Abstract
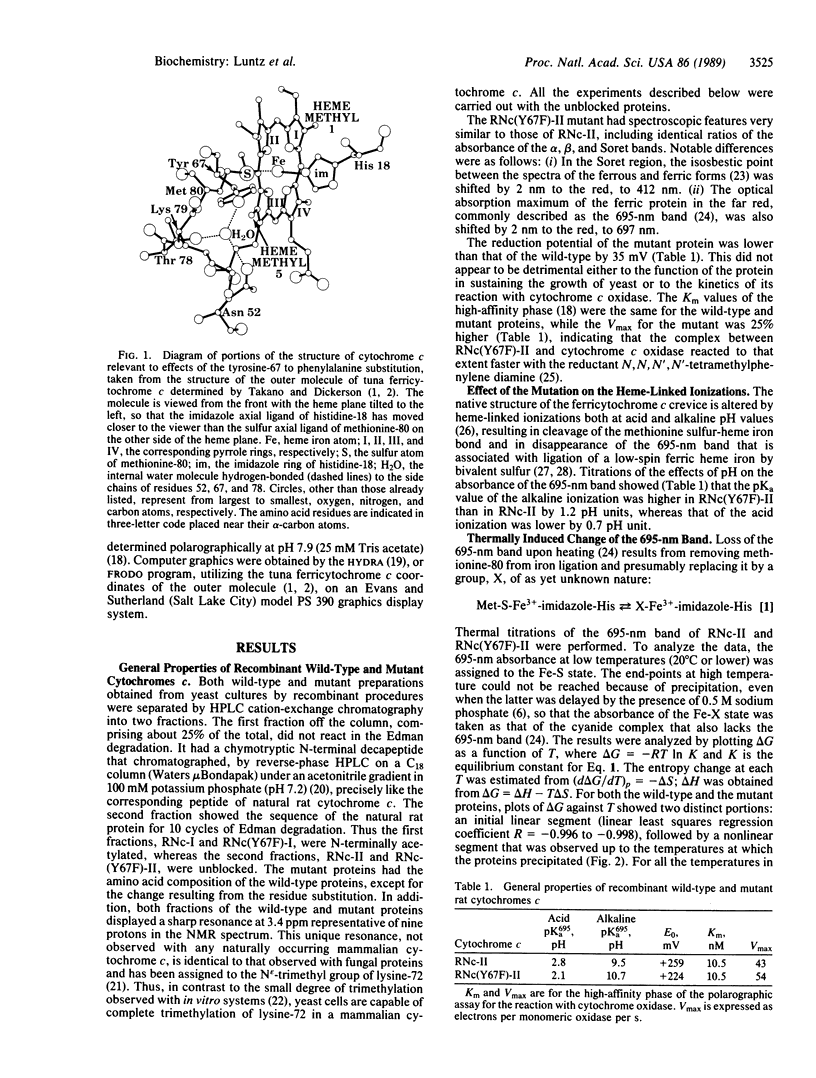
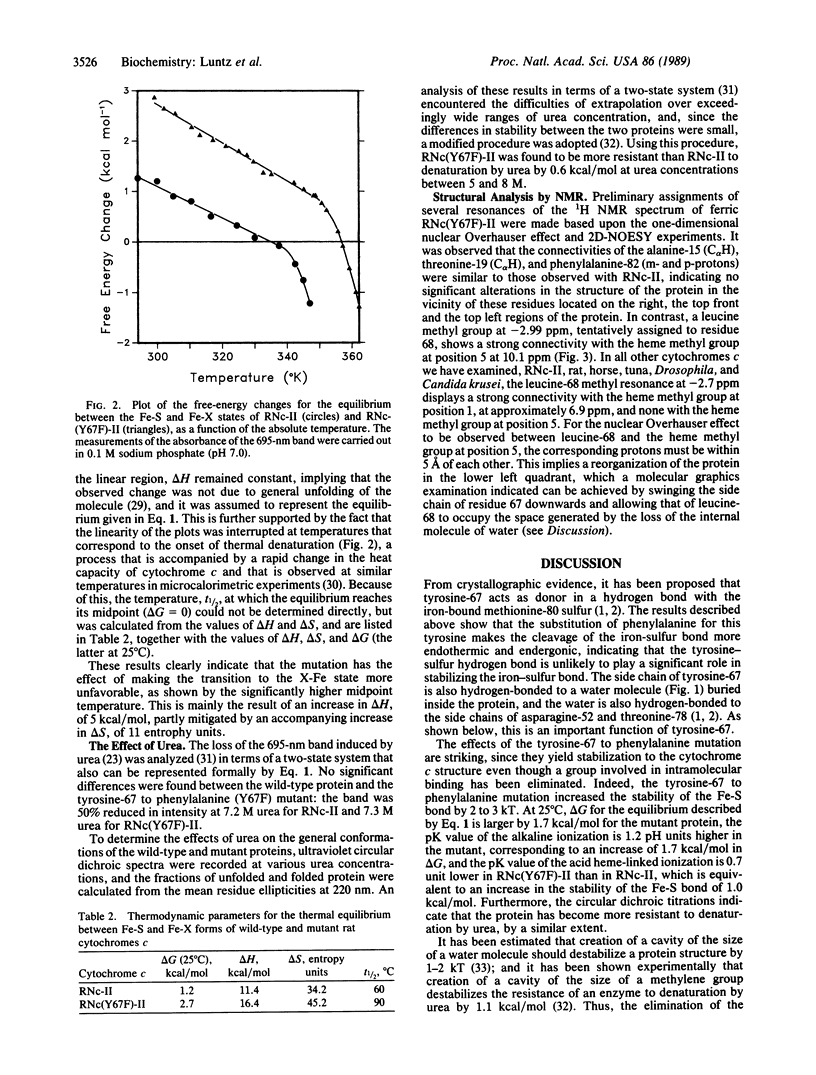
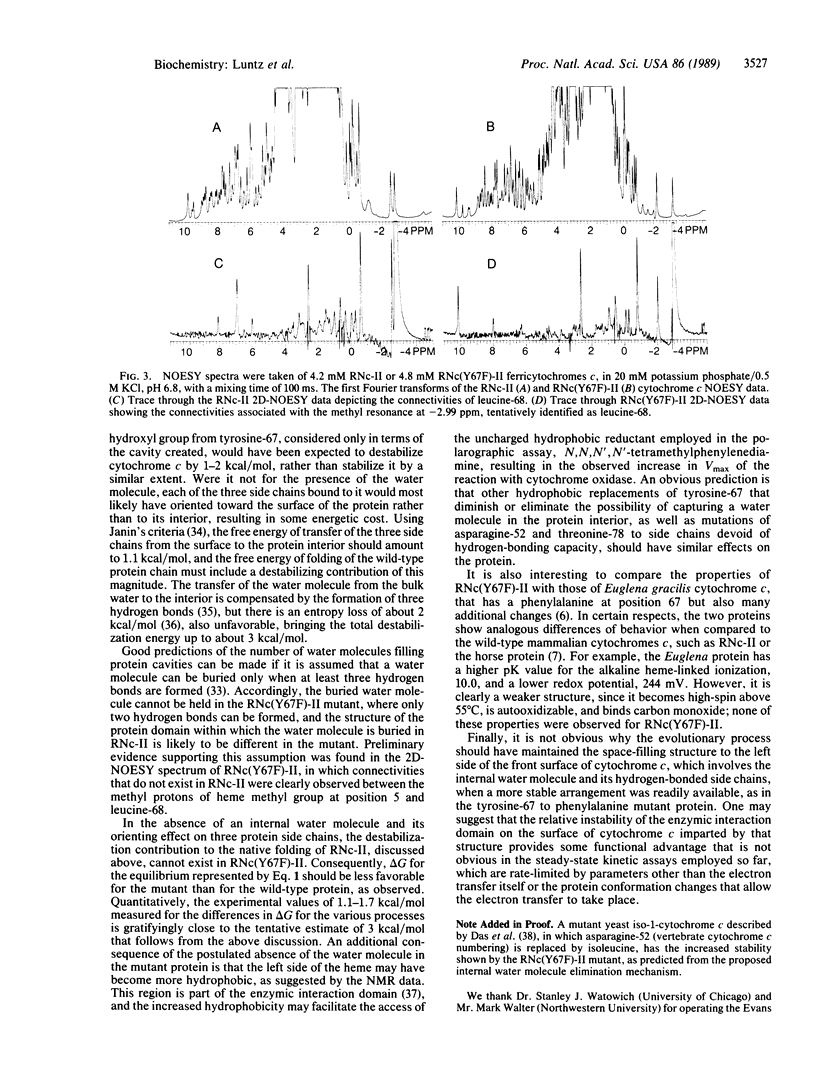
The tyrosine-67 to phenylalanine mutated rat cytochrome c is similar to the unmutated protein in its spectral, reduction potential, and enzymic electron-transfer properties. However, the loss of the 695-nm band, characteristic of the ferric form of the normal low-spin physiologically active configuration, occurs 1.2 pH units higher on the alkaline side and 0.7 pH unit lower on the acid side. Similarly, the heme iron-methionine-80 sulfur bond is more stable to temperature, with the midpoint of the transition being 30 degrees C higher, corresponding to an increase in delta H of 5 kcal/mol (1 cal = 4.184 J), partially mitigated by an increase of 11 entropy units in delta S. Urea has only slightly different effects on the two proteins. These phenomena are best explained by considering that the loss of one of the three hydrogen-bonding side chains, tyrosine-67, asparagine-52, and threonine-78, which hold an internal water molecule on the "left, lower front" side of the protein [Takano, T. & Dickerson, R. E. (1981) J. Mol. Biol. 153, 95-115], is sufficient to prevent its inclusion in the mutant protein, leading to a more stable structure, and, as indicated by preliminary proton NMR two-dimensional phase-sensitive nuclear Overhauser effect spectroscopy analyses, a reorganization of this area. This hypothesis predicts that elimination of the hydrogen-bonding ability of residue 52 or 78 would also result in cytochromes c having similar properties. It is not obvious why the space-filling structure involving the internalized water molecule that leads to a destabilization energy of about 3 kcal/mol should be subject to extreme evolutionary conservation, when a more stable and apparently fully functional structure is readily available.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Das G., Hickey D. R., McLendon D., McLendon G., Sherman F. Dramatic thermostabilization of yeast iso-1-cytochrome c by an asparagine----isoleucine replacement at position 57. Proc Natl Acad Sci U S A. 1989 Jan;86(2):496–499. doi: 10.1073/pnas.86.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durban E., Nochumson S., Kim S., Paik W. K., Chan S. K. Cytochrome c-specific protein-lysine methyltransferase from Neurospora crassa. Purification, characterization, and substrate requirements. J Biol Chem. 1978 Mar 10;253(5):1427–1435. [PubMed] [Google Scholar]
- Faye G., Leung D. W., Tatchell K., Hall B. D., Smith M. Deletion mapping of sequences essential for in vivo transcription of the iso-1-cytochrome c gene. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2258–2262. doi: 10.1073/pnas.78.4.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fersht A. R. Dissection of the structure and activity of the tyrosyl-tRNA synthetase by site-directed mutagenesis. Biochemistry. 1987 Dec 15;26(25):8031–8037. doi: 10.1021/bi00399a001. [DOI] [PubMed] [Google Scholar]
- Janin J. Surface and inside volumes in globular proteins. Nature. 1979 Feb 8;277(5696):491–492. doi: 10.1038/277491a0. [DOI] [PubMed] [Google Scholar]
- Kellis J. T., Jr, Nyberg K., Sali D., Fersht A. R. Contribution of hydrophobic interactions to protein stability. Nature. 1988 Jun 23;333(6175):784–786. doi: 10.1038/333784a0. [DOI] [PubMed] [Google Scholar]
- Knapp J. A., Pace C. N. Guanidine hydrochloride and acid denaturation of horse, cow, and Candida krusei cytochromes c. Biochemistry. 1974 Mar 12;13(6):1289–1294. doi: 10.1021/bi00703a036. [DOI] [PubMed] [Google Scholar]
- Lowry C. V., Weiss J. L., Walthall D. A., Zitomer R. S. Modulator sequences mediate oxygen regulation of CYC1 and a neighboring gene in yeast. Proc Natl Acad Sci U S A. 1983 Jan;80(1):151–155. doi: 10.1073/pnas.80.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIASH E., FROHWIRT N., WIENER E. A study of the cytochrome c haemochromogen. Biochem J. 1959 Mar;71(3):559–570. doi: 10.1042/bj0710559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalit R., Schejter A. Cytochrome c: a thermodynamic study of the relationships among oxidation state, ion-binding and structural parameters. 1. The effects of temperature, pH and electrostatic media on the standard redox potential of cytochrome c. Eur J Biochem. 1973 Feb 1;32(3):492–499. doi: 10.1111/j.1432-1033.1973.tb02633.x. [DOI] [PubMed] [Google Scholar]
- McGowan E. B., Stellwagen E. Reactivity of individual tyrosyl residues of horse heart ferricytochrome c toward iodination. Biochemistry. 1970 Jul 21;9(15):3047–3053. doi: 10.1021/bi00817a017. [DOI] [PubMed] [Google Scholar]
- Moore G. R., Ratcliffe R. G., Williams R. J. NMR and the biochemist. Essays Biochem. 1983;19:142–195. [PubMed] [Google Scholar]
- Pettigrew G. W., Aviram I., Schejter A. Physicochemical properties of two atypical cytochromes c, Crithidia cytochrome c-557 and Euglena cytochrome c-558. Biochem J. 1975 Jul;149(1):155–167. doi: 10.1042/bj1490155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew G. W., Leaver J. L., Meyer T. E., Ryle A. P. Purification, properties and amino acid sequence of atypical cytochrome c from two protozoa, Euglena gracilis and Crithidia oncopelti. Biochem J. 1975 May;147(2):291–302. doi: 10.1042/bj1470291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalov P. L., Khechinashvili N. N. A thermodynamic approach to the problem of stabilization of globular protein structure: a calorimetric study. J Mol Biol. 1974 Jul 5;86(3):665–684. doi: 10.1016/0022-2836(74)90188-0. [DOI] [PubMed] [Google Scholar]
- Ptitsyn O. B. Thermodynamic parameters of helix-coil transitions in polypeptide chains. Pure Appl Chem. 1972;31(1):227–244. doi: 10.1351/pac197231010227. [DOI] [PubMed] [Google Scholar]
- Rashin A. A., Iofin M., Honig B. Internal cavities and buried waters in globular proteins. Biochemistry. 1986 Jun 17;25(12):3619–3625. doi: 10.1021/bi00360a021. [DOI] [PubMed] [Google Scholar]
- SCHEJTER A., GEORGE P. THE 695-MMM. BAND OF FERRICYTOCHROME C AND ITS RELATIONSHIP TO PROTEIN CONFORMATION. Biochemistry. 1964 Aug;3:1045–1049. doi: 10.1021/bi00896a006. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla R. C. Processed pseudogenes for rat cytochrome c are preferentially derived from one of three alternate mRNAs. Mol Cell Biol. 1984 Nov;4(11):2279–2288. doi: 10.1128/mcb.4.11.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schejter A., Aviram I., Sokolovsky M. Nitrocytochrome c. II. Spectroscopic properties and chemical reactivity. Biochemistry. 1970 Dec 22;9(26):5118–5122. doi: 10.1021/bi00828a012. [DOI] [PubMed] [Google Scholar]
- Schejter A., Plotkin B. The binding characteristics of the cytochrome c iron. Biochem J. 1988 Oct 1;255(1):353–356. [PMC free article] [PubMed] [Google Scholar]
- Shechter E., Saludjian P. Conformation of ferricytochrome c. IV. Relationship between optical absorption and protein conformation. Biopolymers. 1967;5(8):788–790. doi: 10.1002/bip.1967.360050812. [DOI] [PubMed] [Google Scholar]
- Sherman F., Stewart J. W., Parker J. H., Inhaber E., Shipman N. A., Putterman G. J., Gardisky R. L., Margoliash E. The mutational alteration of the primary structure of yeast iso-1-cytochrome c. J Biol Chem. 1968 Oct 25;243(20):5446–5456. [PubMed] [Google Scholar]
- Sokolovsky M., Aviram I., Schejter A. Nitrocytochrome c. I. Structure and enzymic properties. Biochemistry. 1970 Dec 22;9(26):5113–5118. doi: 10.1021/bi00828a011. [DOI] [PubMed] [Google Scholar]
- Stewart J. W., Sherman F., Shipman N. A., Jackson M. Identification and mutational relocation of the AUG codon initiating translation of iso-1-cytochrome c in yeast. J Biol Chem. 1971 Dec 25;246(24):7429–7445. [PubMed] [Google Scholar]
- Takano T., Dickerson R. E. Conformation change of cytochrome c. I. Ferrocytochrome c structure refined at 1.5 A resolution. J Mol Biol. 1981 Nov 25;153(1):79–94. doi: 10.1016/0022-2836(81)90528-3. [DOI] [PubMed] [Google Scholar]
- Takano T., Dickerson R. E. Conformation change of cytochrome c. II. Ferricytochrome c refinement at 1.8 A and comparison with the ferrocytochrome structure. J Mol Biol. 1981 Nov 25;153(1):95–115. doi: 10.1016/0022-2836(81)90529-5. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]



