Abstract
Background
Adults with malignant glioma, especially the most common subtype, glioblastoma multiforme, have an unacceptably poor outcome with current therapies. Malignant gliomas are amongst the most angiogenic of cancers, and VEGF is the dominant angiogenic mediator in these tumors.
Objective
To summarize the clinical experience of VEGF-directed treatment for malignant glioma.
Methods
We reviewed the completed, ongoing and planned clinical trials evaluating anti-VEGF strategies for malignant glioma patients.
Results/conclusions
Recent studies incorporating anti-VEGF agents plus cytotoxic therapy among recurrent malignant glioma patients have achieved unprecedented improvements in radiographic response, time to progression and survival. Furthermore, acceptable toxicity was observed. Hence, a major current focus in neuro-oncology is to further develop antiangiogenic strategies for this desperate patient population.
Keywords: angiogenesis, bevacizumab, glioblastoma multiforme, malignant glioma, vascular endothelial growth factor
1. Introduction
Effective therapy for patients with malignant glioma, the most common primary tumor of the CNS, remains elusive. Median survival for parients with glioblastoma multiforme (GBM), the most common malignant glioma, is under 15 months following standard-of-care therapy with surgery, radiation and temozolomide [1], and there is no effective therapy following recurrence [2]. Heterogeneity within and across tumors, high rates of de novo and acquired resistance, and limited delivery are major impediments to cytoxins directly targeting GBM cells. Hence, therapeutics that indirectly attack these tumors by targeting vital components of the supporting extracellular matrix, including neovasculature, are increasingly being explored.
Growth of all tumors is dependent on angiogenesis, the formation of new blood vessels from pre-existing vasculature [3]. Tumor angiogenesis is orchestrated by a simultaneous increase in expression of genes, including VEGF, acidic and basic fibroblast growth factor (FGF), IL-8 and -6, hypoxia-inducible factor 1 alpha (HIF-1α) and the angiopoietins, with downregulation of endogenous angiogenesis inhibitors, such as thrombospondins, angiostatin, endostatin and interferons [4]. VEGF is a paramount common denominator required for tumor angiogenesis and pathogenesis [5,6]. Therapeutic exploitation of the VEGF axis has achieved substantial clinical benefit across many cancer subtypes [7,8]. However, enthusiasm for evaluating these agents among patients with CNS tumors has been dampened by safety concerns, including the risk of intracranial hemorrhage. Nonetheless, recent trials among malignant glioma patients treated with VEGF- or VEGF receptor (VEGFR)-targeting therapeutics plus chemotherapy report unprecedented rates of antitumor benefit, as well as acceptable safety profiles. Specifically, the regimen of bevacizumab (BV), a humanized anti-VEGF monoclonal antibody, plus irinotecan, achieved a 10-fold improvement in radiographic response as well as significant increases in progression-free and overall survival among recurrent GBM patients [9,10], Several additional clinical trials are ongoing, or starting soon, to validate and expand these efforts, including multiple studies to evaluate a variety of VEGF as well as non-VEGF antiangiogenic strategies for malignant glioma patients (Table 1). In this review, we briefly describe angiogenesis in malignant glioma and then detail clinical activities targeting VEGF/VEGFR for malignant glioma patients.
Table 1.
Anti-angiogenic agents currently under evaluation in the treatment of malignant glioma patients.
| Agent | Primary target | Additional targets | Mechanism/classification |
|---|---|---|---|
| ABT-510 | CD36 receptor | – | Synthetic peptide thrombospondin inhibitor |
| AZD2171 (cediranib) | VEGFR2 | PDGFRβ; c-Kit | Tyrosine kinase inhibitor |
| BAY 43-9006 (sorafenib) | BRAF | VEGFR2-3; PDGFRβ; c-Kit;; Ras, p38α | Tyrosine kinase inhibitor |
| CT-322 | VEGFR1-3 | – | Adnectin-based competitive inhibitor |
| EMD 121974 (cilengitide) | Integrins αvβ3, αvβ5 | – | RGD-containing synthetic peptide |
| GW786034 (pazopanib) | VEGFR1-3 | PDGFRβ; c-Kit | Tyrosine kinase inhibitor |
| Interferons α and β | bFGF | – | Suppress expression |
| Metronomic chemotherapy | Endothelial cells | – | Induces apoptosis of endothelial cells |
| PTK787 (Vatalanib) | VEGFR2 | VEGFR1; VEGFR3; PDGFRβ; c-Kit | Tyrosine kinase inhibitor |
| RhuMabVEGF (bevacizumab) | VEGF-A | – | Monoclonal antibody |
| SU11248 (sunitinib) | VEGFR2 | PDGFRβ; FLT3; c-Kit | Tyrosine kinase inhibitor |
| Thalidomide | VEGFR, bFGF | – | Suppress expression |
| VEGF-Trap | VEGFA, B, PLGF | – | Decoy receptor |
| ZD6474 (vandetanib) | VEGFR2 | EGFR; RET | Tyrosine kinase inhibitor |
bFGF: Basic fibroblast growth factor, BRAF: v-Raf murine sarcoma viral oncogene homolog B1; c-Kit. a member of the platelet-derived growth factor receptor family; FLT-3: FMS-related tyrosine kinase 3; PDGFRβ: Platelet-derived growth factor receptor-β; PLGF: Placental growth factor; Ras RAS viral (v-ras) oncogene homolog; RET: Ret proto-oncogene.
2. Angiogenesis in malignant glioma: the perfect storm
Proliferation, survival and invasion of malignant gliomas critically hinge on an adequate blood supply. Malignant gliomas are among the most angiogenic of cancers [11], primarily due to a tumultuous and somewhat redundant constellation of genetic and cellular signaling cues culminating in a remarkably prolific capability for neovascularization. Angiogenesis is fueled by several pro-angiogenic factors in malignant glioma, among which VEGF is dominant Six VEGF isoforms (VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E and placental growth factor) and several additional, biologically active VEGF variants generated by alternative gene splicing or protease cleavage, are secreted by tumor cells, infiltrating inflammatory cells and platelets and can be sequestered in the extracellular matrix [12–18]. Glial tumors exhibit a prototypic ‘angiogenic switch’, in that a hallmark of transformation from low-grade to high-grade gliomas is the induction of pro-angiogenic mediators and new blood vessel formation [19]. Furthermore, increased VEGF expression predicts glioma aggressiveness and poorer outcome [20]. VEGF expression in malignant gliomas is most concentrated adjacent to areas of necrosis and hypoxia, including cellular pseudopalisades at the tumor leading edge [19,21–28]
Several hypoxia-dependent and independent mechanisms converge to produce an abundance of VEGF in the micro-environment of malignant gliomas [5,19,29–32]. Hypoxia, a prominent feature of malignant gliomas [28,33], enhances expression and stabilization of HIF-1α, which acts as a transcription factor to activate myriad target genes regulating tumor angiogenesis, migration and survival, including VEGF and VEGFRs [33–36]. Aberrant activation of multiple growth factor receptors in malignant glioma, including EGFR [37,38], platelet-derived growth factor receptor (PDGFR) [39–41], scatter factor/hepatocyte growth factor receptor (MET) [42], IGF receptor (IGFR) [43,44], stem cell factor receptor (c-Kit) [45], and FGF receptor (FGFR) [46–48], also increases VEGF activity. Dysregulated signaling of the phosphatidylinositol 3-kinase (PI3K/Akt) and Ras/MAPK pathways, which occur frequently among malignant gliomas, also augment VEGF expression [37.49].
Expression of VEGFRs (VEGFR-1, VEGFR-2 and VEGFR-3) and co-receptors, including the neuropilins, although very low in the normal brain, are also markedly increased in malignant gliomas [21,23–25,50,51]. Ligand binding activates VEGFRs, triggering a downstream intracellular signaling cascade that promotes endothelial cell proliferation, survival, activation, invasion, migration and permeability [5,52]. VEGF signaling further fortifies the angiogenic response by triggering endothelial cell nitric oxide synthesis [53], and by mobilizing bone-marrow-derived endothelial cell progenitors [54–57].
Finally, malignant gliomas robustly express several critical mediators of endothelial cell invasion, a critical step in new blood vessel formation, including MMP-2 and -9, urokinase-type plasminogen activator (uPA) and its receptor (uPAR), cathepsin-B, integrins αvβ3 and αvβ5, and tenascin-C [58–63].
3. Targeting VEGF: multiple ways to upset the apple cart
Although the precise antitumor mechanism of VEGF-targeting agents is unknown, increasing amounts of data suggest that effective VEGF inhibition exerts multiple detrimental effects on malignant glioma viability and survival. In addition to ‘choking off’ the blood supply and depriving tumors of vital nutrients and oxygen, preclinical and clinical studies confirm that VEGF-targeting agents are particularly effective when combined with cytotoxins [8–10,64–67]. Potential underlying mechanisms of this enhanced activity include the ability of antiangiogenic agents to sensitize tumor endothelial cells to cytotoxic agents [68,69] and to block a compensatory VEGF surge and/or accelerated tumor cell repopulation induced by rapid cell killing [70,71]. Additionally, appropriately dosed antiangiogenic agents may selectively ‘prune’ tumor vasculature, thereby transiently normalizing perfusion and improving chemotherapy delivery [72–75]. In support of this model, lowered interstitial fluid pressure, higher oxygen content, and decreased permeability were observed in an orthotopic GBM model, following judiciously administered antiangiogenic therapy [76]. Further support for this mechanism of action is emerging from recent clinical studies conducted among patients with colon cancer and GBM undergoing anti-VEGF therapy plus chemotherapy [77,78].
The recent discovery that VEGF-targeting agents can effectively inhibit activity of malignant glioma stem cells provides yet another highly intriguing antitumor mechanism of action for these agents [79]. The self-renewal and tumor-forming abilities of malignant glioma stem cells have recently been shown to be critically dependent on a bi-dimensional interaction with endothelial cells within the immediate microenvironment, referred to as the perivascular niche, which can also be blocked by anti-VEGF agents [80]. In addition, metronomic chemotherapy, felt to block angiogenesis by inducing endothelial cell apoptosis, can also target GBM stem cells [81]. The cumulative findings of these studies suggest that effective antiangiogenic therapy may target GBM stem cells directly and may also critically perturb the perivascular niche required for stem cell well-being [82].
4. Clinical studies: targeting VEGF
4.1 Bevacizumab
Following observations that BV improved outcome when administered with chemotherapy to patients with colorectal, breast, lung and pancreatic cancer patients [67], an initial evaluation of BV plus the topoisomerase-1 inhibitor irinotecan (Camptosar; Pfizer) reported a dramatic rate of radiographic response among recurrent primary CNS tumor patients [83], These results led to a formal Phase II study of this regimen for recurrent malignant glioma patients [9,10,84]. Irinotecan was included in this regimen because it has modest activity as a salvage agent among recurrent malignant glioma patients, including a 5 – 15% radiographic response rate and a median progression-free survival (PFS) of ~ 12 weeks [85–89].
Adult patients with measurable, recurrent Grade 3 or 4 malignant glioma and a Karnofsky performance status of ≥ 60% were eligible. Patients were also required to have adequate bone marrow, hepatic and renal function and to be at least 6 weeks from prior surgery and 4 weeks from prior radiation therapy or chemotherapy (6 weeks for nitrosoureas). Patients with more than three prior episodes of progression, evidence of blood on pretreatment imaging, requirement for warfarin anticoagulation, or previous BV treatment were not eligible. The study primary end point was 6-month PFS.
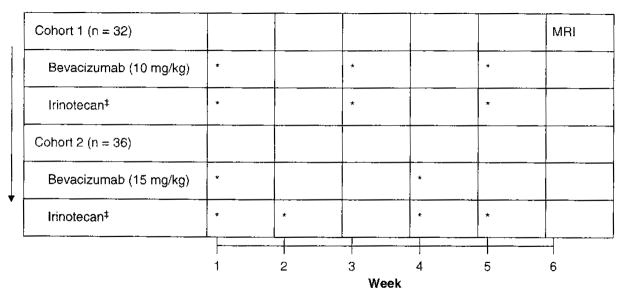
An initial cohort of 32 patients received BV (10 m/kg) and irinotecan every 2 weeks followed by a second cohort of 36 patients treated with BV every three weeks (15 mg/kg) and irinotecan on weeks 1, 2, 4 and 5 of each 6-week cycle (Figure 1). Patients not on concurrent CYP-3A enzyme-inducing antiepileptic drugs (EIAEDs, phenytoin, carbamazepine, oxcarbazepine, phenobarbitol and primidone) received 125 mg/m2 of irinotecan, whereas those on EIAEDs received 340 mg/m2 due to the profound EIAED-induced increase in metabolism of irinotecan and its major metabolite, SN-38 [90]. Patients were evaluated with a complete physical examination and MRI after each cycle. Radiographic response was independently assessed by two study investigators using the modified MacDonald criteria [91]. Of note, for this study, stable or improved T2 and fluid-attenuated inversion recovery (FLAIR; signal abnormalities were also required to define radiographic response in addition to the traditional MacDonald criteria. Treatment was discontinued for progressive disease, unacceptable toxicity or the need to initiate systemic anticoagulation.
Figure 1. Treatment schema for recurrent malignant glioma patients treated with bevacizumab plus irinotecan [9,10].
‡Irinotecan
non-EIAED: 125 mg/m2; EIAED = 340 mg/m2
EIAED. Enzyme-inducing antieptileptic drugs.
Patient characteristics for both cohorts are summarized in Table 2. Of note, all had received prior temozolomide chemoradiation and had a median of two prior episodes of progressive disease (range, 1 – 3).
Table 2.
| Characteristics | Cohort 1 | Cohort 2 |
|---|---|---|
| Number of patients | 32 | 36 |
| Male:female | 21:11 | 24:12 |
| Median age (years; range) | 49 (27 – 66) | 46(18 – 62) |
| Karnofsky performance status | 80 (60 – 100) | 80 (60 – 100) |
| Grade IV:Grade III | 23:9 | 12:24 |
| Median number of progressions (range) | 2 (1 – 3) | 2(1 – 3) |
| Median time from diagnosis (months; range) | 14(3 – 66) | 42 (3 – 165) |
| Anticonvulsant EIAED:non-EIAED | 14:18 | 17:19 |
EIAED: Enzyme-inducmg antiepiileptic drugs.
The study regimen was overall adequately tolerated. Two patients (3%) developed a grade 2 CNS hemorrhage, including a patient in cohort 1 after ten cycles of therapy and a patient on enoxaparin in cohort 2 after nine cycles of therapy. Eight patients (12%), four from each cohort, developed grade 3 – 4 thromboses, including one patient with an arterial cerebrovascular ischemic event, suggesting that BV may further heighten the known risk of thrombosis among malignant glioma patients [92–94], Therapy was discontinued in four patients (6%) due to grade 2 fatigue and in two patients (6% of cohort 1) for grade 2 proteinuria. Four patients (6%) in cohort 2 discontinued therapy due to grade 3 nausea/emesis or diarrhea, most probably due to the more intensive irinotecan schedule.
The rates of radiographic response are summarized in Table 3. For comparison, radiographic response rates among patients treated with temozolomide at first recurrence are included [95,96]. The rate of radiographic response was considerably higher among patients treated with BV plus irinotecan, even though they were more heavily pretreated and had failed prior temozolomide. Overall, 40 of the 68 patients (59%) achieved a radiographic response, including 22 (65%) with grade 3 tumors and 18 (53%) with grade 4 tumors (Figure 2).
Table 3.
Outcome achieved with bevacizumab plus irinotecan among patients with recurrent grade 3 and grade 4 malignant glioma compared to patients enrolled on prior salvage studies.
| Outcome | Grade 3 |
Grade 4 |
||||
|---|---|---|---|---|---|---|
| BV + CPT-11 [9,10] (n = 34) | TMZ 1st PD [96] (n = 162) | Other salvage therapy [97] (n = 150) | BV + CPT-11 [9,10] (n = 34) | TMZ 1st PD [95] (n = 112) | Other salvage therapy [97] (n = 225) | |
| CR/PR(%) | 65 | 35 | 14 | 53 | 5 | 6 |
| SD (%) | 32 | 27 | 34 | 41 | 40 | 27 |
| PD (%) | 3 | 38 | 52 | 6 | 55 | 67 |
| PFS (median, weeks) | 42 | 22 | 13 | 23 | 12 | 9 |
| 6 month PFS (%) | 61 | 46 | 31 | 43 | 21 | 15 |
| Overall survival (median, weeks) | 60 | 54 | 47 | 40 | 30 | 25 |
BV: Bevacizumab; CPT: Irinotecan (Camptosar); CR: Complete response; PD: Progressive disease; PFS: Progression-free survival; PR: Partial response; SD: Stable disease; TMZ: Temozolomide.
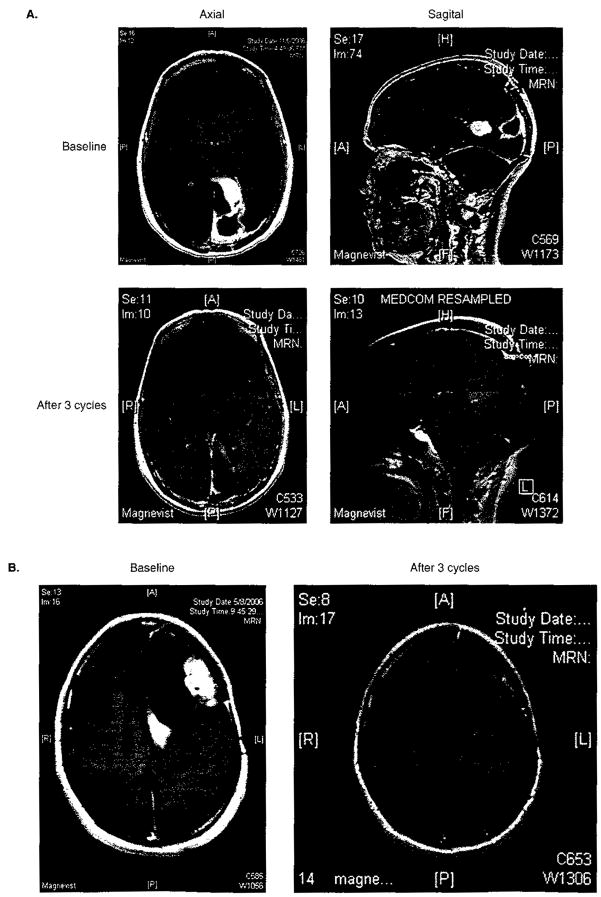
Figure 2.
T-1 weighted MRI scans following gadolinium administration demonstrating representative partial radiographic responses of recurrent GBM patients (A: patient 1; B: patient 2) following treatment with bevacizumab plus irinotecan.
Salvage therapies for GBM patients typically achieve only single-digit rates of radiographic response, while the majority of patients progress as their best response [2,95,97]. In contrast, a majority of patients treated with BV plus irinotecan achieved a radiographic response while only a single-digit rate of progressive disease (6%) was observed. Finally and most encouragingly, clinical and neurological status commonly reflected radiographic findings in that most patients who achieved a radiographic response also noted neurological improvement and were able to taper chronic dexamethasone dosing. Similar rates of radiographic response to BV plus chemotherapy were recently described in two additional reports. Specifically, 4 of 10 recurrent GBM patients (40%) and 3 of 4 recurrent grade 3 patients (75%) achieved a partial response in one study [98]. In a second study, 8 of 21 heavily pretreated patients with recurrent malignant glioma (38%) achieved a radiographic response, including 7 of 18 with GBM (39%) [99].
Median PFS rates for grade 3 and 4 patients reported by Vredenburgh were nearly twofold greater than those achieved with temozolomide at first recurrence (Table 3) [95,96]. In addition, the rates of 6-month progression-free survival (6-PFS) were much higher than those achieved with temozolomide. Furthermore, six patients with recurrent GBM (18%) completed a year of therapy, and five of the six had no hypermetabolic activity noted on companion [18F] fluorodeoxyglucose positron emission tomography (FDG-PET) imaging at therapy completion, suggesting the absence of active tumor. Analogously, seven patients with recurrent grade 3 tumors (21%) completed one year of therapy, and six of these had negative FDG-PET scans at study completion. Similarly, the median overall survival for patients with grade 3 and 4 tumors in this study significantly surpassed the median survival reported for historical temozolomide studies among patients at first recurrence [95,96] and among patients treated on a series of salvage therapies (Table 3) [97]. Although decreased permeability and lowered contrast-agent uptake induced by BV may have contributed to the rate of radiographic response observed among patients treated with BV plus irinotecan, one would predict that an agent/regimen solely capable of decreasing vessel permeability should not meaningfully improve progression-free and overall survival among recurrent malignant glioma patients. Therefore, the improved rates of PFS and overall survival observed among patients treated with BV plus irinotecan, as compared with those achieved historically with other salvage therapies, strongly supports an underlying antitumor action of this regimen.
Following the Vredenburgh study [9,10], additional studies were initiated to determine the contribution of BV compared with the combination of BV plus irinotecan on outcome. Specifically, a randomized Phase II study comparing responses of GBM patients at first or second recurrence to either BV alone or the combination of BV plus irinotecan (CPT-11) recently completed accrual. Preliminary results of this study reveal that patients randomized to BV alone achieved a radiographic response rate of 21% and a 6-PFS of 36%. Patients who received BV plus CPT-11 appeared to fare better in that they achieved a radiographic response rate of 34% and a 6-PFS of 51%. The study results were not sufficiently mature to evaluate overall survival [100]. In addition, single arm studies evaluating BV monotherapy among recurrent malignant glioma patients are underway, including an imaging-intensive trial conducted at the National Cancer Institute. Another ongoing study recently reported preliminary results of 15 recurrent malignant glioma patients treated with 15 mg/kg BV alone every 3 weeks. In this report, only two patients (13%) achieved a partial response, whereas five (33%) were stable, and eight (53%) progressed [101]. Although preliminary, these results suggest an inferior disease control rate for BV alone as compared with that of BV plus irinotecan.
As stated above, most patients with radiographic response reported by Vredenburgh also noted improvement in neurologic status [9,10]. Gonzalez recently reported similar findings among eight patients with CNS tumors and radiation necrosis treated with BV, seven of whom also received chemotherapy. Specifically, a 48% (± 22% SD) average decrease in T1-weighted post-Gadolinium-contrast measurements and a 60% (± 18% SD) decrease in FLAIR changes were reported, while all patients on pretreatment dexamethasone therapy substantially reduced dexamethasone by an average of 8.6 mg (± 3.6 mg) per day [102].
Based on the highly encouraging, initial results reported for BV plus irinotecan, multiple additional studies are ongoing to evaluate different BV-based combinatorial regimens, including separate single-arm studies at Duke University evaluating BV plus protracted, metronomic dosing of either daily temozolomide or etoposide. In addition, the Radiation Therapy Oncology Group (RTOG) is currently conducting a trial randomizing recurrent GBM patients to receive BV with either protracted temozolomide therapy (75 mg/m2/day for 21 days each month) or irinotecan every 2 weeks (RTOG 0625). A study combining BV plus the EGFR inhibitor erlotinib is also underway, based on synergistic antitumor activity observed in preclinical GBM models treated with dual EGFR/VEGF inhibitor therapy [103].
At present, four single-institutional studies are underway to evaluate BV in the treatment of newly diagnosed GBM patients. Investigators at Duke are evaluating the addition of BV to chemoradiation with temozolomide, followed by cycles of BV plus temozolomide and irinotecan. In a separate study, neoadjuvant BV plus temozolomide is being evaluated prior to XRT/temozolomide for patients with bulky, unresectable or multi-focal tumors. A study at the University of California, Los Angeles is evaluating the addition of BV to standard temozolomide chemoradiation, while the combination of BV plus erlotinib plus standard temozolomide chemoradiation is being evaluated at the University of California, San Francisco. Finally, a multi-center, randomized Phase III clinical trial is being planned to evaluate the current temozolomide chemoradiation standard of care with or without BV.
4.2 VEGF receptor tyrosine kinase inhibitors
In contrast to large, growth factor ligand antagonists such as BV that act in the extracellular and intravascular compartments, competitive inhibitors of the intracellular tyrosine kinase domain of VEGFRs are also being evaluated in ongoing clinical trials for malignant glioma patients. Vatalanib (PTK787/ZK222584; Novartis), a potent inhibitor of VEGFR1-3, has been evaluated as a single agent and in combination with either temozolomide or lomustine chemotherapy. Only modest rates of radiographic response and progression-free survival were reported, which may have been influenced by suboptimal, once-daily dosing [104,105]. Subsequent studies have demonstrated more effective pharmacokinetic exposures following twice daily vatalanib dosing [106]. A Phase I study of vatalanib plus the PDGFR inhibitor imatinib mesilate for recurrent malignant glioma patients has recently been completed, and a clinical trial of vatalanib plus temozolomide chemoradiation is ongoing for newly diagnosed GBM patients.
Preliminary results were recently reported for a Phase II trial with cediranib (AZD2171, AstraZeneca, UK), a potent, oral pan-VEGFR, PDGFR, and c-kit inhibitor. Out of 16 patients, 9 (56%) achieved at least a partial response, and 3 additional patients achieved stable disease. The median time to progression was 15.8 weeks. Treatment was associated with meaningful improvements in tumor-associated edema, including the ability to reduce pretreatment corticosteroid dosing in eight of 11 patients (73%). Elegant collaborative imaging studies revealed that decreased contrast enhancement was accompanied by significant decreases in tumor vessel size, permeability, blood volume and blood flow, consistent with vascular normalization, which was also noted to reverse following drug interruption [78]. Although intriguing, this report reflects a small study population and limited follow-up assessments. A multi-center, randomized clinical trial is planned to further evaluate cediranib plus lomustine among recurrent GBM patients.
Additional receptor tyrosine kinase inhibitors targeting VEGF are also under evaluation in ongoing clinical trials for malignant glioma (Table 1).
4.3 Decoy-ligand: VEGF-trap
VEGF-Trap (Regeneron), consisting of portions of human VEGFR1 and VEGFR2 extracellular domains fused to the constant region of human IgG1, acts as a soluble decoy VEGF receptor to bind VEGF and thus prevent it from interacting with VEGFRs on tumor endothelial cells [107,108]. VEGF-Trap potentiates radiotherapy in GBM xenografts [109]. A single-arm Phase II trial of VEGF-Trap for recurrent malignant glioma patients by the North American Brain Tumor Consortium recently completed accrual, and a multi-center clinical trial incorporating VEGF-Trap with temozolomide chemoradiotherapy is planned for newly diagnosed GBM patients.
5. Controversial issues
5.1 Assessment of response
Currently, malignant gliomas are assessed by measuring the largest bidimensional enhancing product following intravenous contrast administration on magnetic resonance imaging (MRI) [91]. However, such assessments may be misleading in that potent anti-VEGF agents can decrease permeability and may lessen contrast enhancement with or without a true underlying antitumor effect. Modification of traditional MRI assessment criteria will probably be necessary to more accurately measure underlying tumor activity following anti-VEGF therapy. Additional imaging approaches may also increase the accuracy of response assessments including positron emission tomography (PET) [110–112], magnetic resonance spectroscopy [113], and complementary MRI techniques, such as dynamic contrast-enhanced MRI [114–116], dynamic susceptibility MRI [117–119], arterial spin labeling [120] and high-resolution magnetic resonance angiography [121,122].
Batchelor et al. recently reported that changes in mean blood vessel size and permeability detected by correlative imaging methods were associated with response among recurrent GBM patients treated with AZD2171 (cediranib) [78]. Similarly, Chen et al. [99] recently reported that recurrent GBM patients who achieved a metabolic response using [18F] fluorothymidine positron emission tomography (FLT PET) following BV plus irinotecan had a threefold longer survival than non-responders (10.8 versus 3.4 months, p = 0.003). Furthermore, early (1–2 weeks after starting therapy) and late (6 weeks after starting therapy) metabolic response correlated more strongly with survival than MRI response.
In addition, circulating biomarkers offer promise to help predict and monitor response to therapy. Clinical evidence is emerging for several potentially valuable circulating biomarkers, including plasma VEGF [78,123–127], basic fibroblast growth factor (bFGF) [78,127], and tumor stromal-derived factor-1 (SDF1α) [78], as well as viable circulating endothelial cells [56,78,124,128].
5.2 Resistance is relevant
Several potential mechanisms of resistance to anti-VEGF agents have recently been identified, including compensatory upregulation of alternative angiogenic factors such as PDGF/PDGFR-β, FGF, SDF-1α and angiopoietin-1 (Ang-1)/Tunica interna endothelial cell kinase homolog (Tie-2) following VEGF inhibition [78,129,130], increased mobilization of pericytes [131], secretion of endothelial cell survival factors [129], and the ability of glioma cells to induce a more invasive phenotype, accompanied by host blood-vessel co-option and eventual gliomatosis [132–136]. Future exploration of clinical strategies to circumvent these mechanisms of resistance-including agents or combinatorial regimens that target multiple angiogenic mediators and regimens that inhibit key mediators of both tumor angiogenesis and invasion [137–139] may prove highly valuable.
6. Conclusion
Malignant gliomas have long been appreciated as highly angiogenic. Insights into the biology of tumor angiogenesis, including the central role of VEGF, as well as the successful application of anti-VEGF agents with chemotherapy in other cancers, has led to the evaluation of VEGF as a therapeutic target for malignant glioma. Although initial results are highly encouraging, validation of these findings and further optimization of antiangiogenic strategies are critically required. Nonetheless, targeting VEGF/VEGFR may represent a major therapeutic advance for patients with these deadly tumors.
7. Expert opinion
Malignant gliomas provide an attractive opportunity for antiangiogenic approaches, and recent clinical trials have demonstrated highly promising therapeutic benefit as well as acceptable safety among patients treated with the humanized anti-VEGF monoclonal antibody bevacizumab plus irinotecan. Specifically, in an initial clinical trial of recurrent GBM patients receiving bevacizumab plus irinotecan who had failed standard-of-care temozolomide chemoradiation and had a median of two prior episodes of progressive disease, the rate of radiographic response was 5- to 10-fold higher than that reported in the literature. Of greater importance, radiographic responses were meaningful in that they were associated with improved neurological function and the ability to successfully taper chronic dexamethasone treatment in most cases. Furthermore, responses were associated with greater durations of progression-free and overall survival than those reported for patients treated historically with alternative salvage agents. Furthermore, a recent study utilizing a small-molecule tyrosine kinase inhibitor to block VEGFR activity has also demonstrated similarly exciting therapeutic benefit and safety for recurrent GBM patients. Additional important clinical trials have recently been completed, are underway or are planned to initiate soon in order to build on the promising initial results and to further define the therapeutic potential of anti-VEGF therapy for recurrent malignant glioma patients.
In addition, investigators are highly enthusiastic to extend this approach to newly diagnosed GBM patients, particularly given the overall poor outcome of current standard therapy. A number of exploratory, single-arm studies have recently initiated to evaluate strategies incorporating BV into the current standard of care with temozolomide chemoradiation. Furthermore, a randomized Phase III study is being planned.
Nonetheless, several challenges exist and will require intensive study in the next few years in order for patients to fully benefit from these promising advances. VEGF-targeting agents decrease permeability and hence contrast enhancement, thereby frequently confounding the assessment of radiographic response. Traditional MRI assessment criteria will probably require modification in order to more accurately measure underlying tumor activity following treatment with these agents. Preliminary studies show that correlative MRI and PET approaches may provide imaging biomarkers to improve the reliability of response assessment. Promising circulating biomarkers of response also warrant further investigation. Antiangiogenic agents also have unique toxicities, including the possibility of an increased risk of thromboembolic events, which must be carefully monitored and addressed, particularly among patients with CNS tumors. Costs of newly developed anti-VEGF agents are significant and currently limit access to these agents for brain tumor patients. Finally, although initial results with anti-VEGF agents are very exciting, nearly all patients eventually relapse, which indicates that mechanisms of resistance must be better understood and circumvented. Optimization of anti-VEGF therapy for malignant glioma patients will require answering a number of key questions:
which agents are safest and most effective?
when are these agents best administered during the overall course of treatment?
should these agents be administered at maximum tolerated dose levels or at a dose level capable of achieving a biologically active parameter?
what are the most effective combinatorial approaches to further improve outcome with acceptable toxicity?
The following key issues have been identified:
Glioblastoma multiforme, the most common primary CNS tumor, continues to have a dismal outcome. Current ‘state-of-the-art’ therapy, including surgery and chemoradiation, achieves a median progression-free-survival of < 7 months and a median overall survival of only 14.7 months.
Salvage therapies for recurrent malignant glioma patients remain ineffective; thus such patients continue to represent a major unmet need in oncology today.
Malignant gliomas are among the most angiogenic of neoplasms, and VEGF is the dominant mediator of glioma angiogenesis.
Initial studies incorporating bevacizumab, a humanized monoclonal antibody against VEGF, plus irinotecan have achieved dramatic rates of durable radiographic response among patients with recurrent grade 3 and 4 malignant glioma. Furthermore, toxicity associated with this treatment regimen was acceptable.
Preliminary encouraging results with the pan-VEGFR oral tyrosine kinase inhibitor AZD2171 suggest that additional strategies to target VEGFR signaling should also be explored for malignant glioma patients.
Validation of the benefit of anti-VEGF treatment strategies for recurrent malignant glioma patients is underway, and approaches that incorporate such strategies into the current treatment paradigm for patients with newly diagnosed GBM are rapidly developing.
Future efforts will also explore additional anti angiogenesis agents, as well as evaluate potentially synergistic combinatorial approaches for this patient population.
Important challenges to address include the evaluation of correlative imaging and circulating biomarkers of response, strategies to minimize toxicity and better understanding of potential mechanisms of therapeutic resistance.
Acknowledgments
This work was supported by National Institutes of Health grant nos. 1-P50-CA108786-01, NS20023, and CA11898 and by grant no. MO1 RR 30 through the General Clinical Research Centers Program, National Center for Research Resources, National Institutes of Health.
Footnotes
Declaration of interest
The authors have no conflict of interest to declare and no fee has been received for preparation of this manuscript.
Bibliography
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Simpson L, Galanis E. Recurrent glioblastoma multiforme: advances in treatment and promising drug candidates. Expert Rev Anticancer Ther. 2006;6(11):1593–607. doi: 10.1586/14737140.6.11.1593. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara N. VEGF as a therapeutic target in cancer. Oncology. 2005;69(Suppl 3):11–6. doi: 10.1159/000088479. [DOI] [PubMed] [Google Scholar]
- 7.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 8.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 9.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–9. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 10.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25(30):4722–9. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 11.Brem S, Cotran R, Folkman J. Tumor angiogenesis: a quantitative method for histologic grading. J Natl Cancer Inst. 1972;48(2):347–56. [PubMed] [Google Scholar]
- 12.Kowanerz M, Ferrara N. Vascular endothelial growth factor signaling pathways: therapeutic perspective. Clin Cancer Res. 2006;12(17):5018–22. doi: 10.1158/1078-0432.CCR-06-1520. [DOI] [PubMed] [Google Scholar]
- 13.Keyt BA, Berleau LT, Nguyen HV, et al. The carboxyl-terminal domain (111–165) of vascular endothelial growth factor is critical for its mitogenic potency. J Biol Chem. 1996;271(13):7788–95. doi: 10.1074/jbc.271.13.7788. [DOI] [PubMed] [Google Scholar]
- 14.Park JE, Keller GA, Ferrara N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell. 1993;4(12):1317–26. doi: 10.1091/mbc.4.12.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergers G, Brekken R, McMahon G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2(10):737–44. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salven P, Orpana A, Joensuu H. Leukocytes and platelets of patients with cancer contain high levels of vascular endothelial growth factor. Clin Cancer Res. 1999;5(3):487–91. [PubMed] [Google Scholar]
- 17.Verheul HM, Hoekman K, Luykx-de Bakker S, et al. Platelet: transporter of vascular endothelial growth factor. Clin Cancer Res. 1997;3(12 Pt 1):2187–90. [PubMed] [Google Scholar]
- 18.Mohle R, Green D, Moore MA, et al. Constitutive production and thrombin-induced release of vascular endothelial growth factor by human megakaryocytes and platelets. Proc Natl Acad Sci USA. 1997;94(2):663–8. doi: 10.1073/pnas.94.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaur B, Tan C, Brat DJ, et al. Genetic and hypoxic regulation of angiogenesis in gliomas. J Neurooncol. 2004;70(2):229–43. doi: 10.1007/s11060-004-2752-5. [DOI] [PubMed] [Google Scholar]
- 20.Leon SP, Folkerth RD, Black PM. Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer. 1996;77(2):362–72. doi: 10.1002/(SICI)1097-0142(19960115)77:2<362::AID-CNCR20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 21.Plate KH, Breier G, Weich HA, et al. Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int J Cancer. 1994;59(4):520–9. doi: 10.1002/ijc.2910590415. [DOI] [PubMed] [Google Scholar]
- 22.Plate KH, Risau W. Angiogenesis in malignant gliomas. Glia. 1995;15(3):339–47. doi: 10.1002/glia.440150313. [DOI] [PubMed] [Google Scholar]
- 23.Plate KH, Breier G, Millauer B, et al. Up-regulation of vascular endothelial growth factor and its cognate receptors in a rat glioma model of tumor angiogenesis. Cancer Res. 1993;53(23):5822–7. [PubMed] [Google Scholar]
- 24.Samoto K, Ikezaki K, Ono M, et al. Expression of vascular endothelial growth factor and its possible relation with neovascularization in human brain tumors. Cancer Res. 1995;55(5):1189–93. [PubMed] [Google Scholar]
- 25.Schmidt NO, Westphal M, Hagel C, et al. Levels of vascular endothelial growth factor, hepatocyte growth factor/scatter factor and basic fibroblast growth factor in human gliomas and their relation to angiogenesis. Int J Cancer. 1999;84(1):10–8. doi: 10.1002/(sici)1097-0215(19990219)84:1<10::aid-ijc3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 26.Fischer I, Gagner JP, Law M, et al. Angiogenesis in gliomas: biology and molecular pathophysiology. Brain Pathol. 2005;15(4):297–310. doi: 10.1111/j.1750-3639.2005.tb00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kargiotis O, Rao JS, Kyritsis AP. Mechanisms of angiogenesis in gliomas. J Neurooncol. 2006;78(3):281–93. doi: 10.1007/s11060-005-9097-6. [DOI] [PubMed] [Google Scholar]
- 28.Rong Y, Durden DL, Van Meir EG, Brat DJ. ‘Pseudopalisading’ necrosis, in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J Neuropathol Exp Neurol. 2006;65(6):529–39. doi: 10.1097/00005072-200606000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359(6398):843–5. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 30.Fukumura D, Xu L, Chen Y, et al. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res. 2001;61(16):6020–4. [PubMed] [Google Scholar]
- 31.Parliament MB, Allalunis-Turner MJ, Franko AJ, et al. Vascular endothelial growth factor expression is independent of hypoxia in human malignant glioma spheroids and tumours. Br J Cancer. 2000;82(3):635–41. doi: 10.1054/bjoc.1999.0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizukami Y, Kohgo Y, Chung DC. Hypoxia inducible factor-1 independent pathways in tumor angiogenesis. Clin Cancer Res. 2007;13(19):5670–4. doi: 10.1158/1078-0432.CCR-07-0111. [DOI] [PubMed] [Google Scholar]
- 33.Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro Oncol. 2005;7(2):134–53. doi: 10.1215/S1152851704001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zagzag D, Zhong H, Scalzitti JM, et al. Expression of hypoxia-inducible factor 1α in brain tumors: association with angiogenesis, invasion, and progression. Cancer. 2000;88(11):2606–18. [PubMed] [Google Scholar]
- 35.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-heiix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92(12):5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lando D, Peet DJ, Whelan DA, et al. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002;295(5556):858–61. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 37.Pore N, Liu S, Haas-Kogan DA, et al. PTEN mutation and epidermal growth factor receptor activation regulate vascular endothelial growth factor (VEGF) mRNA expression in human glioblastoma cells by transactivating the proximal VEGF promoter. Cancer Res. 2003;63(1):236–41. [PubMed] [Google Scholar]
- 38.Maity A, Pore N, Lee J, et al. Epidermal growth factor receptor transcriptionally up-regulates vascular endothelial growth factor expression in human glioblastoma cells via a pathway involving phosphatidylinositol 3′-kinase and distinct from that induced by hypoxia. Cancer Res. 2000;60(20):5879–86. [PubMed] [Google Scholar]
- 39.Ma D, Nutt CL, Shanehsaz P, et al. Autocrine platelet-derived growth factor-dependent gene expression in glioblastoma cells is mediated largely by activation of the transcription factor sterol regulatory element binding protein and is associated with altered genotype and patient survival in human brain tumors. Cancer Res. 2005;65(13):5523–34. doi: 10.1158/0008-5472.CAN-04-2582. [DOI] [PubMed] [Google Scholar]
- 40.Bergers G, Song S, Meyer-Morse N, et al. Benefits of targeting both pericytes and endothelial cells in the tumor vasculature with kinase inhibitors. J Clin Invest. 2003;111(9):1287–95. doi: 10.1172/JCI17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ostman A. PDGF receptors-mediators of autocrine tumor growth and regulators of tumor vasculature and stroma. Cytokine Growth Factor Rev. 2004;15(4):275–86. doi: 10.1016/j.cytogfr.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Abounader R, Laterra J. Scatter factor/hepatocyte growth factor in brain tumor growth and angiogenesis. Neuro Oncol. 2005;7(4):436–51. doi: 10.1215/S1152851705000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trojan J, Cloix JF, Ardourel MY, et al. Insulin-like growth factor type I biology and targeting in malignant gliomas. Neuroscience. 2007;145(3):795–811. doi: 10.1016/j.neuroscience.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 44.Clarke K, Smith K, Gullick WJ, Harris AL. Mutant epidermal growth factor receptor enhances induction of vascular endothelial growth factor by hypoxia and insulin-like growth factor-1 via a PI3 kinase dependent pathway. Br J Cancer. 2001;84(10):1322–9. doi: 10.1054/bjoc.2001.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun L, Hui AM, Su Q, et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9(4):287–300. doi: 10.1016/j.ccr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Li VW, Folkerth RD, Watanabe H, et al. Microvessel count and cerebrospinal fluid basic fibroblast growth factor in children with brain tumours. Lancet. 1994;344(8915):82–6. doi: 10.1016/s0140-6736(94)91280-7. [DOI] [PubMed] [Google Scholar]
- 47.Stefanik DF, Rizkalla LR, Soi A, et al. Acidic and basic fibroblast growth factors are present in glioblastoma multiforme. Cancer Res. 1991;51(20):5760–5. [PubMed] [Google Scholar]
- 48.Lamszus K, Heese O, Westphal M. Angiogenesis-related growth factors in brain tumors. Cancer Treat Res. 2004;117:169–90. doi: 10.1007/978-1-4419-8871-3_12. [DOI] [PubMed] [Google Scholar]
- 49.Pelloski CE, Lin E, Zhang L, et al. Prognostic associations of activated mitogen-activated protein kinase and Akt pathways in glioblastoma. Clin Cancer Res. 2006;12(13):3935–41. doi: 10.1158/1078-0432.CCR-05-2202. [DOI] [PubMed] [Google Scholar]
- 50.Vajkoczy P, Farhadi M, Gaumann A, et al. Microtumor growth initiates angiogenic sprouting with simultaneous expression of VEGF, VEGF receptor-2, and angiopoietin-2. J Clin Invest. 2002;109(6):777–85. doi: 10.1172/JCI14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grau SJ, Trillsch F, Herms J, et al. Expression of VEGFR3 in glioma endothelium correlates with tumor grade. J Neurooncol. 2007;82(2):141–50. doi: 10.1007/s11060-006-9272-4. [DOI] [PubMed] [Google Scholar]
- 52.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20(21):4368–80. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 53.Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100(12):3131–9. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 55.Rajantie I, Ilmonen M, Alminaite A, et al. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104(7):2084–6. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer; towards marker and target identification. Nat Rev Cancer. 2006;6(11):835–45. doi: 10.1038/nrc1971. [DOI] [PubMed] [Google Scholar]
- 57.Santarelli JG, Udani V, Yung CY, et al. Preuss Resident Research Award; bone marrow-derived Flk-1-expressing CD34+ cells contribute to the endothelium of tumor vessels in mouse brain. Clin Neurosurg. 2005;52:384–8. [PubMed] [Google Scholar]
- 58.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev. 2004;5(10):816–26. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 59.Lakka SS, Gondi CS, Rao JS. Proteases and glioma angiogenesis. Brain Pathol. 2005;15(4):327–41. doi: 10.1111/j.1750-3639.2005.tb00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Serini G, Valdembri D, Bussolino F. Integrins and angiogenesis: a sticky business. Expl Cell Res. 2006;312(5):651–8. doi: 10.1016/j.yexcr.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 61.Wang D, Anderson JC, Gladson CL. The role of the extracellular matrix in angiogenesis in malignant glioma tumors. Brain Pathol. 2005;15(4):318–26. doi: 10.1111/j.1750-3639.2005.tb00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bello L, Francolini M, Marthyn P, et al. α(v)β3 and α(v)β5 integrin expression in glioma periphery. Neurosurgery. 2001;49(2):380–9. doi: 10.1097/00006123-200108000-00022. discussion 90. [DOI] [PubMed] [Google Scholar]
- 63.Abdollahi A, Griggs DW, Zieher H, et al. Inhibition of α(v)β3 integrin survival signaling enhances antiangiogenic and antitumor effects of radiotherapy. Clin Cancer Res. 2005;11 (17):6270–9. doi: 10.1158/1078-0432.CCR-04-1223. [DOI] [PubMed] [Google Scholar]
- 64.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–50. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 65.Wedam SB, Low JA, Yang SX, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatorv and locally advanced breast cancer. J Clin Oncol. 2006;24(5):769–77. doi: 10.1200/JCO.2005.03.4645. [DOI] [PubMed] [Google Scholar]
- 66.Kindler HL, Friberg G, Singh DA, et al. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23(31):8033–40. doi: 10.1200/JCO.2005.01.9661. [DOI] [PubMed] [Google Scholar]
- 67.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from Phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3(1):24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 68.Willett CG, Kozin SV, Duda DG, et al. Combined vascular endothelial growth factor-targeted rherapy and radiotherapy for rectal cancer; theory and clinical practice. Semin Oncol. 2006;33(5 Suppl 10):S35–40. doi: 10.1053/j.seminoncol.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kerbel RS. Antiangiogenic rherapy: a universal chemosensitization strategy for cancer? Science. 2006;312(5777):1171–5. doi: 10.1126/science.1125950. [DOI] [PubMed] [Google Scholar]
- 70.Shaked Y, Kerbel RS. Antiangiogenic strategies on defense: on the possibility of blocking rebounds by the tumor vasculature after chemotherapy. Cancer Res. 2007;67(15):7055–8. doi: 10.1158/0008-5472.CAN-07-0905. [DOI] [PubMed] [Google Scholar]
- 71.Gorski DH, Beckett MA, Jaskowiak NT, et al. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects or ionizing radiation. Cancer Res. 1991;59(14):3374–8. [PubMed] [Google Scholar]
- 72.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7(9):987–9. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 73.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 74.Tong RT, Boucher Y, Kozin SV, et al. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64(11):3731–6. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 75.Jain RK, Safabakhsh N, Sckell A, et al. Endothelial cell death, angiogenesis, and microvascular function after castration in an androgen-dependent tumor: role of vascular endothelial growth factor. Proc Natl Acad Sci USA. 1995;95(18):10820–5. doi: 10.1073/pnas.95.18.10820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553–63. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 77.Willett CG, Boucher Y, di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10(2):145–7. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171 a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bao S, Wu Q, Sathornsumetee S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66(16):7843–8. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 80.Calabrese C, Poppleton H, Kocak M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 81.Folkins C, Man S, Xu P, et al. Anticancer therapies combining antiangiogenic and tumor cell cytotoxic effects reduce the tumor stem-like cell fraction in glioma xenograft tumors. Cancer Res. 2007;67(8):3560–4. doi: 10.1158/0008-5472.CAN-06-4238. [DOI] [PubMed] [Google Scholar]
- 82.Gilbertson RJ, Rich JN. Making a tumour’s bed: glioblastoma stem cells and the vascular niche. Nat Rev Cancer. 2007;7(10):733–6. doi: 10.1038/nrc2246. [DOI] [PubMed] [Google Scholar]
- 83.Stark-Vance V. In: Bigner DD, editor. Bevacizumab and CPT-11 in the Treatemtn of Relapsed Malignant Glioma; World Federation of Neuro-Oncology Second Quadrennial Meeting and the Sixth Meeting of the European Association for Neuro-Oncology; 2005; Edinburgh, Scotland: Duke University Press; 2005. p. 369. [Google Scholar]
- 84.Goli KJ, Desjardins A, Herndon JE, et al. Phase II trial of bevacizumab and irinotecan in the treatment of malignant gliomas. 43rd Annual Meeting of Amercian Society of Clinical Oncology; 2007; Chicago, IL: Grunberg MD, Steven; 2007. p. 75S. [Google Scholar]
- 85.Friedman HS, Petros WP, Friedman AH, et al. Irinotecan therapy in adults with recurrent or progressive malignant glioma. J Clin Oncol. 1999;17(5):1516–25. doi: 10.1200/JCO.1999.17.5.1516. [DOI] [PubMed] [Google Scholar]
- 86.Batchelor TT, Gilbert MR, Supko JG, et al. Phase 2 study of weekly irinotecan in adults with recurrent malignant glioma: final report of NABTT 97–11. Neuro Oncol. 2004;6(1):21–7. doi: 10.1215/S1152851703000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cloughesy TF, Filka E, Kuhn J, et al. Two studies evaluating irinotecan treatment for recurrent malignant glioma using an every-3-week regimen. Cancer. 2003;97(9 Suppl):2381–6. doi: 10.1002/cncr.11306. [DOI] [PubMed] [Google Scholar]
- 88.Prados MD, Lamborn K, Yung WK, et al. A phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study. Neuro Oncol. 2006;8(2):189–93. doi: 10.1215/15228517-2005-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chamberlain MC. Salvage chemorherapy with CPT-11 for recurrent glioblastoma multiforme. J Neurooncol. 2002;56(2):183–8. doi: 10.1023/a:1014532202188. [DOI] [PubMed] [Google Scholar]
- 90.Gilbert MR, Supko JG, Batchelor T, et al. Phase I clinical and pharmacokinetic study of irinotecan in adults with recurrent malignant glioma. Clin Cancer Res. 2003;9(8):2940–9. [PubMed] [Google Scholar]
- 91.MacDonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–80. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 92.Marras LC, Geerts WH, Perry JR. The risk of venous thromboembolism is increased throughout the course of malignant glioma; an evidence-based review. Cancer. 2000;89(3):640–6. doi: 10.1002/1097-0142(20000801)89:3<640::aid-cncr20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 93.Semrad TJ, O’Donnell R, Wun T, et al. Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg. 2007;106(4):601–8. doi: 10.3171/jns.2007.106.4.601. [DOI] [PubMed] [Google Scholar]
- 94.Simanek R, Vormittag R, Hassler M, et al. Venous thromboembolism and survival in patients with high-grade glioma. Neuro Oncol. 2007;9(2):89–95. doi: 10.1215/15228517-2006-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yung UK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83(5):588–93. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yung WK, Prados MD, Yaya-Tur R, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol. 1999;17(9):2762–71. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- 97.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17(8):2572–8. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 98.Pope WB, Lai A, Nghiemphu P, et al. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66(8):1258–60. doi: 10.1212/01.wnl.0000208958.29600.87. [DOI] [PubMed] [Google Scholar]
- 99.Chen W, Delaloye S, Silverman DH, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a Pilot Study. J Clin Oncol. 2007;25(30):4714–21. doi: 10.1200/JCO.2006.10.5825. [DOI] [PubMed] [Google Scholar]
- 100.Cloughesy T, Prodos M, Wen P, et al. A phase II, randomized, non-comparative clinical trial of bevacizumab alone or in combination with irinotecan prolongs 6-month PFS in recurrent, treatment-refractory glioblastoma. Presented at the 12th Annual Meeting of the Society for Neuro-Oncologyl; 2007; Dallas, TX. 2007. [Google Scholar]
- 101.Raiser J, Gallot L, Levy RM, et al. In: Yung A, editor. A phase II safety study of bevacizumab in patients with multiplie recurrent or progressive malignant gliomas; Twelfth Annual Meeting of the Society of Neuro-Oncology; 2007; Dallas, TX. [2007]. p. 530. [Google Scholar]
- 102.Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67(2):323–6. doi: 10.1016/j.ijrobp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 103.Rich JN, Sathornsumetee S, Keir ST, et al. ZD6474, a novel tyrosine kinase inhibitor of vascular endothelial growth factor receptor and epidermal growth factor receptor, inhibits tumor growth of multiple nervous system tumors. Clin Cancer Res. 2005;11(22):8145–57. doi: 10.1158/1078-0432.CCR-05-0319. [DOI] [PubMed] [Google Scholar]
- 104.Conrad C, Friedman HS, Reardon DA, et al. A Phase I/II trial of single-agent PTK 787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in patients with recurrent glioblastoma multiforme (GBM). 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition) J Clin Oncol. 2004;22(14S Suppl):1512. [Google Scholar]
- 105.Reardon DA, Friedman HS, Yung WKA, et al. A Phase I/II trial of PTK787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in combination with either temozolomide or lomustine for patients with recurrent glioblastoma multiforme (GBM). 2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition) J Clin Oncol. 2004;22(14S Suppl):1513. [Google Scholar]
- 106.Thomas AL, Morgan B, Horsfield MA, et al. Phase I study of the safety, tolerabiliry, pharmacokinetics, and pharmacodynamics of PTK787/ZK 222584 administered twice daily in patients with advanced cancer. J Clin Oncol. 2005;23(18):4162–71. doi: 10.1200/JCO.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 107.Konner J, Dupont J. Use of soluble recombinant decoy receptor vascular endothelial growth factor trap (VEGF Trap) to inhibit vascular endothelial growth factor activity. Clin Colorectal Cancer. 2004;4(Suppl 2):S81–5. doi: 10.3816/ccc.2004.s.013. [DOI] [PubMed] [Google Scholar]
- 108.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antirumor effects. Proc Natl Acad Sci USA. 2002;99(17):11393–8. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wachsberger PR, Burd R, Cardi C, et al. VEGF trap in combination with radiotherapy improves tumor control in u87 glioblastoma. Int J Radiat Oncol Biol Phys. 2007;67(5):1526–37. doi: 10.1016/j.ijrobp.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 110.Chen W, Silverman DH, Delaloye S, et al. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J Nucl Med. 2006;47(6):904–11. [PubMed] [Google Scholar]
- 111.Popperl G, Kreth FW, Herms J, et al. Analysis of 18F-FET PET for grading of recurrent gliomas: is evaluation of uptake kinetics superior to standard methods. J Nucl Med. 2006;47(3):393–403. [PubMed] [Google Scholar]
- 112.Chen W, Cloughesy T, Kamdar N, et al. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J Nucl Med. 2005;46(6):945–52. [PubMed] [Google Scholar]
- 113.Rock JP, Hearshen D, Scarpace L, et al. Correlations between magnetic resonance spectroscopy and image-guided histopathology, with special attention to radiation necrosis. Neurosurgery. 2002;51(4):912–9. doi: 10.1097/00006123-200210000-00010. discussion 9–20. [DOI] [PubMed] [Google Scholar]
- 114.Cha S, Yang L, Johnson G, et al. Comparison of microvascular permeability measurements, K(trans), determined with conventional steady-state T1-weighted and first-pass T2*-weighted MR imaging methods in gliomas and meningiomas. Am J Neuroradiol. 2006;27(2):409–17. [PMC free article] [PubMed] [Google Scholar]
- 115.Hylton N. Dynamic contrast-enhanced magnetic resonance imaging as an imaging biomarker. J Clin Oncol. 2006;24(20):3293–8. doi: 10.1200/JCO.2006.06.8080. [DOI] [PubMed] [Google Scholar]
- 116.Jackson A, O’Connor JP, Parker GJ, Jayson GC. Imaging tumor vascular heterogeneity and angiogenesis using dynamic contrast-enhanced magnetic resonance imaging. Clin Cancer Res. 2007;13(12):3449–59. doi: 10.1158/1078-0432.CCR-07-0238. [DOI] [PubMed] [Google Scholar]
- 117.Fuss M, Went F, Essig M, et al. Tumor angiogenesis of low-grade astrocytomas measured by dynamic susceptibility contrast-enhanced MRI (DSC-MRI) is predictive of local tumor control after radiation therapy. Int J Radiat Oncol Biol Phys. 2001;51(2):478–82. doi: 10.1016/s0360-3016(01)01691-1. [DOI] [PubMed] [Google Scholar]
- 118.Essig M, Wenz F, Scholdei R, et al. Dynamic susceptibility contrast-enhanced echo-planar imaging of cerebral gliomas. Effect of contrast medium extravasation. Acta Radiol. 2002;43(4):354–9. doi: 10.1080/j.1600-0455.2002.430402.x. [DOI] [PubMed] [Google Scholar]
- 119.Provenzale JM, Wang GR, Brenner T, et al. Comparison of permeability in high-grade and low-grade brain tumors using dynamic susceptibility contrast MR imaging. Am J Roentgenol. 2002;178(3):711–6. doi: 10.2214/ajr.178.3.1780711. [DOI] [PubMed] [Google Scholar]
- 120.Warmuth C, Gunther M, Zimmer C. Quantification of blood flow in brain tumors; comparison of arterial spin labeling and dynamic susceptibility-weighted contrast-enhanced MR imaging. Radiology. 2003;228(2):523–32. doi: 10.1148/radiol.2282020409. [DOI] [PubMed] [Google Scholar]
- 121.Brubaker LM, Bullitt E, Yin C, et al. Magnetic resonance angiography visualizacion of abnormal tumor vasculature in genetically engineered mice. Cancer Res. 2005;65(18):8218–23. doi: 10.1158/0008-5472.CAN-04-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bullitt E, Reardon DA, Smith JK. A review of micro- and macrovascular analyses in the assessment of tumor-associated vasculature as visualized by MR. Neuroimage. 2007;37(Suppl 1):S116–9. doi: 10.1016/j.neuroimage.2007.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jubb AM, Hurwitz HI, Bai W, et al. Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol. 2006;24(2):217–27. doi: 10.1200/JCO.2005.01.5388. [DOI] [PubMed] [Google Scholar]
- 124.Willett CG, Boucher Y, Duda DG, et al. Surrogate markers for antiangiogenic therapy and dose-limiting tonicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J Clin Oncol. 2005;23(31):8136–9. doi: 10.1200/JCO.2005.02.5635. [DOI] [PubMed] [Google Scholar]
- 125.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24(1):25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 126.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24(1):16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 127.Drevs J, Zirrgiebel U, Schmidt-Gersbach CI, et al. Soluble markers for the assessment of biological activity with PTK787/ZK 222584 (PTK/ZK), a vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor in patients with advanced colorectal cancer from two phase I trials. Ann Oncol. 2005;16(4):558–65. doi: 10.1093/annonc/mdi118. [DOI] [PubMed] [Google Scholar]
- 128.Duda DG, Cohen KS, di Tomaso E, et al. Differential CD146 expression on circulating versus tissue endothelial cells in rectal cancer patients: implications for circulating endothelial and progenitor cells as biomarkers for antiangiogenic therapy. J Clin Oncol. 2006;24(9):1449–53. doi: 10.1200/JCO.2005.04.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Erber R, Thurnher A, Katsen AD, et al. Combined inhibition of VEGF and PDGF signaling enforces turnor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004;18(2):338–40. doi: 10.1096/fj.03-0271fje. [DOI] [PubMed] [Google Scholar]
- 130.Huang J, Soffer SZ, Kim ES, et al. Vascular remodeling marks tumors that recur during chronic suppression of angiogenesis. Mol Cancer Res. 2004;2(1):36–42. [PubMed] [Google Scholar]
- 131.Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7(4):452–64. doi: 10.1215/S1152851705000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.De Bouard S, Guillamo JS, Christov C, et al. Antiangiogenic therapy against experimental glioblastoma using genetically engineered cells producing interferon-α, angiostatin, or endostatin. Human Gene Ther. 2003;14(9):883–95. doi: 10.1089/104303403765701178. [DOI] [PubMed] [Google Scholar]
- 133.Kunkel P, Ulbricht U, Bohlen P, et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001;61(18):6624–8. [PubMed] [Google Scholar]
- 134.Pennacchietti S, Michieli P, Galluzzo M, et al. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3(4):347–61. doi: 10.1016/s1535-6108(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 135.Lamszus K, Kunkel P, Westphal M. Invasion as limitation to anti-angiogenic glioma therapy. Acta Neurochir Suppl. 2003;88:169–77. doi: 10.1007/978-3-7091-6090-9_23. [DOI] [PubMed] [Google Scholar]
- 136.Rubenstein JL, Kim J, Ozawa T, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2(4):306–14. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bello L, Carrabba G, Giussani C, et al. Low-dose chemotherapy combined with an antiangiogenic drug reduces human glioma growth in vivo. Cancer Res. 2001;61(20):7501–6. [PubMed] [Google Scholar]
- 138.Farhadi MR, Capelle HH, Erber R, et al. Combined inhibition of vascular endothelial growth factor and platelet-derived growth factor signaling: effects on the angiogenesis, microcirculation, and growth of orthotopic malignant gliomas. J Neurosurg. 2005;102(2):363–70. doi: 10.3171/jns.2005.102.2.0363. [DOI] [PubMed] [Google Scholar]
- 139.de Bouard S, Herlin P, Christensen JG, et al. Antiangiogenic and anti-invasive effects of sunitinib on experimental human glioblastoma. Neuro Oncol. 2007;9(4):412–23. doi: 10.1215/15228517-2007-024. [DOI] [PMC free article] [PubMed] [Google Scholar]