Abstract
Background
Recent studies have demonstrated an association between moderate kidney dysfunction and sudden cardiac death in people with cardiovascular disease.
Methods and Results
The study was a longitudinal analysis among 4465 participants from the Cardiovascular Health Study without prevalent cardiovascular disease at baseline. Cystatin C and creatinine were measured from baseline sera. SCD was defined as a sudden pulseless condition from a cardiac origin in a previously stable individual that occurred out of the hospital or in the emergency room. The association between cystatin C tertiles and SCD was determined with multivariate Cox proportional hazards. A similar analysis compared SCD incidence across creatinine-based eGFR tertiles. Over a median follow-up of 11.2 years, 91 adjudicated SCD events occurred. The annual incidence of SCD events increased across cystatin C tertiles: 10 events per 10,000 person years in tertile 1, 25 events per 10,000 person years in tertile 2 and 32 events per 10,000 person years in the highest cystatin C tertile. These associations persisted after multivariate adjustment: [HR = 2.72, 95% CI (1.44–5.16) in tertile 2 and HR = 2.67, 95% CI (1.33–5.35) in tertile 3]. After multivariate adjustment, the rate of SCD also increased in a linear distribution across creatinine-based eGFR tertiles: 15 events per 10,000 person years in tertile 1, 22 events per 10,000 person years in tertile 2 and 27 events per 10,000 person years in tertile 3. No significant associations, however, remained between creatinine-based eGFR and SCD after multivariable adjustment.
Conclusion
Impaired kidney function, as measured by cystatin C, has an independent association with SCD risk among elderly persons without clinical cardiovascular disease.
Keywords: Cystatin C, kidney, sudden cardiac death, epidemiology
Introduction
Sudden cardiac death (SCD) is an important clinical and public health problem with over 450,000 Americans dying annually from this condition.1 Observational and post-mortem data indicate that coronary arterial abnormalities and their consequences are the cause of 80% of fatal arrhythmias. The presence and severity of underlying heart disease including coronary heart disease, chronic heart failure, and depressed left ventricular ejection fraction, are the most predictive risk factors for the future occurrence of SCD.2–6 In addition, population-based studies have demonstrated an association between traditional coronary heart disease risk factors including dyslipidemia, hypertension, cigarette smoking, physical inactivity, obesity, and a family history of premature coronary heart disease and SCD.7–12 Another population at high risk for SCD are persons with end-stage renal disease (ESRD).5 According to the US Renal Data System, about 22% of all deaths in persons with ESRD are caused by SCD, and the incidence increases with age: 2% per year for ages 20 to 44 years, 3.7% per year for ages 45 to 64 years, and 7% per year for ages 65 years and older.5, 13
Recent observational studies have demonstrated an association between moderate kidney dysfunction and SCD risk in people with cardiovascular disease.14–17 The majority of these participants had a history of myocardial infarction and congestive heart failure; as a result, it is difficult to know whether chronic kidney dysfunction was merely a marker of cardiovascular disease or an independent predictor of risk. As a result, we sought to evaluate whether impaired kidney function predicts SCD events among people in the community without cardiovascular disease. We hypothesized that impaired kidney function measured using creatinine-based estimated glomerular filtration rate (eGFR) and cystatin C would have an independent association with SCD incidence. We investigated this hypothesis among elderly participants in the Cardiovascular Health Study (CHS) without prevalent cardiovascular disease.
Methods
Design
The CHS18 is a community-based study of cardiovascular disease risk in ambulatory elderly persons, sponsored by the National Institute of Health. This analysis evaluates the longitudinal association between baseline kidney function and subsequent sudden cardiac death events.
Study Population
The CHS recruited participants from Medicare eligibility lists in Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Allegheny County, Pennsylvania. Recruiting participants from these four US communities allowed for a mixture of urban and rural populations and adequate numbers of both men and women. To be eligible, persons had to be at least 65 years of age, not institutionalized, expected to remain in the current community for three years or longer, not under active treatment for cancer, and able to provide written informed consent. The initial 5201 participants were enrolled from January 1989 to June 1990; an additional 687 black participants (with race self-reported) were recruited 1992–1993 and enrolled by June 1993. This analysis excluded those persons with prevalent clinical cardiovascular disease, defined as a history of MI or CHF, at entry into the cohort. There remained 4482 participants with baseline measures of kidney function. Missing data constituted 0.4% of the data leaving a sample size of 4465 participants for this project. All participants provided written informed consent, and the institutional review boards at all participating sites approved the study protocol.
Participants underwent a comprehensive examination at baseline, which included a thorough medical history, physical examination, laboratory testing, a 12-lead ECG, and assessment of cardiovascular disease status. Of the participants in this study, 3887 had a baseline transthoracic echocardiographic study to evaluate for left ventricular ejection fraction. The study design, quality-control procedures, laboratory methods, and procedures for blood-pressure measurement have been published previously.18, 19
Sudden cardiac death
Sudden cardiac death (SCD) was defined as a sudden pulseless condition from a cardiac origin in a previously stable individual occurring out of the hospital or in the emergency room. These cases could not have life-threatening, noncardiac comorbidities or be under hospice or nursing home care. SCD cases were identified and adjudicated by a cardiologist’s record review of all cardiac deaths; survivors or successfully resuscitated events were not included in the definition of SCD. A second physician conducted blind review of a sample of 70 potential cases with an 88% inter-reviewer agreement and kappa value of 0.74 for SCD.20
Kidney Function Assays
Measurements were performed on fasting sera specimens that had been stored at −70 degrees Celsius. Cystatin C was measured by a particle-enhanced immunonephelometric assay (N Latex Cystatin C, Dade Behring) with a nephelometer (BNII, Dade Behring).21 The assay range is 0.195 to 7.330 mg/L, with the reference range for young, healthy individuals reported as 0.53–0.95 mg/L. The assay was demonstrated to remain stable over 5 cycles of freeze/thaw without change in the measurement.21, 22
Serum creatinine was assayed by a colorimetric method (Ektachem 700, Eastman Kodak). The mean coefficient of variation for monthly controls was 1.94 percent (range, 1.16 to 3.90). We estimated the creatinine-based GFR with the use of the four-variable version of the Modification of Diet in Renal Disease (MDRD) equation.23 Prior to estimating the GFR, creatinine levels were indirectly calibrated to the Cleveland Clinic lab as previously described.24
Cystatin C and creatinine-based eGFR were categorized into tertiles in order to maintain an equal distribution of participants across kidney function measures and to ensure a sufficient number of SCD events within each group. We also calculated a cystatin C-based eGFR for each cystatin C tertile using a recently derived and validated equation.25 We then defined a subset of participants with preclinical kidney disease using both cystatin C and creatinine-based eGFR measurements. The three groups included the following: chronic kidney disease (eGFR < 60 ml/min/1.73m2), preclinical kidney disease (eGFR > 60 ml/min/1.73m2 and cystatin C >1.0mg/L) and normal kidney function (eGFR > 60 ml/min/1.73m2 and cystatin C <1.0mg/L) as in prior studies.26
Statistical Methods
Baseline characteristics of participants were compared across cystatin C tertiles using chi-squared or ANOVA tests. The association between cystatin C and SCD was determined with multivariate Cox proportional hazards regression models that compared each ascending tertile of cystatin C with the lowest one. A similar evaluation was performed to determine the association between creatinine-based eGFR tertiles and SCD. In these multivariate models, candidate variables for adjustment were retained in the final models if they changed the beta coefficient of the primary predictor of interest (kidney function) by at least 5 percent. In addition, we adjusted for covariates that are known to be associated with sudden cardiac death.
Covariates were selected as candidates for multivariate analysis based on their potential to confound the association of kidney function with SCD or for their independent association with SCD based on prior studies. Specifically, the following variables from table 1 changed the coefficient of cystatin C by at least 5% and were included in the multivariate analysis: diabetes, systolic blood pressure, diastolic blood pressure, LVH, calcium channel blockers, diuretics and aspirin use. In addition age, gender and race (self-reported) were forced into the multivariate models. Finally, current smoking, measures of dyslipidemia including LDL and HDL, body mass index, alcohol use, a family history of cardiovascular disease and physical activity are variables known to be associated with SCD and were included in the multivariate analysis. 7, 8, 11, 12, 27 Other baseline variables such as ECG metrics and medications including ACE inhibitors, beta blockers, diuretics, calcium channel blockers, digitalis, and statins were not included in the multivariate model as they did not effect the association between kidney dysfunction and SCD. Incident MI and CHF were analyzed as time-dependent covariates in the multivariate analysis. Additional adjustment for left ventricular ejection fraction was performed to evaluate SCD risk among those participants who had baseline echocardiograms.
Table 1.
Baseline characteristics across Cystatin C Tertiles
| Cystatin C Tertiles | ||||
|---|---|---|---|---|
| ≤ 0.91 mg/L | 0.92–1.09 mg/L | ≥1.10 mg/L | p-value (trend) | |
| N | 1579 | 1582 | 1304 | |
| Age (yr; mean ± SD) | 71 ± 4 | 72 ± 5 | 75 ± 6 | <0.001 |
| Men (n [%]) | 479 (30) | 605 (38) | 549 (42) | <0.001 |
| Black (n [%]) | 297 (19) | 239 (15) | 198 (15) | 0.006 |
| Smoking | 0.011 | |||
| Former (n [%]) | 656 (42) | 615 (39) | 519 (40) | |
| Current (n [%]) | 158 (10) | 205 (13) | 188 (14) | |
| Alcohol use (beverages/wk; median [interquartile range]) | 0.04 [0, 2.00] | 0.02 [0, 1.25] | 0 [0, 0.75] | <0.001 |
| BMI (kg/m2; mean ± SD) | 25.9 ± 4.2 | 26.8 ± 4.8 | 27.6 ± 5.1 | <0.001 |
| Physical activity (Kcals/wk; median [interquartile range]) | 1238 [489, 2528] | 1134 [438, 2446] | 810 [270, 1835] | <0.001 |
| SBP (mmHg; mean ± SD) | 134 ± 21 | 135 ± 21 | 139 ± 22 | <0.001 |
| DBP (mmHg; mean ± SD) | 71 ± 11 | 71 ± 11 | 71 ± 12 | 0.755 |
| Hypertension (n [%]) | 809 (51) | 869 (55) | 927 (71) | <0.001 |
| Diabetes (n [%]) | 214 (14) | 203 (13) | 221 (17) | 0.027 |
| Potassium (mMol/dl; mean ± SD) | 4.1 ± 0.4 | 4.1 ± 0.4 | 4.2 ± 0.4 | <0.001 |
| LDL (mg/dl; mean ± SD) | 130 ± 34 | 132 ± 36 | 130 ± 37 | 0.541 |
| HDL (mg/dl; mean ± SD) | 60 ± 16 | 55 ±15 | 51 ±15 | <0.001 |
| Family history of CV (n [%]) | 431 (27) | 449 (28) | 394 (30) | 0.073 |
| Electrocardiographic Metrics | ||||
| AF (n [%]) | 14 (1) | 29 (2) | 42 (3) | <0.001 |
| LVH | 54 (3) | 40 (3) | 79 (6) | 0.001 |
| QT interval (msec; mean ± SD) | 416 ± 32 | 415 ± 34 | 414 ± 38 | 0.232 |
| LBBB (n [%]) | 20 (1) | 22 (1) | 24 (2) | 0.212 |
| Medications | ||||
| ACE-I (n [%]) | 79 (5) | 94 (6) | 117 (9) | <0.001 |
| Diuretics (n [%]) | 302 (19) | 377 (24) | 534 (41) | <0.001 |
| B-blockers (n [%]) | 137 (9) | 166 (10) | 218 (17) | <0.001 |
| Calcium-channel blockers (n [%]) | 161 (10) | 158 (10) | 169 (13) | 0.029 |
| Aspirin (n [%]) | 37 (2) | 37 (2) | 50 (4) | 0.047 |
| Statins (n [%]) | 48 (3) | 26 (2) | 20 (2) | 0.005 |
| Measures of kidney function | ||||
| Cystatin C (mg/L; mean ± SD) | 0.81 ± 0.07 | 1.00 ± 0.05 | 1.34 ± 0.35 | NA |
| Creatinine (mg/dL; mean ± SD) | 0.77 ± 0.19 | 0.90 ± 0.20 | 1.18 ± 0.52 | <0.001 |
| Creatinine-based eGFR (ml/min per 1.73 m2; mean ± SD) | 94 ± 23 | 79 ± 17 | 62 ± 18 | <0.001 |
BMI indicates body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; LVH, left ventricular hypertrophy; LBBB, left bundle branch block; ACE-I, angiotensin converting enzyme inhibitors; and eGFR, estimated glomerular filtration rate
The proportional hazards assumption was not violated in any of these analyses. Model predictiveness was evaluated using the c-statistic. Bootstrapping methods were also implemented to evaluate over-fitting of the data. S-Plus (release 6.1, Insightful Inc, Seattle, WA) and SPSS statistical software (release 15.0, SPSS Inc, Chicago, IL) were used for the analyses. A P value <0.05 was considered statistically significant.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Baseline Characteristics
Participants in the highest cystatin C tertile were on average older and more likely to be male and white instead of black (table 1). In addition, they had higher BMI, higher systolic blood pressure, a lower HDL and were more likely to have a history of hypertension, diabetes, atrial fibrillation and left ventricular hypertrophy (LVH). Participants with higher cystatin C were more likely to be on ACE inhibitors, diuretics, beta blockers, calcium channel blockers, digitalis and aspirin. They also had a higher likelihood of having a family history of cardiovascular disease. Creatinine levels increased and eGFR decreased across cystatin C tertiles. Participants in the highest cystatin C tertile had a mean creatinine-based eGFR of 63 ml/min/1.73m2. Finally, nearly all participants had normal left ventricular ejection fraction as assessed by transthoracic echocardiography.
SCD Incidence
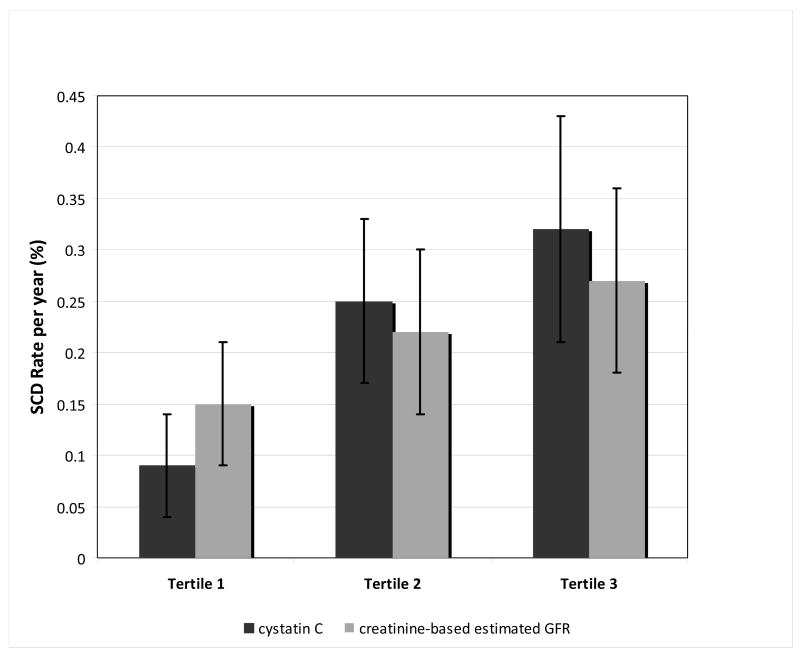
Among the 4465 subjects, 91 adjudicated SCD events occurred over a median follow-up of 11.2 years. The incidence of SCD events increased across cystatin C tertiles: 0.10% (per year), 0.25%, and 0.32% (figure 1). An increase in SCD risk was also observed across creatinine-based eGFR tertiles: 0.15%/year, 0.22%/year, and 0.27%/year. SCD risk remained increased in those with elevated cystatin C concentrations in both unadjusted and adjusted analysis. The risk for SCD increased more than 3-fold across cystatin C tertiles in unadjusted analysis (table 2). After adjusting for multiple variables including incident CHF and MI, cystatin C tertiles retained an independent risk of SCD. In addition, among the 3887 participants with baseline echocardiograms, cystatin C remained an independent predictor of SCD after adding left ventricular ejection fraction to the multivariate model: [HR = 3.25, 95% CI (1.54–6.85) in tertile 2; HR = 2.82, 95% CI (1.26–6.32) in tertile 3]. Finally, only the highest creatinine-based eGFR tertile was predictive of SCD risk in unadjusted analysis; however, it did not achieve statistical significance after multivariate adjustment (table 3).
Figure 1. Sudden cardiac death rate per year.
x-axis: cystatin C and creatinine-based estimated GFR tertiles
y-axis: sudden cardiac death rates per year (% per year)
Table 2.
Association of Cystatin C with Sudden Cardiac Death
| Measure and Outcome Cystatin C and SCD | Tertile 1 | Tertile 2 | Tertile 3 |
|---|---|---|---|
| Cystatin C (mg/liter) | ≤ 0.91 | 0.92–1.09 | ≥1.10 |
| Cystatin-based eGFR* (ml/min/1.73m2) | ≥86 | 69–85 | ≤68 |
| No. of participants | 1579 | 1582 | 1304 |
| No of SCD events | 15 | 40 | 36 |
| Hazard Ratio (95% CI) | |||
| Unadjusted | 1.00 | 3.13 (1.67, 5.86) | 3.92 (2.05, 7.47) |
| Adjusted† | 1.00 | 2.72 (1.44, 5.16) | 2.67 (1.33, 5.35) |
Cystatin based estimated glomerular filtration rate (eGFR) = 76.7 × cystatin C−1.19
Adjusted for age, gender, race, current smoking, alcohol use, LDL, HDL, diabetes, systolic blood pressure, diastolic blood pressure, calcium channel blockers, diuretics, aspirin, left ventricular hypertrophy, physical activity, family history of cardiovascular disease, and myocardial infarction and congestive heart failure as time dependent covariates
Table 3.
Association of creatinine-based estimated GFR with Sudden Cardiac Death
| Measure and Outcome Creatinine-based eGFR and SCD | Tertile 1 | Tertile 2 | Tertile 3 |
|---|---|---|---|
| Range of Values (ml/min/1.73m2) | ≥88 | 68–87 | ≤67 |
| No. of participants | 1497 | 1481 | 1487 |
| No of SCD events | 23 | 31 | 37 |
| Hazard Ratio (95% CI) | |||
| Unadjusted | 1.00 | 1.55 (0.88, 2.75) | 2.00 (1.16, 3.47) |
| Adjusted* | 1.00 | 1.31 (0.73, 2.36) | 1.65 (0.93, 2.94) |
Adjusted for age, gender, race, current smoking, alcohol use, LDL, HDL, diabetes, systolic blood pressure, diastolic blood pressure, calcium channel blockers, diuretics, aspirin, left ventricular hypertrophy, physical activity, family history of cardiovascular disease, and myocardial infarction and congestive heart failure as time dependent covariates
To assess the predictiveness of this model, the c-statistic was applied to a fully adjusted model with and without cystatin C. There was a significant improvement upon adding cystatin C to the fully adjusted model (p=0.002) with a c-statistic of 0.6735 (fully adjusted) versus 0.6847 (with the addition of cystatin C). In addition, bootstrapping of the fully adjusted Cox model was performed to assess over-fitting of the data. A histogram of the mean deviance residuals from the resultant bootstrapped sample centered on the vertical line indicates that the model behaves well. Further, the bootstrap estimates (using 100 re-samples) indicate that the model was not over-fitted (original Somer’s D = −0.37 versus corrected Somer’s D = −0.35).
SCD risk among those with preclinical kidney disease
After excluding persons with CKD (creatinine-based eGFR < 60 ml/min/1.73m2), participants with preclinical kidney disease (creatinine-based eGFR ≥ 60 ml/min/1.73m2 and cystatin C ≥ 1.0 mg/L) had a two-fold SCD risk compared to those without CKD and low cystatin C concentrations [HR 2.68, 95% CI (1.53 – 4.69) in unadjusted analysis and HR 1.99, 95% CI (1.11 – 3.57) after multivariate adjustment]. This risk increase was similar to that of CKD compared with the normal kidney function (creatinine-based eGFR ≥ 60 mL/min/1.73m2 and cystatin C < 1.0 mg/L) referent group (HR=2.37; 95% CI, 1.41 to 3.98 in unadjusted analysis and HR=2.09; 95% CI, 1.21 to 3.61 after multivariate adjustment).
Discussion
In this community-based study of ambulatory older adults, impaired kidney function was associated with increased risk of SCD. There was an increased SCD risk among those participants with elevated cystatin C concentrations after multivariate analysis including adjustment for left ventricular ejection fraction. This elevated risk is similar in the second and third cystatin C tertiles. Whether tertile 1 is protective or tertiles 2 and 3 are at greater risk is difficult to distinguish. The cystatin C levels in tertile 2 (0.92 – 1.09 mg/L), however, would generally be considered abnormal and likely reflect the adverse cardiovascular consequences of impaired kidney function. Interestingly, participants meeting the definition of “preclinical kidney disease” had elevated risks of SCD that were equivalent to participants with CKD.
These findings extend the range of adverse cardiovascular events associated with elevated cystatin C concentrations also to include sudden cardiac death. Prior studies have demonstrated an independent association between elevated cystatin C concentrations and both cardiovascular and non-cardiovascular mortality.22, 26, 28 The spontaneous nature of SCD events, however, suggests a distinct process from other types of cardiovascular death where long-standing symptoms associated with pump failure and ischemia are often present. In addition the adjudication for SCD focused on people who were not hospitalized, which distinguishes it from all other cardiovascular events. Further, we excluded participants with prevalent cardiovascular disease to enhance the specificity for identifying arrhythmic events such as ventricular tachycardia or ventricular fibrillation. To further minimize the confounding effects of cardiovascular disease, we also adjusted for incident myocardial infarction and congestive heart failure. Finally, these findings extend the association of kidney dysfunction and sudden cardiac death risk to the setting of preclinical kidney disease. Previous studies have demonstrated an association between end stage and chronic kidney disease to SCD15–17 especially in patients with advanced heart failure and coronary disease. Even mild kidney dysfunction is associated with a range of adverse cardiovascular events including SCD.
Several findings from our study also suggest that SCD may be a direct result of kidney dysfunction. The temporal association between kidney disease and SCD in this cohort study suggests that it may be a causal one. The increased risk of SCD observed in this study may have been attributable to higher rates of malignant ventricular arrhythmias.29 Prior studies have demonstrated the increased prevalence of left ventricular hypertrophy, systolic dysfunction and diastolic dysfunction among patients with kidney disease including those with elevated cystatin C concentrations.30 Though we adjusted for LVH and systolic dysfunction, other structural changes may be present in the absence of clinical heart failure and may contribute to greater cardiac fibrosis and arrhythmia risk.30, 31 In addition, autonomic dysfunction, myocyte dysfunction and altered electrolyte metabolism may contribute to arrhythmic risk in patients with kidney dysfunction.32–34
Our findings suggest that cystatin C levels and the corresponding cystatin-based eGFR provide a stronger estimate of the risk of sudden cardiac death among elderly persons than creatinine-based estimates. Creatinine is an insensitive measure of kidney function in elderly persons among whom lean muscle mass comprises a smaller and unpredictable proportion of body mass. Prior studies have also demonstrated that cystatin C levels are superior to creatinine and creatinine-based estimates of GFR for predicting adverse cardiovascular events among elderly individuals.22 In this analysis, we also found cystatin C to have a linear association with the risk of sudden cardiac death. This finding contrasts with traditional measures of renal function where the risk of adverse events increased only when GFR dropped below a threshold of 60 ml per minute per 1.73 m2 of body-surface area.35–37 Cystatin C is most likely a stronger predictor of adverse cardiovascular events in the elderly because it provides a better approximation of GFR across the spectrum of kidney function.
Elevated cystatin C concentrations also capture a state of preclinical kidney disease that is highly prevalent among this population-based cohort of ambulatory, elderly persons. A preclinical state refers to a specific condition that precedes the development of overt, clinical disease. Preclinical disease states have been associated with adverse health consequences;26, 38–42 therefore, their identification can have important clinical consequences. Participants in our study without chronic kidney disease (estimated GFR ≥60 ml/min per 1.73m2) and elevated cystatin C levels were at substantially increased risk for sudden cardiac death events during the follow-up period. Whether kidney disease is labeled as preclinical or undetected, elevated cystatin C concentrations were independently associated with sudden cardiac death events among participants without chronic kidney disease. Because an estimated GFR less than 60 ml/min per 1.73 m2 seems specific for defining abnormal kidney function in elderly persons, we believe it remains the appropriate initial screening measure for kidney dysfunction. Measuring cystatin C concentrations in persons with an estimated GFR greater than 60 mL/min per 1.73 m2 may be a useful test for further defining kidney function and for distinguishing levels of cardiovascular risk.
Several limitations of the current study should be considered. Since CHS consisted solely of elderly persons, the described associations of cystatin C with SCD cannot necessarily be extrapolated to younger populations. In addition, as with any observational study, there is likely to be residual confounding in the association of cystatin C with SCD. For example, a variety of unmeasured factors might modify the associations between higher cystatin C concentrations and sudden death. Finally, this study cannot distinguish the extent to which the observed associations of cystatin C with SCD reflect its approximation of GFR versus a direct or indirect pathologic link from cystatin C to cardiovascular risk that is independent of kidney function.
The findings from our study provide insight that SCD may be related to impaired kidney function. These results suggest that even mild reductions in kidney function, evidenced by higher cystatin C levels, can alter the electrophysiologic properties of the myocardium and increase the risk for SCD, without any clinical evidence of CHF, coronary disease or cardiac structure. Future studies should investigate candidate mechanisms to explain the SCD risk of mild to moderate kidney dysfunction.
Acknowledgments
Funding Sources
The research reported in this article was supported by contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke and R01 AG027002. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
Footnotes
Disclosures: None
References
- 1.Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 2.Traven ND, Kuller LH, Ives DG, Rutan GH, Perper JA. Coronary heart disease mortality and sudden death: trends and patterns in 35- to 44-year-old white males, 1970–1990. Am J Epidemiol. 1995;142:45–52. doi: 10.1093/oxfordjournals.aje.a117544. [DOI] [PubMed] [Google Scholar]
- 3.Gillum RF. Sudden coronary death in the United States: 1980–1985. Circulation. 1989;79:756–765. doi: 10.1161/01.cir.79.4.756. [DOI] [PubMed] [Google Scholar]
- 4.Cupples LA, Gagnon DR, Kannel WB. Long- and short-term risk of sudden coronary death. Circulation. 1992;85:I11–18. [PubMed] [Google Scholar]
- 5.Collins AJ, Kasiske B, Herzog C, Chen SC, Everson S, Constantini E, Grimm R, McBean M, Xue J, Chavers B, Matas A, Manning W, Louis T, Pan W, Liu J, Li S, Roberts T, Dalleska F, Snyder J, Ebben J, Frazier E, Sheets D, Johnson R, Dunning S, Berrini D, Guo H, Solid C, Arko C, Daniels F, Wang X, Forrest B, Gilbertson D, St Peter W, Frederick P, Eggers P, Agodoa L. Excerpts from the United States Renal Data System 2003 Annual Data Report: atlas of end-stage renal disease in the United States. Am J Kidney Dis. 2003;42:A5–7. S1–230. [PubMed] [Google Scholar]
- 6.Kannel WB, Wilson PW, D’Agostino RB, Cobb J. Sudden coronary death in women. American Heart Journal. 1998;136:205–212. doi: 10.1053/hj.1998.v136.90226. [DOI] [PubMed] [Google Scholar]
- 7.Albert CM, Chae CU, Grodstein F, Rose LM, Rexrode KM, Ruskin JN, Stampfer MJ, Manson JE. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107:2096–2101. doi: 10.1161/01.CIR.0000065223.21530.11. [DOI] [PubMed] [Google Scholar]
- 8.Friedlander Y, Siscovick DS, Weinmann S, Austin MA, Psaty BM, Lemaitre RN, Arbogast P, Raghunathan TE, Cobb LA. Family history as a risk factor for primary cardiac arrest. Circulation. 1998;97:155–160. doi: 10.1161/01.cir.97.2.155. [DOI] [PubMed] [Google Scholar]
- 9.Jouven X, Desnos M, Guerot C, Ducimetiere P. Predicting sudden death in the population: the Paris Prospective Study I. Circulation. 1999;99:1978–1983. doi: 10.1161/01.cir.99.15.1978. [DOI] [PubMed] [Google Scholar]
- 10.Kannel WB, Thomas HE., Jr Sudden coronary death: the Framingham Study. Annals of the New York Academy of Sciences. 1982;382:3–21. doi: 10.1111/j.1749-6632.1982.tb55203.x. [DOI] [PubMed] [Google Scholar]
- 11.Siscovick DS, Weiss NS, Fletcher RH, Lasky T. The incidence of primary cardiac arrest during vigorous exercise. The New England Journal of Medicine. 1984;311:874–877. doi: 10.1056/NEJM198410043111402. [DOI] [PubMed] [Google Scholar]
- 12.Siscovick DS, Weiss NS, Hallstrom AP, Inui TS, Peterson DR. Physical activity and primary cardiac arrest. JAMA. 1982;248:3113–3117. [PubMed] [Google Scholar]
- 13.Meier P, Vogt P, Blanc E. Ventricular arrhythmias and sudden cardiac death in end-stage renal disease patients on chronic hemodialysis. Nephron. 2001;87:199–214. doi: 10.1159/000045917. [DOI] [PubMed] [Google Scholar]
- 14.Deo R, Fyr CL, Fried LF, Newman AB, Harris TB, Angleman S, Green C, Kritchevsky SB, Chertow GM, Cummings SR, Shlipak MG. Kidney dysfunction and fatal cardiovascular disease-an association independent of atherosclerotic events: results from the Health, Aging, and Body Composition (Health ABC) study. Am Heart J. 2008;155(1):62–68. doi: 10.1016/j.ahj.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Goldenberg I, Moss AJ, McNitt S, Zareba W, Andrews ML, Hall WJ, Greenberg H, Case RB. Relations among renal function, risk of sudden cardiac death, and benefit of the implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am J Cardiol. 2006;98:485–490. doi: 10.1016/j.amjcard.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 16.Saxon LA, Bristow MR, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, Feldman AM, Galle E, Ecklund F. Predictors of sudden cardiac death and appropriate shock in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Trial. Circulation. 2006;114:2766–2772. doi: 10.1161/CIRCULATIONAHA.106.642892. [DOI] [PubMed] [Google Scholar]
- 17.Deo R, Lin F, Vittinghoff E, Tseng ZH, Hulley SB, Shlipak MG. Kidney dysfunction and sudden cardiac death among women with coronary heart disease. Hypertension. 2008;51:1578–1582. doi: 10.1161/HYPERTENSIONAHA.107.103804. [DOI] [PubMed] [Google Scholar]
- 18.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 19.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–270. [PubMed] [Google Scholar]
- 20.Sotoodehnia N, Siscovick DS, Vatta M, Psaty BM, Tracy RP, Towbin JA, Lemaitre RN, Rea TD, Durda JP, Chang JM, Lumley TS, Kuller LH, Burke GL, Heckbert SR. Beta2-adrenergic receptor genetic variants and risk of sudden cardiac death. Circulation. 2006;113:1842–1848. doi: 10.1161/CIRCULATIONAHA.105.582833. [DOI] [PubMed] [Google Scholar]
- 21.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59:1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 22.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C. Cystatin C and the risk of death and cardiovascular events among elderly persons. The New England Journal of Medicine. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 24.Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, Bleyer A, Newman A, Siscovick D, Psaty B. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA. 2005;293:1737–1745. doi: 10.1001/jama.293.14.1737. [DOI] [PubMed] [Google Scholar]
- 25.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shlipak MG, Katz R, Sarnak MJ, Fried LF, Newman AB, Stehman-Breen C, Seliger SL, Kestenbaum B, Psaty B, Tracy RP, Siscovick DS. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Annals of Internal Medicine. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 27.Whang W, Manson JE, Hu FB, Chae CU, Rexrode KM, Willett WC, Stampfer MJ, Albert CM. Physical exertion, exercise, and sudden cardiac death in women. JAMA. 2006;295:1399–1403. doi: 10.1001/jama.295.12.1399. [DOI] [PubMed] [Google Scholar]
- 28.Fried LF, Katz R, Sarnak MJ, Shlipak MG, Chaves PH, Jenny NS, Stehman- Breen C, Gillen D, Bleyer AJ, Hirsch C, Siscovick D, Newman AB. Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol. 2005;16:3728–3735. doi: 10.1681/ASN.2005040384. [DOI] [PubMed] [Google Scholar]
- 29.McCullough PA, Sandberg KR. Chronic kidney disease and sudden death: strategies for prevention. Blood Purif. 2004;22:136–142. doi: 10.1159/000074934. [DOI] [PubMed] [Google Scholar]
- 30.Ix JH, Shlipak MG, Chertow GM, Ali S, Schiller NB, Whooley MA. Cystatin C, left ventricular hypertrophy, and diastolic dysfunction: data from the Heart and Soul Study. J Card Fail. 2006;12:601–607. doi: 10.1016/j.cardfail.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levin A, Singer J, Thompson CR, Ross H, Lewis M. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. Am J Kidney Dis. 1996;27:347–354. doi: 10.1016/s0272-6386(96)90357-1. [DOI] [PubMed] [Google Scholar]
- 32.Tamura K, Tsuji H, Nishiue T, Tokunaga S, Yajima I, Higashi T, Iwasaka T. Determinants of ventricular arrhythmias in hemodialysis patients. Evaluation of the effect of arrhythmogenic substrate and autonomic imbalance. Am J Nephrol. 1998;18:280–284. doi: 10.1159/000013351. [DOI] [PubMed] [Google Scholar]
- 33.Tun A, Khan IA, Wattanasauwan N, Win MT, Hussain A, Hla TA, Cherukuri VL, Vasavada BC, Sacchi TJ. Increased regional and transmyocardial dispersion of ventricular repolarization in end-stage renal disease. Can J Cardiol. 1999;15:53–56. [PubMed] [Google Scholar]
- 34.Roithinger FX, Punzengruber C, Rossoll M, Pachinger O, Kramar R, Prischl FC. Ventricular late potentials in haemodialysis patients and the risk of sudden death. Nephrol Dial Transplant. 1992;7:1013–1018. [PubMed] [Google Scholar]
- 35.Fried LP, Kronmal RA, Newman AB, Bild DE, Mittelmark MB, Polak JF, Robbins JA, Gardin JM. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 36.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 37.Shlipak MG, Smith GL, Rathore SS, Massie BM, Krumholz HM. Renal function, digoxin therapy, and heart failure outcomes: evidence from the digoxin intervention group trial. J Am Soc Nephrol. 2004;15:2195–2203. doi: 10.1097/01.ASN.0000135121.81744.75. [DOI] [PubMed] [Google Scholar]
- 38.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 39.Vasan RS, Larson MG, Leip EP, Kannel WB, Levy D. Assessment of frequency of progression to hypertension in non-hypertensive participants in the Framingham Heart Study: a cohort study. Lancet. 2001;358:1682–1686. doi: 10.1016/S0140-6736(01)06710-1. [DOI] [PubMed] [Google Scholar]
- 40.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002;19:708–723. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 41.Kanaya AM, Herrington D, Vittinghoff E, Lin F, Bittner V, Cauley JA, Hulley S, Barrett-Connor E. Impaired fasting glucose and cardiovascular outcomes in postmenopausal women with coronary artery disease. Ann Intern Med. 2005;142:813–820. doi: 10.7326/0003-4819-142-10-200505170-00006. [DOI] [PubMed] [Google Scholar]
- 42.Hu FB, Stampfer MJ, Haffner SM, Solomon CG, Willett WC, Manson JE. Elevated risk of cardiovascular disease prior to clinical diagnosis of type 2 diabetes. Diabetes Care. 2002;25:1129–1134. doi: 10.2337/diacare.25.7.1129. [DOI] [PubMed] [Google Scholar]