Abstract
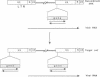
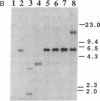
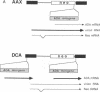
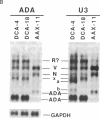
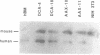
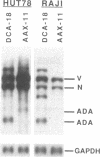
This study describes a type of retroviral vector called double-copy (DC) vector that was designed to improve the expression of transduced genes. The unique feature of DC vectors is that the transduced gene is inserted within the U3 region of the 3' long terminal repeat (LTR). Consequently, in the infected cell the gene is duplicated and transferred to the 5' LTR. The important result is that in its new position the gene is placed outside the retroviral transcriptional unit, eliminating or at least reducing the negative effects of the retroviral transcriptional unit. The utility of the DC vector design was tested by using a 2.1-kilobase-pair (kbp)-long adenosine deaminase (ADA; EC 3.5.4.4) minigene that was inserted into the 3' LTR of the N2 retroviral vector, generating a 2.7-kbp-long chimeric LTR. DNA blot analysis was used to show that the chimeric LTR was faithfully duplicated in cells infected with the corresponding virus, generating two copies of the ADA minigene, one copy in each LTR. Insertion of the ADA minigene into the 3' LTR of the N2 vector led to a 10- to 20-fold increase in ADA transcripts and human ADA isozyme synthesized in NIH 3T3 cells as compared to cells harboring the same vector in which the ADA minigene was inserted between the two LTRs. A similar increase in ADA expression was observed in two human lymphoid cell lines tested, HUT 78 and Raji. These results are consistent with previous observations that upstream promoters exert an inhibitory effect on promoters placed downstream and bear out the predictions used in the design of DC vectors. The use of DC vectors may contribute to the solution of the problems encountered in expressing retrovirally transduced genes in cultured cells and, in particular, when introduced into the live animal.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armentano D., Yu S. F., Kantoff P. W., von Ruden T., Anderson W. F., Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J Virol. 1987 May;61(5):1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowtell D. D., Cory S., Johnson G. R., Gonda T. J. Comparison of expression in hemopoietic cells by retroviral vectors carrying two genes. J Virol. 1988 Jul;62(7):2464–2473. doi: 10.1128/jvi.62.7.2464-2473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cone R. D., Weber-Benarous A., Baorto D., Mulligan R. C. Regulated expression of a complete human beta-globin gene encoded by a transmissible retrovirus vector. Mol Cell Biol. 1987 Feb;7(2):887–897. doi: 10.1128/mcb.7.2.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Lomedico P. T., Ju G. Transcriptional interference in avian retroviruses--implications for the promoter insertion model of leukaemogenesis. Nature. 1984 Jan 19;307(5948):241–245. doi: 10.1038/307241a0. [DOI] [PubMed] [Google Scholar]
- Dougherty J. P., Temin H. M. A promoterless retroviral vector indicates that there are sequences in U3 required for 3' RNA processing. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1197–1201. doi: 10.1073/pnas.84.5.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierzak E. A., Papayannopoulou T., Mulligan R. C. Lineage-specific expression of a human beta-globin gene in murine bone marrow transplant recipients reconstituted with retrovirus-transduced stem cells. Nature. 1988 Jan 7;331(6151):35–41. doi: 10.1038/331035a0. [DOI] [PubMed] [Google Scholar]
- Emerman M., Temin H. M. Genes with promoters in retrovirus vectors can be independently suppressed by an epigenetic mechanism. Cell. 1984 Dec;39(3 Pt 2):449–467. [PubMed] [Google Scholar]
- Fan H., Mittal S., Chute H., Chao E., Pattengale P. K. Rearrangements and insertions in the Moloney murine leukemia virus long terminal repeat alter biological properties in vivo and in vitro. J Virol. 1986 Oct;60(1):204–214. doi: 10.1128/jvi.60.1.204-214.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver R. I., Jr, Chytil A., Karlsson S., Fells G. A., Brantly M. L., Courtney M., Kantoff P. W., Nienhuis A. W., Anderson W. F., Crystal R. G. Production of glycosylated physiologically "normal" human alpha 1-antitrypsin by mouse fibroblasts modified by insertion of a human alpha 1-antitrypsin cDNA using a retroviral vector. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1050–1054. doi: 10.1073/pnas.84.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa E. Retrovirus vectors and their uses in molecular biology. Bioessays. 1986 Dec;5(6):252–257. doi: 10.1002/bies.950050605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley R. G., Covarrubias L., Hawley T., Mintz B. Handicapped retroviral vectors efficiently transduce foreign genes into hematopoietic stem cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2406–2410. doi: 10.1073/pnas.84.8.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadesch T., Berg P. Effects of the position of the simian virus 40 enhancer on expression of multiple transcription units in a single plasmid. Mol Cell Biol. 1986 Jul;6(7):2593–2601. doi: 10.1128/mcb.6.7.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lobel L. I., Patel M., King W., Nguyen-Huu M. C., Goff S. P. Construction and recovery of viable retroviral genomes carrying a bacterial suppressor transfer RNA gene. Science. 1985 Apr 19;228(4697):329–332. doi: 10.1126/science.2984770. [DOI] [PubMed] [Google Scholar]
- McIvor R. S., Johnson M. J., Miller A. D., Pitts S., Williams S. R., Valerio D., Martin D. W., Jr, Verma I. M. Human purine nucleoside phosphorylase and adenosine deaminase: gene transfer into cultured cells and murine hematopoietic stem cells by using recombinant amphotropic retroviruses. Mol Cell Biol. 1987 Feb;7(2):838–846. doi: 10.1128/mcb.7.2.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera Khan P. Enzyme electrophoresis on cellulose acetate gel: zymogram patterns in mgh-mouse and man--Chinese hamster somatic cell hybrids. Arch Biochem Biophys. 1971 Aug;145(2):470–483. doi: 10.1016/s0003-9861(71)80007-3. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Eckner R. J., Jolly D. J., Friedmann T., Verma I. M. Expression of a retrovirus encoding human HPRT in mice. Science. 1984 Aug 10;225(4662):630–632. doi: 10.1126/science.6377498. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J. Transcriptional interference and termination between duplicated alpha-globin gene constructs suggests a novel mechanism for gene regulation. Nature. 1986 Aug 7;322(6079):562–565. doi: 10.1038/322562a0. [DOI] [PubMed] [Google Scholar]
- Reik W., Weiher H., Jaenisch R. Replication-competent Moloney murine leukemia virus carrying a bacterial suppressor tRNA gene: selective cloning of proviral and flanking host sequences. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1141–1145. doi: 10.1073/pnas.82.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode B. W., Emerman M., Temin H. M. Instability of large direct repeats in retrovirus vectors. J Virol. 1987 Mar;61(3):925–927. doi: 10.1128/jvi.61.3.925-927.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiginton D. A., Adrian G. S., Friedman R. L., Suttle D. P., Hutton J. J. Cloning of cDNA sequences of human adenosine deaminase. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7481–7485. doi: 10.1073/pnas.80.24.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiginton D. A., Kaplan D. J., States J. C., Akeson A. L., Perme C. M., Bilyk I. J., Vaughn A. J., Lattier D. L., Hutton J. J. Complete sequence and structure of the gene for human adenosine deaminase. Biochemistry. 1986 Dec 16;25(25):8234–8244. doi: 10.1021/bi00373a017. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Lemischka I. R., Nathan D. G., Mulligan R. C. Introduction of new genetic material into pluripotent haematopoietic stem cells of the mouse. Nature. 1984 Aug 9;310(5977):476–480. doi: 10.1038/310476a0. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Lim B., Spooncer E., Longtine J., Dexter T. M. Restriction of expression of an integrated recombinant retrovirus in primary but not immortalized murine hematopoietic stem cells. Blood. 1988 Jun;71(6):1738–1743. [PubMed] [Google Scholar]
- Williams D. A., Orkin S. H., Mulligan R. C. Retrovirus-mediated transfer of human adenosine deaminase gene sequences into cells in culture and into murine hematopoietic cells in vivo. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2566–2570. doi: 10.1073/pnas.83.8.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee J. K., Moores J. C., Jolly D. J., Wolff J. A., Respess J. G., Friedmann T. Gene expression from transcriptionally disabled retroviral vectors. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5197–5201. doi: 10.1073/pnas.84.15.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S. F., von Rüden T., Kantoff P. W., Garber C., Seiberg M., Rüther U., Anderson W. F., Wagner E. F., Gilboa E. Self-inactivating retroviral vectors designed for transfer of whole genes into mammalian cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3194–3198. doi: 10.1073/pnas.83.10.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]