Abstract
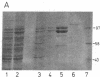
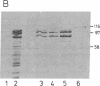
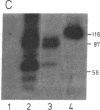
The gene for human nuclear NAD+ ADP-ribosyltransferase [NAD+:poly(adenosine diphosphate D-ribose) ADP-D-ribosetransferase, EC 2.4.2.30; pADPRT] was localized to chromosome 1 at q41-q42 by in situ hybridization with a pADPRT-specific cDNA probe. Expression of a pADPRT cDNA under control of the lac promoter in Escherichia coli induces the synthesis of a group of related proteins that were immunoreactive with pADPRT antibody and that had catalytic properties very similar to those of the human enzyme. Purification of this enzymatic activity was performed essentially as described for the human enzyme. The Km, pH optimum, optimal reaction temperature, and inhibition by 3-aminobenzamide and 3-methoxybenzamide were found to be similar for the recombinant and the human enzymes. The purified recombinant enzyme consists of two major proteins of Mr 99,000 and Mr 89,000. Both proteins show pADPRT activity in activity gel analysis with [32P]NAD+ as substrate. Microsequencing of these two proteins isolated by denaturing gel electrophoresis and deletion mutagenesis of the pADPRT expression plasmid shows that the Mr 99,000 and Mr 89,000 proteins derive from initiation of translation at internal translational start signals located within the pADPRT cDNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkhatib H. M., Chen D. F., Cherney B., Bhatia K., Notario V., Giri C., Stein G., Slattery E., Roeder R. G., Smulson M. E. Cloning and expression of cDNA for human poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1224–1228. doi: 10.1073/pnas.84.5.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J. M. Potential metal-binding domains in nucleic acid binding proteins. Science. 1986 Apr 25;232(4749):485–487. doi: 10.1126/science.2421409. [DOI] [PubMed] [Google Scholar]
- Burtscher H. J., Auer B., Klocker H., Schweiger M., Hirsch-Kauffmann M. Isolation of ADP-ribosyltransferase by affinity chromatography. Anal Biochem. 1986 Feb 1;152(2):285–290. doi: 10.1016/0003-2697(86)90410-0. [DOI] [PubMed] [Google Scholar]
- Burtscher H. J., Klocker H., Schneider R., Auer B., Hirsch-Kauffmann M., Schweiger M. ADP-ribosyltransferase from Helix pomatia. Purification and characterization. Biochem J. 1987 Dec 15;248(3):859–864. doi: 10.1042/bj2480859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Cherney B. W., McBride O. W., Chen D. F., Alkhatib H., Bhatia K., Hensley P., Smulson M. E. cDNA sequence, protein structure, and chromosomal location of the human gene for poly(ADP-ribose) polymerase. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8370–8374. doi: 10.1073/pnas.84.23.8370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutrillaux B., Viegas-Pequignot E. High resolution R- and G-banding on the same preparation. Hum Genet. 1981;57(1):93–95. doi: 10.1007/BF00271176. [DOI] [PubMed] [Google Scholar]
- Eckerskorn C., Mewes W., Goretzki H., Lottspeich F. A new siliconized-glass fiber as support for protein-chemical analysis of electroblotted proteins. Eur J Biochem. 1988 Oct 1;176(3):509–519. doi: 10.1111/j.1432-1033.1988.tb14308.x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gaal J. C., Pearson C. K. Eukaryotic nuclear ADP-ribosylation reactions. Biochem J. 1985 Aug 15;230(1):1–18. doi: 10.1042/bj2300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S., Halpern E., Trisler P. Role of sulA and sulB in filamentation by lon mutants of Escherichia coli K-12. J Bacteriol. 1981 Oct;148(1):265–273. doi: 10.1128/jb.148.1.265-273.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. E., Saunders G. F. Localization of single copy DNA sequences of G-banded human chromosomes by in situ hybridization. Chromosoma. 1981;83(3):431–439. doi: 10.1007/BF00327364. [DOI] [PubMed] [Google Scholar]
- Klocker H., Auer B., Hirsch-Kauffmann M., Altmann H., Burtscher H. J., Schweiger M. DNA repair dependent NAD+ metabolism is impaired in cells from patients with Fanconi's anemia. EMBO J. 1983;2(3):303–307. doi: 10.1002/j.1460-2075.1983.tb01423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosaki T., Ushiro H., Mitsuuchi Y., Suzuki S., Matsuda M., Matsuda Y., Katunuma N., Kangawa K., Matsuo H., Hirose T. Primary structure of human poly(ADP-ribose) synthetase as deduced from cDNA sequence. J Biol Chem. 1987 Nov 25;262(33):15990–15997. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Naylor S. L., McGill J. R., Zabel B. U. In situ hybridization of metaphase and prometaphase chromosomes. Methods Enzymol. 1987;151:279–292. doi: 10.1016/s0076-6879(87)51024-2. [DOI] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poustka A., Rackwitz H. R., Frischauf A. M., Hohn B., Lehrach H. Selective isolation of cosmid clones by homologous recombination in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4129–4133. doi: 10.1073/pnas.81.13.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R., Auer B., Kühne C., Herzog H., Klocker H., Burtscher H. J., Hirsch-Kauffmann M., Wintersberger U., Schweiger M. Isolation of a cDNA clone for human NAD+: protein ADP-ribosyltransferase. Eur J Cell Biol. 1987 Oct;44(2):302–307. [PubMed] [Google Scholar]
- Scovassi A. I., Stefanini M., Bertazzoni U. Catalytic activities of human poly(ADP-ribose) polymerase from normal and mutagenized cells detected after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1984 Sep 10;259(17):10973–10977. [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida K., Morita T., Sato T., Ogura T., Yamashita R., Noguchi S., Suzuki H., Nyunoya H., Miwa M., Sugimura T. Nucleotide sequence of a full-length cDNA for human fibroblast poly(ADP-ribose) polymerase. Biochem Biophys Res Commun. 1987 Oct 29;148(2):617–622. doi: 10.1016/0006-291x(87)90921-1. [DOI] [PubMed] [Google Scholar]
- Ueda K., Hayaishi O. ADP-ribosylation. Annu Rev Biochem. 1985;54:73–100. doi: 10.1146/annurev.bi.54.070185.000445. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel B. U., Naylor S. L., Sakaguchi A. Y., Bell G. I., Shows T. B. High-resolution chromosomal localization of human genes for amylase, proopiomelanocortin, somatostatin, and a DNA fragment (D3S1) by in situ hybridization. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6932–6936. doi: 10.1073/pnas.80.22.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]