Abstract
While the cerebellum contains the highest density of cannabinoid receptor (CB1) in the brain, no studies have assessed the effect of exogenous cannabinoids on cerebellar-dependent learning in humans. The current study therefore examined the effect of chronic cannabis use on classical eyeblink conditioning (EBC), a cerebellar-mediated task which has been shown to be disrupted in CB1 knockout mice. Chronic cannabis users (24 hours abstinence prior to study; positive THC urine drug test) free of DSM-IV Axis I or Axis II disorders, were evaluated. A delay EBC task was utilized, in which a conditioned stimulus (CS; 400 ms tone) co-terminated with a corneal air puff unconditioned stimulus (US; 50 ms), thus eliciting a conditioned blink response (CR). The cannabis group exhibited markedly fewer, and more poorly timed CRs as compared to drug-nal̈ve controls. There were no differences between the groups in either the UR or an EEG measure of selective attention to the CS (N100 auditory ERP), indicating that the disruption observed in the cannabis group was specific to CR acquisition. These results suggest that cannabis use is associated with functional deficits in the cerebellar circuitry underlying EBC, a finding which corroborates recent work in CB1 knockout mice.
Keywords: THC, Cannabinoid, Cerebellum, Eyeblink Conditioning, Behavior, Human
INTRODUCTION
Cannabis sativa remains one of the most widely used psychoactive substances in the world. In the U.S alone, over 94 million individuals have used cannabis at least once in their lifetime (approximately 40 % of the population; SAMHSA, 2004). The principal psychoactive constituent in cannabis, Δ-9-tetrahydrocannabinol (THC; Gaoni and Mechoulam, 1964), affects the brain via the action of central cannabinoid receptors (CB1; Devane et al, 1988). The subsequent discovery of several endogenous cannabinoid neuromodulators, namely anandamide and 2-arachidonylglycerol (2-AG) has lead to an exponential growth in cannabinoid research (Devane et al, 1992; Stella et al, 1997). However, despite substantial advances in the molecular and biochemical aspects of endocannabinoid function, little is known about the specific role that this system plays at behavioral level, particularly in the context of chronic cannabis use.
To date, the majority of studies assessing the effects of cannabis on human brain function have focused on higher sensory, attentional, and memory processes (Patrick et al., 1995; 1997; Iversen, 2003; Skosnik et al, 2001; 2006a; 2006b; Ramaekers et al., 2006; Jager et al., 2007). However, several of these studies have lead to ambiguous and equivocal results, which may be due to the fact that tasks evaluating higher cognitive processes probe the integrity of distributed neocortical networks (i.e. neocortical areas contain relatively moderate CB1 densities compared with structures such as the basal ganglia and the cerebellum). Tasks which examine cerebellar function should be particularly useful indices of the effects of exogenous cannabinoids, as the cerebellum contains the highest density of CB1 receptors in the brain (Herkenham et al, 1990; Pertwee, 1997; Tsou et al, 1998). In addition to its role in motor coordination, the cerebellum is thought to play a key role in temporal operations such as rhythm production, time estimation, and some forms of associative learning (Ivry and Keele, 1989; Katz and Steinmetz, 2002; Leiner et al, 1991). Indeed, altered time perception is one of the most frequently reported subjective experiences associated with acute cannabis intoxication (Hicks et al, 1994; Mathew et al, 1998), which is thought to be modulated by the cerebellum (O’Leary et al, 2003). Thus, studies examining cerebellar-mediated processes may provide important clues in relation to the subjective effects of cannabis and the role of the endocannabinoid system in normal brain function.
Of the experiments which have evaluated cannabinoid effects on time estimation, several have shown that direct cannabis administration induces temporal disintegration, which is typically exhibited in the form of increased internal clock speeds (the experience of time passing more slowly; Hicks et al, 1994; Mathew et al, 1998; O’Leary et al, 2003). In relation to cerebellar modulation of cannabis’ effect on time perception, a study integrating behavioral measures of temporal processing with neuroimaging methodologies has yielded positive results. Using PET, O’Leary et al (2003) demonstrated that cannabis-induced alterations in a self-paced timing task were correlated with increased activity of medial cerebellar cortex. Interestingly, cerebellar metabolism during acute cannabis administration has been shown to correlate with subjective ratings of intoxication (Volkow et al, 1996). While it is clear that cannabis use in humans affects cerebellar function, time estimation tasks such as those described above probably recruit a distributed neural network including such structures as basal ganglia and neocortex (Bengtsson et al, 2005), making specific interpretations regarding cannabinoid effects on cerebellar function difficult. What is needed is a probe specific to cerebellar function that is based upon well-established neural circuitry, while solely dependent on the cerebellum.
One such task which has been shown to be modulated almost completely by the cerebellum is classical eyeblink conditioning (EBC). EBC is an associative motor learning task which involves the paired presentation of a neutral conditioned stimulus (CS), such as a tone, followed by an unconditioned stimulus (US), such as an ocular air puff. The US air puff evokes a reflexive eyeblink, the unconditioned response (UR), and after repeated CS-US paired presentations, a conditioned response (CR), a blink of the eye, forms subsequent to CS presentation. After repeated pairings the CR is timed so that the peak eyelid closure occurs near the onset of the US (Gormezano et al, 1983)
All forms of EBC are dependent on cerebellar function, though some forms, such as trace conditioning, also critically depend on the function of the hippocampus (Steinmetz, 2000). The importance of the cerebellum in the acquisition of the nictitating membrane/eyeblink response in non-human animals has been clearly demonstrated (McCormick et al, 1981; 1984a; 1984b; Thompson, 1986; Yeo et al, 1985a; 1985b). Although it is difficult to directly assess the neural substrates of EBC in humans, increasing evidence corroborates the non-human animal literature (Daum et al, 1993; Topka et al, 1993; Woodruff-Pak et al, 1996). For instance, a PET study showed activation in the inferior cerebellar cortex and deep cerebellar nuclei in humans during eyeblink classical conditioning (Logan and Grafton, 1995). Furthermore, cerebellar morphology and volume in both animals and humans are related to the magnitude of eyeblink conditioning (Woodruff-Pak et al, 2000).
EBC methodology is well suited for examining cerebellar-based timing deficits in cannabis users. The neural circuitry associated with conditioning is distinct, well characterized, and includes structures and pathways modulated by the cannabinoid system. For example, at several critical nodes in this circuit, particularly granule cell (parallel fiber) to Purkinje cell synapses, excitatory glutamatergic release is modulated (inhibited) by presynaptic CB1 activation (Kreitzer and Regehr, 2001a). More specifically, Purkinje cells activated by glutamatergic granule cells normally serve to inhibit cerebellar deep nuclei (i.e. interpositus nucleus) thus modulating the timing of conditioned blink responses (Steinmetz, 2000). Chronic cannabis use, which is known to produce behavioral tolerance and may induce long-term endocannabinoid compensatory changes (e.g. CB1 downregulation; Sim et al, 1996; Romero et al, 1998), could result in increased granule cell glutamate release, and hence overactive inhibitory Purkinje inputs to the interpositus. This position is in agreement with a recent study of CB1 knockout animals, in which mice lacking the CB1 receptor exhibit severely disrupted EBC (Kishimoto and Kano, 2006). However, despite knowledge of dense CB1 presence in the cerebellum, the effect of cannabis use on learning-related changes in this area in humans is unknown.
In order to elucidate the long-term effect of cannabinoids on the cerebellum in humans, the current study assessed chronic cannabis users utilizing cerebellar-dependent single-cue delay EBC. Fourteen current cannabis users and 10 healthy drug-free control participants were evaluated. Drug-use status was determined through clinical interviews, drug use questionnaires, and urine toxicology screens. The primary dependent measures for the eyeblink procedure were percent conditioned responses (% CRs) and CR peak latency. In addition, fronto-central electroenphalographic (EEG) data were collected in order to assess attention-dependent event-related potentials (ERPs) to the tone CS. An EEG measure was included in order to address the possible confound that cannabis-related alterations in sensory/attentional function could affect processing of the tone CS. The N100 component was evaluated, as this ERP is known to be reliably elicited by discreet auditory stimuli (i.e. tones), and is highly sensitive to attentional allocation (Hillyard et al., 1973; Coull, 1998). As previously observed in CB1 knockout mice (Kishimoto and Kano, 2006), it was hypothesized that cannabis users would show deficits in the acquisition and timing of the conditioned blink response.
METHODS
Participants
Current cannabis users (n = 14) and healthy drug-naive controls (n = 10) were assessed. This study was approved by the Indiana University Bloomington Human Subjects Committee. Participants were recruited from the local university community, were paid for their participation, and written informed consent was obtained from each. Recruitment consisted of flyers that were posted on the Indiana University campus and in the local Bloomington area which advertised for a paid psychology study for individuals currently using marijuana, but who were free of heavy alcohol use or use of other drugs. The control flyer simply advertised for individuals with no history of illicit drug use and only moderate alcohol use. Potential participants were first screened over the phone to determine if they met our inclusion criteria (see below). After phone screening (of approximately 50 potential subjects), 28 individuals met our inclusion criteria and were invited to participate in the study. Four of these were rejected after the initial clinical interview due to past histories of psychopathology. Table 1 illustrates basic demographic information as well as drug/alcohol use rates. There were no significant differences between the groups in age, years of education, or WAIS scores (Table 1). All participants but two were right handed.
Table 1.
Demographic and drug use histories for the cannabis group (n=14) and control participants (n=10). There were no differences between the groups in age, education, or WAIS scores
| Variable | Cannabis Group (n = 14) |
Control Group (n = 10) |
Value* | p* |
|---|---|---|---|---|
| Mean (S.D.) | Mean (S.D.) | |||
| Age (Years) | 20.8 (2.5) | 21.4 (2.8) | F=0.32 | 0.58 |
| Educational Level (Years) | 14.3 (1.3) | 15.1 (1.5) | F=2.04 | 0.17 |
| WAIS (Picture Completion) | 12.2 (2.4) | 13.1 (3.5) | F=0.54 | 0.47 |
| WAIS (Digit Symbol) | 10.9 (2.4) | 11.7 (2.5) | F=0.97 | 0.34 |
| WAIS (Similarities) | 11.2 (2.8) | 12.9 (2.6) | F=2.23 | 0.15 |
| WAIS (Digit Span) | 11.9 (3.1) | 12.4 (1.9) | F=0.18 | 0.67 |
| Ave. Cannabis (Joints) Per Week (Past Month) | 9.7 (6.0) | 0 (0) | ||
| Total Cannabis Use Past Month | 37.1 (18.5) | 0 (0) | ||
| Total Cannabis Use Past 6 Months | 224.3 (120.8) | 0 (0) | ||
| Age of First Cannabis Use | 15.3 (1.5) | 0 (0) | ||
| Total Years of Cannabis Use | 5.6 (3.5) | 0 (0) | ||
| Ave. Alcoholic Drinks Per Week (Past Month) | 5.6 (4.0) | 1.1 (1.5) | F=11.4 | 0.003 |
| Ave. Number of Cigarettes Per day (Past Month) | 8.4 (9.2) | 2.0 (6.3) | F=3.6 | 0.07 |
| N (%) | N (%) | |||
| Diagnosis of Cannabis Abuse | 5 (38%) | 0 (0%) | ||
| Diagnosis of Cannabis Dependence | 3 (21%) | 0 (0%) | ||
| Handedness | ||||
| Right | 12 (92%) | 10 (100%) | ||
| Left | 2 (8%) | 0 (0%) | ||
| Gender | ||||
| Male | 11 (79%) | 6 (60%) | ||
| Female | 3 (21%) | 4 (40%) | ||
One-way ANOVA for the cannabis group versus controls
The inclusion criteria were as follows: 1) For the cannabis group: current cannabis consumption (smoked joints) at the rate of at least once per week during the past month, a positive urine toxicology screen for THC, no other illicit substance use during the past six months (including a negative urine toxicology screen for other illicit drugs), and no DSM-IV diagnosis of Axis I or II disorders except cannabis abuse or dependence; 2) For the control group: no history of illicit substance use, a negative urine toxicology screen for all drugs tested, and no history of psychiatric illness (Axis I or II); 3) For all participants: ages 18-35, completion of high school education, no family history of Axis I or II disorders, no history of cardiovascular disease, hearing problems, neurological disease, learning disability, or head injury resulting in loss of consciousness. In addition, participants were excluded if they reported consumption of more than 2 alcoholic drinks per day (1 per day for females). The cannabis group drug-use inclusion criteria (cannabis use at least once per week; 24 hour abstinence) was chosen to eliminate acute cannabis effects, while retaining neurophysiological effects from altered CB1 activity. Human studies indicate that 80%-90% of the total amount of THC is excreted within 5 days, so a minimum use of once per week enabled detection of THC metabolites.
Clinical Interviews, Questionnaires, and Drug Use Assessment
The following psychometric questionnaires and clinical interviews were administered to all participants: a substance use questionnaire, The Drug Abuse Screening Test (DAST; Skinner, 1982), and The Short Michigan Alcoholism Screening Test (SMAST; Selzer et al., 1975). In addition, The Structured Clinical Interview for DSM-IV Axis I and II Disorders (SCID I and SCID II; First et al., 1997; 2002) and subscales of the Wechsler Adult Intelligence Scale III (Picture completion, Digit Symbol, Similarities, and Digit Span; Wechsler, 1997) were used to rule out previous psychiatric conditions and general deficits in neuropsychological function.
The clinical interview was used to ascertain current and past diagnoses for alcohol and substance abuse and dependence. Measures of frequency, quantity, and density of cannabis consumption were determined via the interview for the past six months, then for one month prior to the test session. Participants were instructed to consider each day of the week and indicate, for an average week, how much they consumed per drug-use occasion over the past six months, and then for the month prior to testing. Recency and density of last use was assessed using the past month section of the interview. Overall chronicity was assessed using a time-line follow-back procedure (Skosnik et al., 2006a), total months of active use since the onset of initial drug use (of any drug), which accounts for different periods of non-use, and a measure of overall density of use (total amount ever used/total months active use).
Urine screens (Q10-1, Proxam) were administered immediately preceding all testing in order to corroborate self-reports from the drug questionnaire and clinical interviews. The Q10-1 kit screens for cannabis, opiates, amphetamines, cocaine, MDMA (ecstasy), tricyclic antidepressants, phencyclidine, benzodiazepines, methamphetamines, and barbituates.
EBC Stimuli and Procedure
Participants engaged in a 148-trial delay EBC paradigm similar to Sears et al. (2000) and Brown et al. (2005). A visual overview of the experimental setup can be seen in Figure 1. Initially, 8 unconditioned stimulus (US) alone trials were presented, with an intertrial interval (ITI) of 15 s. Subsequently, the acquisition phase commenced consisting of 10 blocks of trials (mean ITI = 15 s, range = 10 to 20s). Each block contained 9 CS-US paired trials and 1 CS-alone trial, which were randomly presented within the last 5 trials of each block. Paired CS-US trials consisted of a 400 ms, 1000 Hz tone (80 dB SPL) with a coterminating 50 ms air puff. The extinction phase included 25 CS alone and 25 US alone trials, randomly presented across five additional blocks with a mean ITI of 15 s (range = 10 to 20 s). In order to maintain attention throughout the procedure, participants were asked to rate the pleasantness of neutral pictures selected from the International Affective Picture System (Lange and Greenwald, 1988). Pictures were presented for 2s in between each trial, and participants rated the images on a scale of 1 to 10 using a button response pad.
Figure 1.
Experimental setup. Visual overview of the experimental setup showing method and timing of US and CS presentation, and EEG/EMG recording.
Eye-blinks were recorded using pairs of electromyographic (EMG) electrodes (8 mm AG/AG-Cl; Model TD-23; MedAssociated, St. Albans, VT). The electrodes were placed on the orbicularis palpebrarum muscle below each eye, with a ground electrode on the forehead. All electrode impedances were maintained below 5 kΩ. The US consisted of a 10 psi (50 ms duration) puff of medical-grade air presented to the left eye with copper tubing affixed to eye-glass rims and placed 1 cm away from the inner canthus of the eye. Foam ear inserts were used for presentation of the CS tones (E-A-RLINK - Aearo Company Auditory Systems, Indianapolis, IN). EMG data were continuously recorded at 2.5 kHz with a Sensorium EPA-6 bioamplifier (highpass filter = 1 Hz, 12 dB/octave; lowpass filter = 300 Hz, 8th order elliptic; gain = 5000) and acquired using the software Neuroscan (v.4.2, El Paso, TX).
EBC Data Processing
Individual trials were epoched (1086 ms) from the continuous EMG data file beginning 500 ms prior to presentation of the CS (Using Neuroscan Edit software), and high pass filtered (10 Hz, 6 dB/octave) before being rectified and smoothed using a 41 point Gaussian weighted moving average. Data were then entered into the software DataMunch for further analysis (King and Tracy, 1999; Tracy et al., 2001). For each subject, responses were recorded as blinks if the amplitude exceeded 5 standard deviations above the baseline (baseline window for each trial = 125 ms prior to CS presentation). Conditioned responses (CRs) were recorded if the blink occurred between 100 and 350 ms after CS onset (corresponding to a period beginning 250 ms before US onset). Trials in which spontaneous blinks occur within a window from 75 ms prior to CS presentation to 25 ms following the CS were labeled bad trials and excluded from further analysis.
EEG Recording and Processing
EEG data was concurrently collected during the EBC paradigm. An examination of the N100 ERP component was assessed, which is sensitive to selective attention. This EEG measure was utilized to rule out the possibility that group differences might be due to altered CS encoding resulting from cannabis-related attentional impairments (Skosnik et al, 2001). The EEG was recorded continuously (band pass 0.1 – 100 Hz; sampling rate 1000 Hz) from the scalp at electrode site FCz with a nose reference, along with additional electrodes to record the vertical electrooculogram (VEOG). The recorded EEG was segmented into epochs consisting of the 400 ms during stimulus presentation, along with a 50 ms baseline, with any epoch containing a voltage greater than ± 100 μV excluded. Ocular movement artifact correction and averages were computed for each frequency block using commercially available software (Neuroscan). For analysis of the transient evoked response (N100), epochs were lowpass filtered at 20 Hz (24 dB/octave) prior to averaging, and baseline corrected (50 ms prestimulus/pre CS baseline) after averaging. The time window for the N100 component was defined as the largest peak occurring between 90 – 150 ms after onset of the tone CS. Finally, all experimenters responsible for EBC and EEG data analysis were blind to which experimental group each subject was assigned.
Statistical Analysis
The primary dependent measures for the eye-blink procedure were % CRs and CR peak latency. For % CRs and CR Peak latency during the acquisition phase (ten blocks of paired CS-US trials), a repeated measures ANOVA was used to assess the between participants effect of group (2) and the within participants effect of block (10). During the five block extinction phase, a repeated measures ANOVA was used to assess the between participant effect of group (2) and block (5). For the ten block acquisition phase, a similar repeated measures ANOVA was used to assess the effect of group (2) and block (10) on CR and UR amplitude, and UR onset latency. For the primary dependent variables of % CRs and CR peak latency, effect sizes are reported (partial η2), where small effect sizes are less than .06, moderate effect sizes range from .06 to .14, and large effect sizes are greater than .14 (Cohen, 1973). Possible group differences in EEG N100 amplitude/latency and questionnaire scores were determined using one-way ANOVAs. All statistical tests used an alpha level of p < 0.05 to determine significance (two-tailed), and all tests were performed using the software package SPSS 14.0.
RESULTS
Raw EBC data
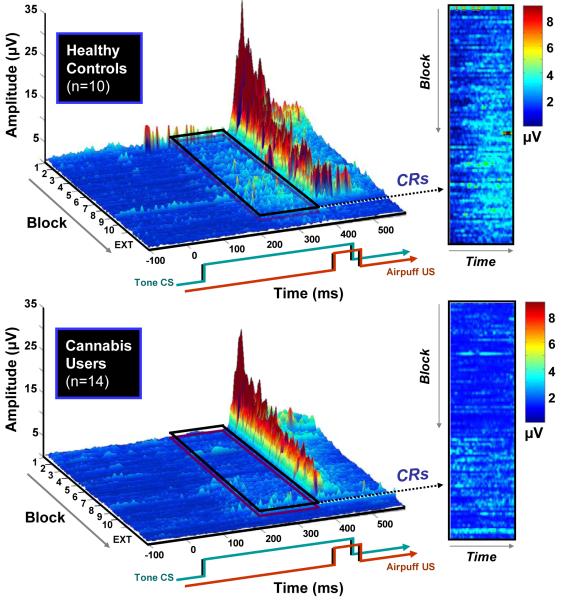
The primary dependent variables of interest were % CRs and CR peak latencies during paired trials across the duration of the experiment. Plots of the grand-averaged trial-by-trial EMG data from all the paired CS-US trials can be seen in Figure 2. As can be seen in the outlined sections representing the CR time window (approximately 220 – 350 ms), cannabis use was associated with a pronounced deficit in the acquisition of the CR compared to the control participants, particularly from about block 3 to block 10. Both groups showed comparable UR amplitudes (at approximately 400 ms), which steadily decreased in amplitude across the acquisition phase due to habituation.
Figure 2.
Grand averaged trial-by-trial EMG data from all paired CS–US trials. The 400-ms tone CS (1 kHz, 80 dB) co-terminates with a 50 ms (10 psi) airpuff. The outlined sections and panels display the time window where conditioned responses (CRs) occurred (approximately 220 – 350 ms). At around block 3, the control participants (top) begin to show robust CRs, as evidenced by the increased amplitude in the period shortly before US onset. Conversely, cannabis users (bottom) are impaired in the acquisition and timing of the CR. Both groups show decreased unconditioned response (UR) amplitudes as the experiment progresses due to habituation.
EBC Acquisition Phase
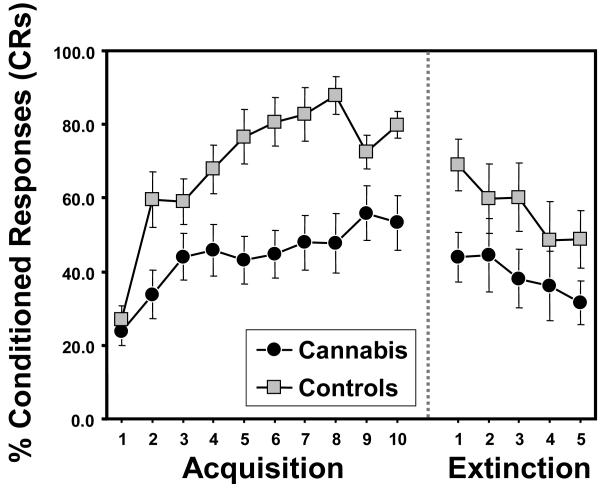
Percent CRs and CR peak latency during each 10-trial block (plus extinction) were calculated for each group. For % CRs (Figure 3), a repeated measures ANOVA revealed a main effect of block [F(9,198) = 8.67, p < 0.0001; partial η2 = 0.85] and a main effect of group [F(1,22) = 14.36, p < 0.001; partial η2 = 0.40]. However, there was no group X block interaction [F(9,198) = 1.89, p = 0.14; partial η2 = 0.55]. Given that the cannabis group exhibited slightly higher alcohol use rates than controls (see Table 1), the average number of drinks per week (during the past month) was included in the repeated measures ANOVA as a covariate. The main effect of group remained significant after covarying for alcohol use [F(1,21) = 9.63, p < 0.005; partial η2 = 0.31]. Likewise, the group differences also remained significant after covarying for tobacco use per day [F(1,21) = 12.80, p < 0.002; partial η2 = 0.38]. For CR peak latency, there was also a main effect of block [F(9,198) = 5.17, p < 0.003; partial η2 = 0.77] and a main effect of group [F(1,22) = 7.23, p < 0.01; partial η2 = 0.25]. There was also a trend towards a group X block interaction [F(9,198) = 2.31, p = 0.08; partial η2 = 0.60]. The main effect of group remained significant after covarying for tobacco use [F(1,21) = 7.85, p < 0.01; partial η2 = 0.27], and fell to a trend level difference after covarying for alcohol use [F(1,21) = 2.79, p < 0.10; partial η2 = 0.12]. For UR amplitude, no group differences were observed during paired trials [F(1,22) = 0.71, p = 0.41] or in UR latency during paired trials [F(1,22) = 0.02, p = 0.9]. Taken together, the significant group differences in % CRs and CR peak latency during acquisition demonstrates that the cannabis group showed impaired learning of the conditioned eyeblink response compared to controls (Figure 3), and shorter (less adaptive) peak CR latencies.
Figure 3.
Mean % conditioned responses (CRs) across blocks (1 block = 9 CS-US paired trials) for cannabis users versus controls. Both groups displayed approximately equal % CRs in the first block of the learning phase, with controls showing greater learning across time. Both groups show similar declines in the percentage of CRs across the extinction blocks, indicating similar rates of extinction of the CR. Error bars indicate ± SEM.
EBC Extinction Phase and Auditory Tone CS ERP
A repeated-measures ANOVA on % CRs during the last five extinction blocks revealed a main effect of group [F(1,22) = 4.23, p < 0.05; partial η2 = 0.16] and a main effect of block [F(4,88) = 3.61, p < 0.02; partial η2 = 0.43], which demonstrates that while both groups displayed decreasing % CRs across extinction, the cannabis group remained significantly below control levels. However, there was no group X block interaction [F(4,88) = 0.35, p = 0.84; partial η2 = 0.07], demonstrating that extinction rates for the two groups were equal.
For CR peak latency during extinction, there was no effect of group [F(1,22) = 1.8, p = 0.21; partial η2 = 0.12], block [F(4,88) = 0.27, p = 0.89; partial η2 = 0.10], or group X block [F(4,88) = 0.74, p = 0.59; partial η2 = 0.23], indicating that timing for the remaining CRs was the same in both groups. Likewise for CR amplitude, there was no effect of group [F(1,22) = 0.28, p = 0.6], block [F(4,88) = 1.3, p = 0.32], or group X block [F(4,88) = 1.2, p = 0.36].
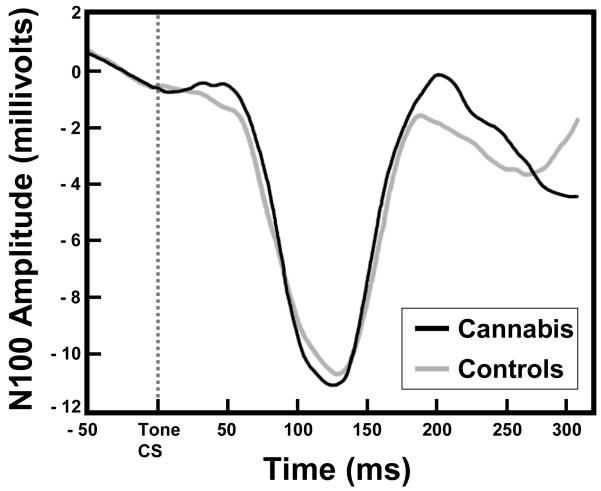
Finally, for the ERP measure, a one-way ANOVA revealed that there were no differences in N100 amplitude between the groups [F(1,23) = 0.11, p = 0.75]. The grand-averaged EEG waveforms illustrating the N100 ERPs for each group can be seen in Figure 4.
Figure 4.
Grand averaged transient ERPs elicited by tone CS onset for both cannabis users and control participants at electrode FCz. The similar N100 component in the cannabis users and controls provide evidence of intact acoustic stimulus processing and CS encoding in both groups.
DISCUSSION
The current study was the first to assess cannabinoid modulation of cerebellar-dependent delay EBC in human volunteers. These results are in agreement with previous human work showing altered cerebellar activity during cannabis administration (Mathew et al, 1998; O’Leary et al, 2003). In addition to providing further evidence that cannabis use disrupts cerebellar function, the current findings provide an important clue about the specific role endocannabinoids may play in normal cerebellar-dependent learning, particularly in light of the recent study of delay EBC in CB1 knockout animals (Kishimoto and Kano, 2006). Using an animal analogue of the delay EBC learning paradigm, Kishimoto and Kano (2006) have shown that mice lacking the CB1 receptor display highly impaired acquisition of the CR compared to wild-type animals. Interestingly, CB1 knockouts showed normal UR amplitudes, CS processing, and unaltered EBC extinction phases, patterns which are also identical to the current human EBC results. It therefore appears that the cannabinoid system is primarily involved in the acquisition phase of EBC, with little involvement in CS sensory processing or UR blink generation.
As to the mechanism of CB1 effects on EBC, both the well-established circuitry mediating conditioning (Steinmetz, 2000) and the specified role of endocannabinoids in cerebellar synaptic function allow for specific hypotheses regarding the role of cannabinoids in EBC. It is thought that information about the CS is projected into the cerebellum via mossy fibers which in turn activate granule cells, while the US is projected into the cerebellum along climbing fibers. Plasticity is thought to occur at neuronal junctions that receive convergent CS-US input (the interpositus nucleus, and Purkinje cells in cerebellar cortex). The Purkinje cells that receive convergent CS-US input from granule cells (i.e. CS) and climbing fibers (i.e. US) project to the neurons in the interpositus nucleus that also receive convergent CS-US input. The inhibitory Purkinje cells tonically suppress interpositus activity, and it is thought that for conditioning to occur, the GABAergic Purkinje cells must themselves be inhibited, which in turn disinhibits interpositus activity, thus affecting the timing and gain of CRs (Steinmetz, 2000).
In regard to cannabinoid modulation of this circuit, CB1 receptors are known to reside on glutamatergic granule cell axons (parallel fiber terminals). Purkinje cells synthesize and release retrograde endocannabinoids following bursts of parallel fiber stimulation (Brown et al, 2003). Thus, during EBC, endocannabinoids may operate by activating presynaptic CB1 receptors on parallel fiber terminals, which reduce Ca2+ influx, ultimately inhibiting Purkinje cell activity by decreasing glutamate release from parallel fiber terminals. Indeed, it has recently been shown that concurrent parallel fiber/climbing fiber activation of Purkinje cells (which is analogous to convergent CS-US input) leads to short-term depression of the parallel fiber synapse, an effect which is mediated by endocannabinoids (Brenowitz and Regehr, 2005). Such an effect may be crucial for the induction of longer-lasting synaptic plasticity, such as endocannabinoid-mediated long-term depression (LTD; Safo and Regehr, 2005), which itself may represent the neural substrate of EBC (Thompson and Kim, 1996).
In the context of chronic cannabis use, long-term activation of presynaptic parallel fiber CB1 by exogenous THC may act to desensitize or downregulate the receptors, which could disrupt the short-term plasticity and/or LTD necessary for learning in the cerebellum. It has already been established that chronic cannabis users assessed during periods of abstinence demonstrate hypoactive cerebellar activity, which could be interpreted as neuroadaptive endocannabinoid downregulation (Volkow et al, 1996; Block et al, 2000; Chang et al, 2006). Indeed, animal studies have shown that behavioral tolerance to the motor effects of THC is likely mediated by CB1 downregulation throughout the cerebellum (Sim et al, 1996; Romero et al, 1998). More recently, it has been found that chronic cannabis use in humans does in fact lead to significant CB1 downregulation as determined via post-mortem [3H]SR141716A binding (Villares, 2007). These data provide indirect evidence that cerebellar changes in CB1 induced by chronic cannabis intake may partly explain the impaired EBC performance observed in the current study.
Several limitations of the current study warrant further discussion. First, since participants were tested after 24 hours of abstinence, it is unclear whether THC withdrawal may have accounted for the observed findings. However, most heavy cannabis users do not display peak withdrawal symptoms until 2-3 days following cessation of use (Budney et al., 2004), which most likely precludes the possibility that the current results were due to cannabis withdrawal. However, a related confound is that participants in the current experiment were not monitored during the 24 hour abstinence window. Thus, the veracity of participants’ self-reported cannabis use 24 hours pre-experiment could be unreliable, meaning that acute intoxication or residual THC may have contributed to EBC learning deficits.
A second limitation of the current study is that even though both groups displayed moderate to low levels of alcohol and tobacco use, the cannabis group had slightly increased rates of consumption of these substances. However, current weekly levels of alcohol and tobacco use were included as covariates in each of the analyses of EBC performance (% CRs and CR peak latency), which in turn did not influence the observed effects. In relation to tobacco use, nicotine administration has actually been shown to increase EBC performance in animals (Woodruff-Pak, et al., 1994; 2000). This, along with the fact that the onset of nicotine withdrawal is 2 hours and peaks around 36 hours (tobacco use was permitted in our subjects up to 1 hour prior to testing; Hughes et al., 1994; Kenny and Markou, 2001), most likely rules out that possibility that tobacco use or withdrawal contributed to the current findings.
Thirdly, since the current study did not directly administer cannabis or THC, it remains possible that some pre-existing condition contributed to both cannabis drug-seeking and altered EBC performance (e.g. premorbid alterations in CB1 or endocannabinoids). However, since all of the current cannabis subjects tested positive for THC, and had a mean of 5.6 years of cannabis use, the informed assumption of CB1 downregulation discussed above is likely more proximal and salient than subtle pre-existing individual differences in endocannabinoid activity. Nonetheless, since there was no way of directly assessing CB1 densities in the current sample, possible preexisting differences cannot be ruled out using the current methodology.
Several additional limitations of the current experiment exist which could be addressed by future studies including the small sample size, the generalizability of the findings given the homogeneity of the groups, and whether the observed deficits would normalize following longer periods of abstinence. This last issue regarding whether EBC performance is recoverable will be important to elucidate from a public health perspective. Current neuropsychological data suggest a recovery of cannabis-induced cognitive impairments after one month of abstinence (Pope et al., 2001; Harrison et al., 2002). However, these neuropsychological tasks are typically sensitive to distributed cortical networks not specific to cerebellar processes, as is the EBC procedure. It therefore remains feasible that compensatory mechanisms of diverse brain regions (i.e. neocortical) may partially explain recovery of function on classical neuropsychological tasks. Future studies are planned which will attempt to address these issues, including assessing past cannabis users on EBC, and the use of alternate cerebellar tasks (e.g. paced finger tapping and postural sway) which could corroborate the finding of altered cerebellar-dependent learning using EBC. Prospective experiments on chronic cannabis users would also benefit from identified behavioral patterns of CB1 knockout animals. Given that CB1 knockout mice exhibit alterations on tests assessing extinction of fear conditioning, pain thresholds, locomotor activity, and spatial memory, developing human analogues of these tasks for application in cannabis users would seem prudent.
In summary, the overall implications and relevance of the current study are twofold: First, the observed effects provide evidence that endocannabinoids may be critically involved in cerebellar EBC. Second, the fact that chronic cannabis users exhibit learning patterns similar to CB1 knockout animals suggests that long-term use may produce physiological alterations (i.e. CB1 downregulation) of a magnitude previously unreported. Given that the cerebellum is reciprocally connected to vast regions of brain (Dum and Strick, 2003) and is now implicated in a host of non-motor functions including perception and cognition (Katz and Steinmetz, 2002), it is imperative that future human and animal studies further assess the role of cannabinoids in cerebellar function.
ACKNOWLEDGEMENTS
This work was supported in part by a grant from NIDA (NIDA 1 R03 DA019630-01) and a NARSAD Young Investigator Award. The authors wish to thank Michael Walker, Jo Anne Tracy, Jennifer Vollmer, Ken Mackie, and Michael Paulin for their help throughout the project.
Footnotes
COMPETING INTEREST STATEMENT
The authors declare that they have no competing financial interests.
REFERENCES
- Ashton JC, Appleton I, Darlington CL, Smith PF. Immunohistochemical localization of cannabinoid CB1 receptor in inhibitory interneurons in the cerebellum. Cerebellum. 2004;3(4):222–6. doi: 10.1080/14734220410019011. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Ehrsson HH, Forssberg H, Ullen F. Effector-independent voluntary timing: behavioural and neuroimaging evidence. Eur J Neurosci. 2005;22(12):3255–65. doi: 10.1111/j.1460-9568.2005.04517.x. [DOI] [PubMed] [Google Scholar]
- Block RI, O’Leary DS, Hichwa RD, Augustinack JC, Ponto LL, Ghoneim MM, Arndt S, Ehrhardt JC, Hurtig RR, Watkins GL, Hall JA, Nathan PE, Andreasen NC. Cerebellar hypoactivity in frequent marijuana users. Neuroreport. 2000;11(4):749–53. doi: 10.1097/00001756-200003200-00019. [DOI] [PubMed] [Google Scholar]
- Brenowitz SD, Regehr WG. Associative short-term synaptic plasticity mediated by endocannabinoids. Neuron. 2005;45(3):419–31. doi: 10.1016/j.neuron.2004.12.045. [DOI] [PubMed] [Google Scholar]
- Brown SM, Kieffaber PD, Carroll CA, Vohs JL, Tracy JA, Shekhar A, O’Donnell BF, Steinmetz JE, Hetrick WP. Eye-blink conditioning deficits indicate timing and cerebellar abnormalities in schizophrenia. Brain Cog. 2005;58:94–108. doi: 10.1016/j.bandc.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Brown SP, Brenowitz SD, Regehr WG. Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat Neurosci. 2003;6(10):1048–57. doi: 10.1038/nn1126. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Hughes JR, Moore BA, Vandrey RG. A review of the validity and significance of the cannabis withdrawal syndrome. Am J Psychiatr. 2004;161:1967–1977. doi: 10.1176/appi.ajp.161.11.1967. [DOI] [PubMed] [Google Scholar]
- Chang L, Yakupov R, Cloak C, Ernst T. Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain. 2006;129(Pt 5):1096–112. doi: 10.1093/brain/awl064. [DOI] [PubMed] [Google Scholar]
- Cohen J. Eta-Squared and Partial Eta-Squared in Communication Science. Hum Commun Res. 1973;28:473–490. [Google Scholar]
- Coull JT. Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol. 1998;55(4):343–61. doi: 10.1016/s0301-0082(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Daniel H, Rancillac A, Crepel F. Mechanisms underlying cannabinoid inhibition of presynaptic Ca2+ influx at parallel fibre synapses of the rat cerebellum. J Physiol. 2004;557(Pt 1):159–74. doi: 10.1113/jphysiol.2004.063263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum I, Schugens M, Ackermann H, Lutzenberger W, Dichgans J, Birbaumer N. Classical conditioning after cerebellar lesions in humans. Behav Neurosci. 1993;107:748–756. doi: 10.1037//0735-7044.107.5.748. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34(5):605–13. [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–9. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol. 2003;89(1):634–9. doi: 10.1152/jn.00626.2002. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Personality Disorders, (SCID-II) American Psychiatric Press, Inc.; Washington, D.C.: 1997. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem So. 1964;86:1646–1647. [Google Scholar]
- Gormezano I, Kehoe EJ, Marshall BS. Twenty years of classical conditioning with the rabbit. Prog Psychobiol Physiol Psychol. 1983;10:197–275. [Google Scholar]
- Harrison GP, Jr, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Cognitive measures in long-term cannabis users. J Clin Pharmacol. 2002;42(11 Suppl):41S–47S. doi: 10.1002/j.1552-4604.2002.tb06002.x. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87(5):1932–6. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks RE, Gualtieri CT, Mayo JP, Jr, Perez-Reyes M. Cannabis, atropine, and temporal information processing. Neuropsychobiol. 1984;12(4):229–37. doi: 10.1159/000118144. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, Picton TW. Electrical signs of selective attention in the human brain. Science. 1973;182(108):177–80. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Bickel WK. Nicotine withdrawal versus other drug withdrawal syndromes: similarities and dissimilarities. Addiction. 1994;89(11):1461–70. doi: 10.1111/j.1360-0443.1994.tb03744.x. [DOI] [PubMed] [Google Scholar]
- Iversen L. Cannabis and the brain. Brain. 2003;126(Pt 6):1252–70. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Jager G, Van Hell HH, De Win MM, Kahn RS, Van Den Brink W, Van Ree JM, Ramsey NF. Effects of frequent cannabis use on hippocampal activity during an associative memory task. Eur Neuropsychopharmacol. 2007;17(4):289–97. doi: 10.1016/j.euroneuro.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Ivry RB, Keele SW. Timing of functions of the cerebellum. J Cog Neurosci. 1989;1:136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- Katz DB, Steinmetz JE. Psychological functions of the cerebellum. Behav Cog Neurosci Rev. 2002;1:229–241. doi: 10.1177/1534582302001003004. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Neurobiology of the nicotine withdrawal syndrome. Pharmacol Biochem Behav. 2001;70(4):531–49. doi: 10.1016/s0091-3057(01)00651-7. [DOI] [PubMed] [Google Scholar]
- King DAT, Tracy J. DataMunch: A Matlab m-file collection for the analysis of trial-based spike and behavioral data. 1999 Available from: http://www.novl.indiana.edu/~dmunch/
- Kishimoto Y, Kano M. Endogenous cannabinoid signaling through the CB1 receptor is essential for cerebellum-dependent discrete motor learning. J Neurosci. 2006;26(34):8829–37. doi: 10.1523/JNEUROSCI.1236-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001a;29(3):717–27. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci. 2001b;21(20):RC174. doi: 10.1523/JNEUROSCI.21-20-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK. The International Affective Picture System Standardization Procedure and Initial Group Results for Affective Judgments: Technical Report 1A. The Center for Research in Psychophysiology, University of Florida; Gainesville, FL: 1988. [Google Scholar]
- Leiner HC, Leiner AL, Dow RS. The human cerebrocerebellar system: its computing, cognitive, and language skills. Behav Brain Res. 1991;44:113–128. doi: 10.1016/s0166-4328(05)80016-6. [DOI] [PubMed] [Google Scholar]
- Logan CG, Grafton ST. Functional anatomy of human eyeblink conditioning determined with regional cerebral glucose metabolism and positron-emission tomography. Proc Natl Acad Sci USA. 1995;92(16):7500–7504. doi: 10.1073/pnas.92.16.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH, Turkington TG, Coleman RE. Cerebellar activity and disturbed time sense after THC. Brain Res. 1998;797(2):183–9. doi: 10.1016/s0006-8993(98)00375-8. [DOI] [PubMed] [Google Scholar]
- McCormick DA, et al. The engram found? Role of the cerebellum in classical conditioning of nictitating membrane and eyelid responses. Bull Psychonomic Soc. 1981;18(3):103–105. [Google Scholar]
- McCormick DA, Thompson RF. Cerebellum essential involvement in the classically conditioned eyelid response. Science. 1984a;223:296–299. doi: 10.1126/science.6701513. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Thompson RF. Neuronal responses of the rabbit cerebellum during acquisition and performance of a classically conditioned nictitating membrane-eyelid response. J Neurosci. 1984b;4:2811–2822. doi: 10.1523/JNEUROSCI.04-11-02811.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary DS, Block RI, Turner BM, Koeppel J, Magnotta VA, Ponto LB, Watkins GL, Hichwa RD, Andreasen NC. Marijuana alters the human cerebellar clock. Neuroreport. 2003;14(8):1145–51. doi: 10.1097/00001756-200306110-00009. [DOI] [PubMed] [Google Scholar]
- Patrick G, Straumanis JJ, Struve FA, Nixon F, Fitz-Gerald MJ, Manno JE, Soucair M. Auditory and visual P300 event related potentials are not altered in medically and psychiatrically normal chronic marihuana users. Life Sci. 1995;56(23-24):2135–40. doi: 10.1016/0024-3205(95)00199-g. [DOI] [PubMed] [Google Scholar]
- Patrick G, Straumanis JJ, Struve FA, Fitz-Gerald MJ, Manno JE. Early and middle latency evoked potentials in medically and psychiatrically normal daily marihuana users: a paucity of significant findings. Clin Electroencephalogr. 1997;28(1):26–31. doi: 10.1177/155005949702800105. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Therapeut. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58(10):909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Kauert G, van Ruitenbeek P, Theunissen EL, Schneider E, Moeller MR. High-potency marijuana impairs executive function and inhibitory motor control. Neuropsychopharmacology. 2006;31(10):2296–303. doi: 10.1038/sj.npp.1301068. [DOI] [PubMed] [Google Scholar]
- Romero J, Berrendero F, Garcia-Gil L, Ramos JA, Fernandez-Ruiz JJ. Cannabinoid receptor and WIN-55,212-2-stimulated [35S]GTP gamma S binding and cannabinoid receptor mRNA levels in the basal ganglia and the cerebellum of adult male rats chronically exposed to delta 9-tetrahydrocannabinol. J Mol Neurosci. 1998;11(2):109–19. doi: 10.1385/JMN:11:2:109. [DOI] [PubMed] [Google Scholar]
- Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48(4):647–59. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Sears LL, Andreasen NC, O’Leary DS. Cerebellar functional abnormalities in schizophrenia are suggested by classical eyeblink conditioning. Biol Psychiatry. 2000;48(3):204–9. doi: 10.1016/s0006-3223(00)00247-x. [DOI] [PubMed] [Google Scholar]
- Selzer ML, Vinokur A, van Rooijen L. A self-administered Short Michigan Alcoholism Screening Test (SMAST) J Stud Alcohol. 1975;36(1):117–26. doi: 10.15288/jsa.1975.36.117. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Hampson RE, Deadwyler SA, Childers SR. Effects of chronic treatment with delta9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci. 1996;16(24):8057–66. doi: 10.1523/JNEUROSCI.16-24-08057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA. The drug abuse screening test. Addict Behav. 1982;7(4):363–71. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Krishnan G, Aydt AE, Kuhlenschmidt H, O’Donnell BF. Psychophysiological Evidence of Altered Neural Synchronization in Cannabis Use: Relationship to Schizotypy. Am J Psychiatr. 2006a;163:1798–1805. doi: 10.1176/ajp.2006.163.10.1798. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Krishnan G, Vohs J, O’Donnell BF. The Effect of Cannabis Use and Gender on the Visual Steady State Evoked Potential. Clin Neurophysiol. 2006b;117(1):144–56. doi: 10.1016/j.clinph.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Skosnik PD, Spatz-Glenn L, Park S. Cannabis use is associated with schizotypy and attentional disinhibition. Schizophr Res. 2001;48(1):83–92. doi: 10.1016/s0920-9964(00)00132-8. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE. Brain substrates of classical eyeblink conditioning: a highly localized but also distributed system. Behav Brain Res. 2000;110:13–24. doi: 10.1016/s0166-4328(99)00181-3. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388(6644):773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2003 National Survey on Drug Use and Health: National Findings. SAMHSA; Rockville, MD: 2004. NSDUH Series H-25. DHHS Pub. No. (SMA) 04-3964. [Google Scholar]
- Thompson RF. The neurobiology of learning and memory. Science. 1986;223:941–947. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Kim JJ. Memory systems in the brain and localization of a memory. Proc Natl Acad Sci USA. 1996;93(24):13438–44. doi: 10.1073/pnas.93.24.13438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topka H, Valls-Sole J, Massaquoi SG, Hallett M. Deficit in classical conditioning in patients with cerebellar degeneration. Brain. 1993;116:961–969. doi: 10.1093/brain/116.4.961. [DOI] [PubMed] [Google Scholar]
- Tracy JA, Britton GB, Steinmetz JE. Comparison of single unit responses to tone, light, and compound conditioned stimuli during rabbit classical eyeblink conditioning. Neurobiol Learn Mem. 2001;76(3):253–67. doi: 10.1006/nlme.2001.4024. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neurosci. 1998;83(2):393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Villares J. Chronic use of marijuana decreases cannabinoid receptor binding and mRNA expression in the human brain. Neuroscience. 2007;145(1):323–34. doi: 10.1016/j.neuroscience.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, Valentine A, Hollister L. Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatr Res. 1996;67(1):29–38. doi: 10.1016/0925-4927(96)02817-x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. Ed 3. The Psychological Corporation; San Antonio. TX: 1997. [Google Scholar]
- Woodruff-Pak DS, Goldenberg G, Downey-Lamb MM, Boyko OB, Lemieux SK. Cerebellar volume in humans related to magnitude of classical conditioning. Neuroreport. 2000;11:609–615. doi: 10.1097/00001756-200002280-00035. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Papka M, Ivry RB. Cerebellar involvement in eyeblink classical conditioning in humans. Neuropsychol. 1996;10:443–458. [Google Scholar]
- Woodruff-Pak DS, Li YT, Kem WR. A nicotinic agonist (GTS-21), eyeblink classical conditioning, and nicotinic receptor binding in rabbit brain. Brain Res. 1994;645(1-2):309–17. doi: 10.1016/0006-8993(94)91665-9. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Green JT, Coleman-Valencia C, Pak JT. A nicotinic cholinergic agonist (GTS-21) and eyeblink classical conditioning: acquisition, retention, and relearning in older rabbits. Exp Aging Res. 2000;26(4):323–36. doi: 10.1080/036107300750015723. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hardiman MJ, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. I. Lesions of the cerebellar nuclei. Exper Brain Res. 1985a;60(1):87–98. doi: 10.1007/BF00237022. [DOI] [PubMed] [Google Scholar]
- Yeo CH, Hardiman MJ, Glickstein M. Classical conditioning of the nictitating membrane response of the rabbit. II. Lesions of the cerebellar cortex. Exper Brain Res. 1985b;60(1):99–113. doi: 10.1007/BF00237023. [DOI] [PubMed] [Google Scholar]