara T, Simms LA, Whitehall VLJ, et al. BRAF mutation is associated with DNA methylation in serrated polyps and cancers of the colorectum. Gut. 2004;53:1137–1144. doi: 10.1136/gut.2003.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O'Brien MJ, Yang S, Mack C, et al. Comparison of microsatellite instability, CpG island methylation phenotype, BRAF and KRAS status in serrated polyps and traditional adenomas indicates separate pathways to distinct colorectal carcinoma end points. Am J Surg Pathol. 2006;30:1491–1501. doi: 10.1097/01.pas.0000213313.36306.85. [DOI] [PubMed] [Google Scholar]
- 65.Chan TL, Zhao W, Leung SY, Yuen ST Cancer Genoma Project. BRAF and KRAS mutations in colorectal hyperplastic polyps and serrated adenomas. Cancer Res. 2003;63:4878–4881. [PubMed] [Google Scholar]
- 66.Huang CS, O'Brien MJ, Yang S, Farraye FA. Hyperplastic polyps, serrated adenomas, and the serrated polyp neoplasia pathway. Am J Gastroenterol. 2004;99:2242–2255. doi: 10.1111/j.1572-0241.2004.40131.x. [DOI] [PubMed] [Google Scholar]
- 67.Dong SM, Lee EJ, Jeon ES, Park CK, Kim K-M. Progressive methylation during the serrated neoplasia pathway of the colorectum. Mod Pathol. 2005;18:170–178. doi: 10.1038/modpathol.3800261. [DOI] [PubMed] [Google Scholar]
- 68.Yashiro M, Laghi L, Saito K, et al. Serrated adenomas have a pattern of genetic alterations that distinguishes them from other colorectal polyps. Cancer Epidemiol Biomarkers Prev. 2005;14:2253–2256. doi: 10.1158/1055-9965.EPI-04-0790. [DOI] [PubMed] [Google Scholar]
- 69.Hoffmann MJ, Schulz WA. Causes and consequences of DNA hypomethylation in human cancer. Biochem Cell Biol. 2005;83:296–321. doi: 10.1139/o05-036. [DOI] [PubMed] [Google Scholar]
- 70.Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301:89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- 71.Bariol C, Suter C, Cheong K, et al. The relationship between hypomethylation and CpG island methylation in colorectal neoplasia. Am J Pathol. 2003;162:1361–1371. doi: 10.1016/S0002-9440(10)63932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamada Y, Jackson-Grusby L, Linhart H, et al. Opposing effects of DNA hypomethylation on intestinal and liver carcinogenesis. Proc Natl Acad Sci USA. 2005;102:13580–13585. doi: 10.1073/pnas.0506612102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lengauer C, Kinzler KW, Vogelstein B. DNA methylation and genetic instability in colorectal cancer cells. Proc Natl Acad Sci USA. 1997;94:2545–2550. doi: 10.1073/pnas.94.6.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deng G, Nguyen A, Tanaka H, et al. Regional hypermethylation and global hypomethylation are associated with altered chromatin conformation and histone acetylation in colorectal cancer. Int J Cancer. 2006;118:2999–3005. doi: 10.1002/ijc.21740. [DOI] [PubMed] [Google Scholar]
- 75.Kawakami K, Ruszkiewicz A, Bennett G, et al. DNA hypermethylation in the normal colonic mucosa of patients with colorectal cancer. Br J Cancer. 2006;94:593–598. doi: 10.1038/sj.bjc.6602940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Issa JP, Ahuja N, Toyota M, Bronner MP, Brentnall TA. Accelerated age-related CpG island methylation in ulcerative colitis. Cancer Res. 2001;61:3573–3577. [PubMed] [Google Scholar]
- 77.Grady WM, Raiput A, Lutterbaugh JD, Markowitz SD. Detection of aberrantly methylated hMLH1 promoter DNA in the serum of patients with microsatellite unstable colon cancer. Cancer Res. 2001;61:900–902. [PubMed] [Google Scholar]
- 78.Laird PW, Jackson-Grusby L, Fazeli A, et al. Suppression of intestinal neoplasia by DNA hypomethylation. Cell. 1995;81:197–205. doi: 10.1016/0092-8674(95)90329-1. [DOI] [PubMed] [Google Scholar]
- 79.Cormier RT, Dove WF. Dnmt1N/+ reduces the net growth rate and multiplicity of intestinal adenomas in C57BL/6-multiple intestinal neoplasia (Min)/+ mice independently of p53 but demonstrates strong synergy with the modifier of Min 1 (AKR) resistance allele. Cancer Res. 2000;60:3965–3970. [PubMed] [Google Scholar]
- 80.Suzuki H, Gabrielson E, Chen W, et al. A genomic screen for genes upregulated by demethylation and histone deacetylase inhibition in human colorectal cancer. Nat Genet. 2002;31:141–149. doi: 10.1038/ng892. [DOI] [PubMed] [Google Scholar]
- 81.Riester D, Hildmann C, Schwienhorst A. Histone deacetylase inhibitors-turning epigenetic mechanisms of gene regulation into tools of therapeutic intervention in malignant and other diseases. Appl Microbiol Biotechnol. 2007;75:499–514. doi: 10.1007/s00253-007-0912-1. [DOI] [PubMed] [Google Scholar]
- 82.Rhee I, Bachman KE, Park BH, et al. DNMT1 and DNMT3b cooperate to silence genes in human cancer cells. Nature. 2002;416:552–556. doi: 10.1038/416552a. [DOI] [PubMed] [Google Scholar]
- 83.Cote RJ, Laird PW, Datar RH. Promoter hypermethylation: a new therapeutic target emerges in urothelial cancer. J Clin Oncol. 2005;23:2879–2881. doi: 10.1200/JCO.2005.11.923. [DOI] [PubMed] [Google Scholar]